Abstract
The factor II G20210A mutation is a recently identified congenital risk factor for venous thrombosis. Its role in artery disease is still undefined. We investigated 72 patients (35 male and 37 female) with documented ischemic stroke occurred before 50 years of age and without risk factors such as diabetes, hypertension, and hyperlipidemia; 198 thrombosis-free individuals were investigated as the control group. We found 7 heterozygotes (9.7%) and 2 homozygotes (2.7%) for the mutant factor II allele among the patients and 5 heterozygotes (2.5%) among the controls; the mutant factor II allele frequency in the patient group (7.6%, 95% confidence interval [CI], 3.3 to 11.9) was significantly higher than in the controls (1.2%; 95% CI, 0.1 to 2.3;P = .0001). The prevalence of other investigated mutant alleles (factor V G1691A, methylenetetrahydrofolate reductase C677T) did not significantly differ between the two groups. The odds ratio for ischemic stroke associated with the carriership of the mutant factor II allele (both heterozygous and homozygous genotypes) was 5.1 (95% CI, 1.6 to 16.3). Heterozygous genotype was associated with a 3.8-fold increased risk for cerebral ischemia (95% CI, 1.1 to 13.1); in particular, assuming an expected prevalence of homozygotes in the general population of 1.6 to 10,000 according to the Hardy-Weinberg equilibrium, the risk associated with the homozygous genotype was estimated exceedingly high, being increased 208-fold.
RECENTLY, A NOVEL mutation in the 3′-untranslated region of the prothrombin gene (20210G to A) associated with high levels of plasma prothrombin has been reported to be a moderate risk factor for venous thrombosis, being present in heterozygous form in 5.4% to 7.3% of the patients and in 1.2% to 2.3% of the controls, with an increase in risk by threefold to fivefold.1-4 The association of the mutant factor II allele with arterial thrombosis has been reported in patients with myocardial infarction weaker than in venous thrombosis (2-fold increase in risk), without reaching statistical significance5; in a more recent report, the heterozygous mutant factor II genotype was found associated with a significant increased risk (4-fold) for myocardial infarction in young women.6 The investigations so far conducted on unselected patients with cerebral ischemia did not show any increase in risk associated with the mutant factor II allele.4 7 In the present study, we investigated the prevalence of the mutant factor II allele in selected young patients with ischemic stroke.
PATIENTS AND METHODS
We investigated 72 patients (35 males and 37 females) with ischemic stroke occurred before 50 years of age (mean age, 33.9; median age, 37 years; age range, 2 to 50 years) and without risk factors such as hypertension, diabetes, and increased levels of cholesterol or triglycerides. Complete information about smoking habit (current and former habit and number of cigarettes smoken per day) was not available for the totality of the patients and the controls investigated, so that this was not considered in the final interpretation of the results. The patients were consecutively referred for laboratory evaluation since November 1994 through October 1997; 60 of them had been admitted to the Catholic University hospital (Policlinico Gemelli, Rome) because of acute cerebral ischemia, whereas the remaining 12 had a past history of cerebral ischemia and were referred by other hospitals or by their family physicians. The patients were selected for the laboratory investigation only if the ischemic event was documented by computer tomography (CT) scan and/or nuclear magnetic resonance (NMR) of the brain; blood was collected in sodium citrate and plasma as well as genomic DNA were stored at −80°C until assayed. In 10 of the 37 female patients (27%), intake of oral contraceptives was present at the moment of the acute event; in another 2 patients, ischemic stroke occurred during pregnancy (n = 1) or puerperium (n = 1). In all patients, the presence of the 20210 G to A substitution in the prothrombin gene was investigated on genomic DNA prepared by standard procedures; mutant factor II was detected by amplification by polymerase chain reaction (PCR) and digestion of the fragment with HindIII according to the original method of Poort et al.1 Laboratory evaluation included also evaluation of natural coagulation inhibitors (antithrombin and protein C functional levels and free protein S antigen levels) as well as detection of the G1691A mutation in the factor V gene (factor V Leiden)8 and the C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene (thermolabile variant), a possible cause of mild hyperhomocysteinemia in homozygous individuals.9 The mutant alleles were investigated also in a control group of 198 thrombosis-free individuals (78 males and 120 females; mean age, 49 years; median age, 49 years; age range, 16 to 81 years). All of the patients and the control individuals were of Italian ancestry (Middle and Southern Italy).
The differences between the patient and the control groups were evaluated using the χ2 test. The univariate odds ratio was calculated as an approximation of relative risk for putative risk factors by simple cross-tabulation, with 95% confidence intervals (CI).
RESULTS
No significant variation was found between the patient group and the controls as regards the prevalence of factor V Leiden carriers, the overall allele frequency of the factor V Leiden, the prevalence of homozygous carriers of the MTHFR C677T mutation, and the overall allele frequency of the MTHFR C677T mutation (Table 1). One patient had levels of free protein S antigen below the normal range.
Prevalence of Mutant Genotypes in 72 Individuals With Ischemic Stroke Before 50 Years of Age and in 198 Controls
| Carriers . | Patients (n = 72) . | Controls (n = 198) . | P Value . |
|---|---|---|---|
| Factor V G/A | 4 (5.5%) | 5 (2.5%) | .2199 |
| Factor V A/A | 0 | 1 (0.5%) | .5457 |
| Factor II G/A | 6 (8.3%) | 5 (2.5%) | .0328 |
| Factor II A/A | 2 (2.8%) | 0 | .0186 |
| FV G/A + FII G/A | 1 (1.4%) | 0 | .0966 |
| MTHFR C/T | 27 (37.5%) | 98 (49.5%) | .0805 |
| MTHFR T/T | 17 (23.6%) | 35 (17.7%) | .2742 |
| Allele Frequency | Patients | Controls | P Value |
| Factor V A allele (95% CI) | 3.4% (0.4-6.4) | 1.7% (0.4-3.0) | .2347 |
| Factor II A allele (95% CI) | 7.6% (3.3-11.9) | 1.2% (0.1-2.3) | .0001 |
| MTHFR T allele (95% CI) | 42.3% (34.2-50.4) | 42.4% (37.5-47.2) | .9895 |
| Carriers . | Patients (n = 72) . | Controls (n = 198) . | P Value . |
|---|---|---|---|
| Factor V G/A | 4 (5.5%) | 5 (2.5%) | .2199 |
| Factor V A/A | 0 | 1 (0.5%) | .5457 |
| Factor II G/A | 6 (8.3%) | 5 (2.5%) | .0328 |
| Factor II A/A | 2 (2.8%) | 0 | .0186 |
| FV G/A + FII G/A | 1 (1.4%) | 0 | .0966 |
| MTHFR C/T | 27 (37.5%) | 98 (49.5%) | .0805 |
| MTHFR T/T | 17 (23.6%) | 35 (17.7%) | .2742 |
| Allele Frequency | Patients | Controls | P Value |
| Factor V A allele (95% CI) | 3.4% (0.4-6.4) | 1.7% (0.4-3.0) | .2347 |
| Factor II A allele (95% CI) | 7.6% (3.3-11.9) | 1.2% (0.1-2.3) | .0001 |
| MTHFR T allele (95% CI) | 42.3% (34.2-50.4) | 42.4% (37.5-47.2) | .9895 |
Odds ratio for ischemic stroke: Carriership of factor II A allele (both G/A and A/A genotypes): 5.5 (95% CI, 1.7-17.0). After adjustment for other inherited causes of thrombophilia: carriership of factor II A allele: 5.1 (95% CI, 1.6-16.3); heterozygous genotype: 3.8 (95% CI, 1.1-13.1); homozygous genotype: 208.0 (95% CI, 14.4-2,957.2).
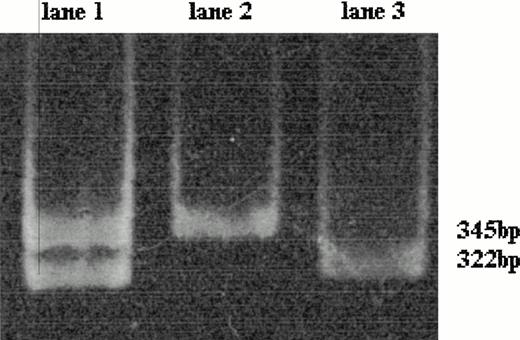
The normal sequence of the prothrombin gene displayed a band of 345 bp after HindIII digestion. The mutant factor II gene heterozygous genotype is characterized by two fragments of 345 and 322 bp; the absence of the fragment of 345 bp and the presence of only the fragment of 322 bp is distinctive of the homozygous genotype (Fig 1).
Polyacrylamide gel electrophoresis of the amplified 345-bp fragment of factor II gene after HindIII digestion; the mutant factor II gene has a restriction site for HindIII leading to the production of two 322- and 23-bp fragments (the latter not detectable here). The wild-type factor II gene is characterized by the presence of the only 345-bp fragment (lane 2). The mutant factor II gene heterozygous genotype (lane 1) is characterized by two fragments of 345 bp (wild-type allele) and 322 bp (mutant allele); the homozygous genotype shows only a 322-bp fragment (lane 3).
Polyacrylamide gel electrophoresis of the amplified 345-bp fragment of factor II gene after HindIII digestion; the mutant factor II gene has a restriction site for HindIII leading to the production of two 322- and 23-bp fragments (the latter not detectable here). The wild-type factor II gene is characterized by the presence of the only 345-bp fragment (lane 2). The mutant factor II gene heterozygous genotype (lane 1) is characterized by two fragments of 345 bp (wild-type allele) and 322 bp (mutant allele); the homozygous genotype shows only a 322-bp fragment (lane 3).
In the patient group, we found 9 individuals carriers of the factor II mutant allele (12.5%; 95% CI, 4.8 to 20.1): 7 heterozygotes (2 males and 5 females) and 2 homozygotes (2 males and 0 females). In the control group, 5 individuals were heterozygous for the mutant factor II allele (2.5%; 95% CI, 0.3 to 4.7). One of the heterozygous patients had associated heterozygosity for factor V Leiden mutation (Table 1).
One homozygous patient was a 26-year old man having suffered at 24 years of age from ischemic stroke in the left thalamic region. At 26 years of age, he had an ischemic recurrence in the left calcarine cortex leading to hemianopsia and afterwards underwent aspirin treatment. Family investigation demonstrated heterozygosity for mutant factor II allele both in the father (54 years of age) and in the mother (52 years of age) of the proband; also, her sister (20 years of age) was heterozygous for mutated factor II gene. All of these family members did not experience thrombosis. The other homozygous individual was a 26-year-old man with acute ischemia involving the basilar artery documented by NMR; he was treated with intravenous heparin and afterwards with oral anticoagulants. Transesophageal echocardiography demonstrated the presence of a patent foramen ovale. Both the homozygous patients were nonsmokers. Three of the 7 heterozygotes identified were nonsmokers (overall percentage of nonsmokers among the carriers of the factor II mutant allele was 55.5%).
Among the 10 patients taking oral contraceptives at the moment of the acute event, 1 was protein S-deficient, 1 was heterozygous for factor V Leiden, and 2 were homozygous for MTHFR C677T mutation. No alteration was found in the 2 women having suffered from cerebral ischemia during pregnancy or puerperium. Among the other 25 female patients, 2 were heterozygous for factor V Leiden and 4 were homozygous for MTHFR C677T mutation, with a distribution of mutant genotypes almost identical to that found in the group of women with ischemic stroke after oral contraceptives. No significant difference was found between the prevalence of heterozygous mutant factor II genotype among the female patients having suffered from cerebral ischemia after exposure to a circumstantial risk factor (1/12 [8.3%]) and the prevalence among women without such exposure (4/25 [16%]; P = .5231). Considering the 60 patients (35 males and 25 females) without a concomitant risk situation, the overall prevalence of affected individuals was 13.3% (11.4% among male patients and 16% among female patients).
The mutant factor II allele frequency in the patient group was 7.6%, at significant variance with the control group (1.2%, P = .0001; Table 1). The risk for ischemic stroke associated with the carriership (both heterozygous and homozygous) of the mutant prothrombin allele was 5.5-fold increased. After exclusion of the patient with protein S deficiency and of the individual carriers of the factor V Leiden, the risk for carriers of the factor II mutant allele was substantially unchanged (Table 1). The adjusted odds ratio associated with the isolated heterozygous factor II mutant genotype was 3.8 (95% CI, 1.1 to 13.1). No homozygous individual carrier of mutant factor II allele was found in the control group; therefore, the odds ratio associated with the homozygous genotype was estimated calculating from Hardy-Weinberg equilibrium the expected number of homozygotes among the controls, following a procedure previously described in detail.10 According to the Hardy-Weinberg equilibrium, the expected number of homozygous individuals in the normal population is 1.58/10,000 (mutant allele frequency 0.01262), leading to an expected number of 0.031 homozygous individuals among the 198 controls.
Accordingly, in our series, the odds ratio associated with homozygous mutant factor II genotype after exclusion of the 12 individuals with protein S deficiency (n = 1), factor V Leiden (n = 4), heterozygous factor II mutant genotype (n = 6), combined heterozygosity for factor V Leiden and mutant factor II (n = 1) resulted in 208, with a 95% CI of 14 to 2,957 estimated according to Rosendaal et al10 (Table 1).
DISCUSSION
Mutation in the 3′-untranslated region of the prothrombin gene is a congenital risk factor for venous thrombosis recently identified1; it is associated with high levels of prothrombin and is present in 1% to 3.2% of the general population with European ancestry.1-4,7 Recently, the factor II mutated allele was demonstrated to be associated with an increased risk for myocardial infarction in young women, especially if they are smokers.6 We found that 12.5% (9/72) of patients with ischemic stroke before reaching 50 years of age and with absence of hypertension or evident metabolic risk factors are carriers of the factor II G20210A gene mutation; in the control group, the prevalence of the carriers was 2.5%. The prevalence of affected individuals among the patients is very comparable with that found in patients with venous thrombotic disease, which has been reported to be from 5% to 7%.1-4 In the present series, the mutant prothrombin II allele frequency is the only putative risk factor for ischemic stroke, which was significantly different from the control group (7.6%v 1.2%); we found an increased prevalence of factor V Leiden carriers and of homozygous carriers of the C677T MTHFR gene mutation, but without reaching the statistical significance. Carriership of the mutant factor II allele (both heterozygous and homozygous genotypes) was found to be associated with a 5.1-fold increase in risk for ischemic stroke. The risk associated with the heterozygous genotype was 3.8-fold increased. This finding disagrees with the results of two recent studies that failed to demonstrate in patients with cerebral ischemia any increase in risk associated with the factor II mutant allele.4,7 In both investigations, the patients were not selected according to age; however, in the study of Martinelli et al,7 the young age was not found to be associated with an increased prevalence of the mutant factor II allele. Yet, the clinical manifestations of such patients were not homogeneous, including also transient ischemic attacks; moreover, no selection criterium, such as the absence of classic vascular risk factors, was adopted.4,7 In contrast, we investigated a population of patients highly selected, with documented ischemic stroke, age younger than 50 years, and no evident constitutional risk factor for arterial disease. In the patient group, we found 2 homozygous carriers of the factor II gene mutation (2.7%). This is quite surprising, because pooling the data obtained from six investigations published in detail, no homozygous individual was detected among 1,741 healthy controls, 871 patients with venous thrombotic disease, and 439 patients with arterial thrombotic disease.1-4,6,7 Moreover, we did not find any homozygote in a series of 343 patients with venous thrombotic disease investigated in our laboratory (unpublished results). To the best of our knowledge, only 2 homozygous individuals have been so far reported. One of them was the sister of a proband patient investigated by Poort et al1; she was heterozygous for factor V Leiden too and experienced thrombotic symptoms.1The other one was a 18-year-old woman suffering from deep vein thrombosis during pregnancy.11 According to the Hardy-Weinberg equilibrium, the mutant factor II allele frequency found in our control population leads to an expected prevalence of homozygous carriers in Italians of 1.6 to 10,000, so that the finding of 2.7% homozygous genotype among young patients with ischemic stroke strongly suggests a relationship between the inherited condition and the thrombotic symptoms. No evident risk factor for artery disease was found in the 2 young homozygous patients that we identified; in 1 of them, the presence of a patent foramen ovale was demonstrated, but the role of this anatomic variant as main cause by itself of cardioembolic stroke is controversial.12 To estimate the risk for ischemic stroke associated with the mutant factor II homozygous genotype, we applied a procedure previously described by Rosendaal et al10 for investigating the thrombotic risk related to homozygous factor V Leiden genotype. The increase in risk we found was 208-fold, which was statistically significant yet with a large range of the 95% CI. We conclude that the 12.5% prevalence of carriers of mutant factor II allele among young patients with ischemic stroke is at least comparable with that found in patients with venous thrombotic disease and that it is associated with an increase in risk, so that genotyping for this mutation should be included in the diagnostic panel of cerebral ischemia. In particular, the homozygous genotype seems highly predisposing to ischemic stroke and further investigations on larger series of patients are advisable.
Address reprint requests to Valerio De Stefano, MD, Divisione di Ematologia, Istituto Semeiotica Medica, UniversitàCattolica, Largo Gemelli 8, 00168 Roma, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal