Abstract
Induction of chemokine gene expression from peripheral blood mononuclear cells (PBMCs) stimulated by proinflammatory cytokines plays an important role in both wound repair and response to infectious agents. In the present study, we show that the proinflammatory cytokine interleukin-6 (IL-6) potently induced mRNA expression and secretion of the CC chemokine monocyte chemotactic protein 1 (MCP-1) in PBMCs. In addition, because human immunodeficiency virus (HIV) infection in vivo and in vitro has been shown to dysregulate the production of and/or the response to cytokines, PBMCs from both healthy uninfected and HIV-infected individuals were studied for their constitutive and IL-6–induced expression of MCP-1. No substantial differences were observed between the two groups of individuals. In addition, IL-6 upregulated MCP-1 expression in the promonocytic cell line U937 and in its chronically HIV-infected counterpart, U1. In these cell lines, IL-6 selectively induced MCP-1 and not other chemokines, including regulated upon activation normal T cells expressed and secreted (RANTES), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and IL-8. IL-6 induction of MCP-1 was partially inhibited by hydrocortisone in U1 cells. Thus, IL-6 activates PBMCs to secrete MCP-1, a CC chemokine pivotal for monocyte recruitment in tissue and organs in which important inflammatory events occur.
MONOCYTE CHEMOTACTIC protein-1 (MCP-1) belongs to the β or CC family of chemotactic cytokines, or chemokines,1 which stimulate the migration of monocytic cells, whereas α or CXC chemokines predominantly activate polymorphonuclear leukocytes.2,3 The coordinated synthesis and release of MCP-1 plays a central role in both acute and chronic inflammatory processes by controlling the influx of phagocytic cells and also their state of activation in concert with primary inflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and IL-6.2,3 It is of interest that a selective accumulation of MCP-1 in the cerebrospinal fluid (CSF) of AIDS patients with cytomegalovirus (CMV) encephalitis, but not with other opportunistic infections (OI) or primary lymphomas of the central nervous system (CNS), has been recently described.4 In addition, we have recently observed that a similar selective accumulation of MCP-1 in the CSF occurred in patients with human immunodeficiency virus (HIV) encephalitis (Cinque et al, manuscript submitted). These findings suggest a potential role of MCP-1 in monocyte recruitment to the brain in this acquired immunodeficiency syndrome (AIDS)-related disease. It is conceivable that the accumulation of MCP-1 in the CSF may be secondary to the known ability of CMV to induce several proinflammatory cytokines, including TNF-α, IL-1β, leukemia inhibitory factor (LIF), and IL-6.5-8 All these cytokines, as well as IL-4 and interferon-γ (IFN-γ), have been shown to induce MCP-1 expression in human endothelial cells (ECs), mononuclear phagocytes, and fibroblasts.9-15 Because both LIF and IL-6 share a common gp130 transducing chain,16 the observation that LIF can induce MCP-1 from human monocytes supports the hypothesis that IL-6 may exert a similar regulatory effect in cells of monocytic lineage. In support of this hypothesis, IL-6 has been reported to induce MCP-1 in rat brain macrophages17 and also in mesenchymal cells such as murine fibroblasts18 and human osteoblast cell lines.19
IL-6 is a multifunctional cytokine that plays an important role in several physiologic processes, including hematopoiesis, B-cell proliferation, plasma cell differentiation, and hepatocyte growth.16,20,21 Furthermore, IL-6 is a component of the inflammatory cascade,22-25 and it is a critical factor for triggering the acute-phase response.20,26,27 In addition to inflammatory diseases, increased levels of IL-6 have been described in the plasma and CSF of HIV-infected individuals.28-31
In the present study, we have investigated whether IL-6 could induce MCP-1 expression in primary peripheral blood mononuclear cells (PBMCs) obtained both from healthy HIV-seronegative individuals and from HIV-infected individuals and in the promonocytic cell lines U937 (uninfected) and U1 (chronically HIV-infected).
MATERIALS AND METHODS
Patients.
The HIV-seropositive individuals who donated their blood for PBMC studies were observed at the Division of Infectious Diseases of the San Raffaele Scientific Institute (Milan, Italy). They had progressive disease, with CD4+ counts of less than 200/μL, and were undergoing different therapeutic regimens.
Cell cultures.
All cultures were maintained in RPMI 1640 supplemented with glutamine (2 mmol/L), penicillin (100 U/mL), streptomycin (100 μg/mL; Biowhittaker, Verviers, Belgium), and 10% fetal calf serum (Hyclone Europe, Ltd, Cramlington, UK).
Cell lines.
U937 is a well-known human promonocytic cell line32 that can be induced to differentiate into mature macrophages by several agents, including phorbol 12-myristate 13-acetate (PMA). The chronically infected U1 cell line was obtained by limiting dilution cloning of U937 cells acutely infected with HIV-1 (IIIB/LAI strain).33 Both U937 and U1 cells were seeded at the initial concentration of 4 × 105 cells/mL in 48-well polystirene culture plates (Falcon, Becton Dickinson Labware, Lincoln Park, NJ), and culture supernatants were harvested at the indicated time points and stored at −80°C until tested for the presence of cytokines, chemokines, and, in the case of U1 cells, HIV.
Primary cells.
Whole venous blood and buffy coats donated by the transfusional center of the San Raffaele Scientific Institute were obtained from HIV-infected individuals and healthy donors, respectively. PBMCs, which were isolated by centrifugation on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient, were cultured at the concentration of 1.5 × 106 cells/mL in 24-well culture plates (Falcon) in the absence and presence of IL-6 (10 ng/mL) or lipopolysaccharide (LPS; 1 μg/mL), and supernatants were harvested at the indicated time points.
Reagents.
LPS from Escherichia coli (serotype 055:B55) and PMA (Sigma Chemical Corp, St Louis, MO) were used at the fixed final concentrations of 1 μg/mL and 10 nmol/L, respectively. One hundred, 10, and 1 nmol/L final concentrations of hydrocortisone (Sigma) were used in some experiments. Human recombinant TNF-α, IL-6, and IFN-γ were purchased from R&D Systems (Minneapolis, MN) and were used at the concentrations of 0.02 nmol/L (1 ng/mL), 0.24 nmol/L (10 ng/mL), and 1.5 nmol/L (50 ng/mL), respectively.
Measurement of cytokine and chemokine contents.
Reverse transcriptase-polymerase chain reaction (RT-PCR) detection of MCP-1 mRNA.
Total cellular RNA was isolated from 3 to 6 × 106PBMCs with RNA-zol B (Duotech S.r.l., Milan, Italy), following the manufacturer's protocol. RNA (1 μg) was reverse transcribed by incubation at 42°C for 40 minutes and at 94°C for 5 minutes in the presence of 1× RT buffer (GIBCO BRL, Life Technologies, Paisley, UK), 800 mmol/L dNTPs (Pharmacia), 20 μg/mL random hexamers (Promega, Madison, WI), 4 mmol/L dithiothreitol (GIBCO BRL), 12 U RNA guard (Promega), and 400 U of Moloney murine leukemia virus (M-MLV) RT (GIBCO BRL) in a 50-μL reaction. One twentieth of the cDNA obtained from the RT reaction was amplified with primers specific for MCP-1 (forward, 5′-CAATAGGAAGATCTCAGTGC; reverse, 5′-GTGTTCAAGTCTTCGGAGTT)35 in the presence of 2.5 U of Taq polymerase (Ex Taq; Taqara Biomedical Europe S.A., Gennevilliers, France), following the instructions of the manufacturer. To verify that equal amounts of cDNA were added in each PCR reaction, the housekeeping gene GAPDH was amplified from 1/20 of the same cDNA, using specific primers (forward, 5′-CCATGGAGAAGGCTGGGG; reverse, 5′-CAAAGTTGTCATGGATGACC).36 Amplification (30 seconds of denaturation at 94°C, 45 seconds of annealing at 60°C, and 60 seconds of extension at 72°C) was performed for 32 cycles with MCP-1 primers and for 28 cycles with GAPDH primers; amplifications were preceded by a denaturation step (90 seconds at 94°C) and followed by a final extension period (7 minutes at 72°C). PCR amplification products were analyzed by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
HIV-RT activity.
HIV expression, monitored as Mg2+-dependent RT activity released into supernatants of chronically infected U1 cells, was measured as previously described.37
Northern blot analyses.
A total of 107 U1 cells (4 × 105 cells/mL in 25 mL per flask; Falcon) were seeded in culture for each condition and time point. Northern blot analyses were performed according to standard procedures.38 Briefly, 10 μg of total RNA was isolated by the guanidium isothiocyanate method and analyzed by electrophoresis through 1% agarose/formaldehyde gel, followed by Northern blot transfer to Gene Screen Plus membranes (New England Nuclear, Boston, MA). The probe, human MCP-1 cDNA (0.672-kb PstI-Pst I fragment)39 was labeled by Megaprime DNA labeling system (Amersham, Buckinghamshire, UK) with α32P-dCTP (3,000 Ci/mmol; Amersham).
RESULTS
IL-6 induces MCP-1 expression in PBMCs of healthy seronegative and HIV-infected individuals.
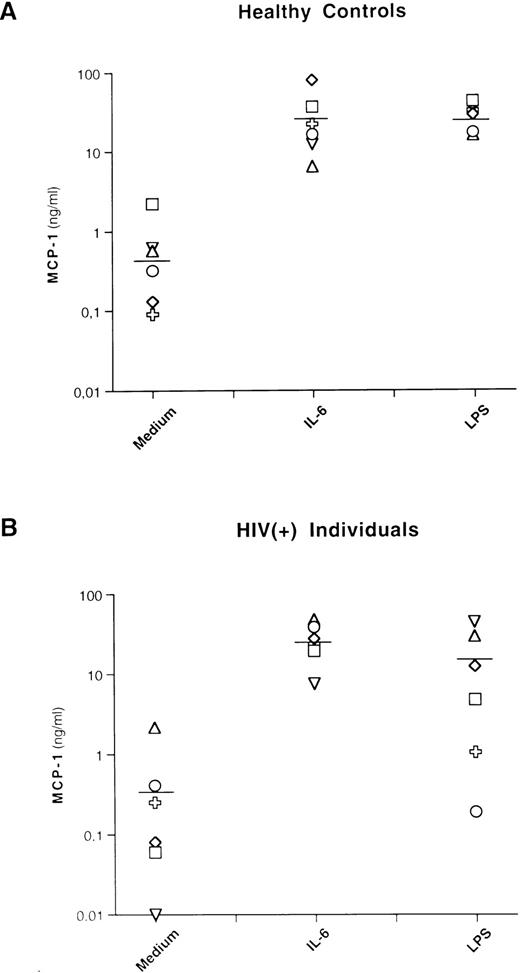
Basal levels of MCP-1 were detectable, with a mean of 0.7 ng/mL (±0.8 standard deviation [SD]) and 0.5 ng/mL (±0.8 SD) for healthy controls and HIV-seropositive individuals, respectively. IL-6 stimulation induced substantial levels of MCP-1 in PBMCs from both groups of individuals, with a mean of 29 ng/mL (±26 SD) and 27.3 ng/mL (±14.3 SD) for uninfected and HIV-infected individuals, respectively (Fig 1). These levels were comparable to those induced by LPS in control individuals (mean, 28 ng/mL; ±10.1 SD) and were even higher, although not statistically significant, than those induced by LPS in HIV-infected individuals (mean, 14.9 ng/mL; ±16.9 SD).
IL-6 and LPS induce MCP-1 secretion in healthy and HIV-seropositive individuals. PBMCs from 6 healthy controls and 6 HIV-seropositive individuals were cultivated with medium alone (Medium), IL-6 (0.24 nmol/L), or LPS (1 μg/mL). After 72 hours of culture, supernatants were harvested and frozen at −80°C until tested for MCP-1 content. Each symbol corresponds to a culture established from a single individual. The lines indicate the mean MCP-1 concentrations: 0.7 (medium), 29 (IL-6), and 28 (LPS) ng/mL for healthy controls and 0.5 (medium), 27.3 (IL-6), and 14.9 (LPS) ng/mL for HIV-seropositive individuals, respectively. No statistical differences in terms of MCP-1 production after stimulation with LPS were observed between cultures established from HIV-negative and HIV-seropositive individuals (P = .15, nonparametric Rank Sums test). A total of 27 ng/mL of MCP-1 was produced from 1 patient (○) after 8 days of stimulation with LPS.
IL-6 and LPS induce MCP-1 secretion in healthy and HIV-seropositive individuals. PBMCs from 6 healthy controls and 6 HIV-seropositive individuals were cultivated with medium alone (Medium), IL-6 (0.24 nmol/L), or LPS (1 μg/mL). After 72 hours of culture, supernatants were harvested and frozen at −80°C until tested for MCP-1 content. Each symbol corresponds to a culture established from a single individual. The lines indicate the mean MCP-1 concentrations: 0.7 (medium), 29 (IL-6), and 28 (LPS) ng/mL for healthy controls and 0.5 (medium), 27.3 (IL-6), and 14.9 (LPS) ng/mL for HIV-seropositive individuals, respectively. No statistical differences in terms of MCP-1 production after stimulation with LPS were observed between cultures established from HIV-negative and HIV-seropositive individuals (P = .15, nonparametric Rank Sums test). A total of 27 ng/mL of MCP-1 was produced from 1 patient (○) after 8 days of stimulation with LPS.
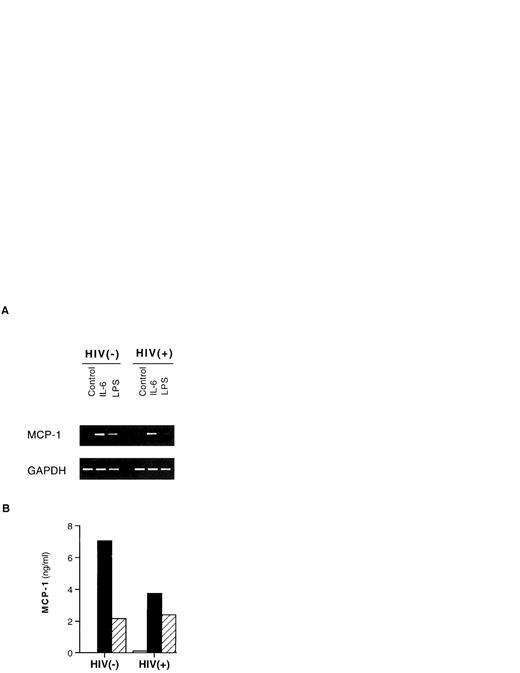
IL-6 caused an increase of the levels of MCP-1 mRNA as detected at 24 hours (Fig 2A) in the very same cell cultures in which the chemokine was simultaneously released in the supernatants (Fig 2B).
Induction of MCP-1 mRNA and protein by IL-6 and LPS in PBMCs. One HIV-negative and one HIV-positive individual are shown. PBMCs were cultivated for 24 hours with medium alone (control), IL-6 (0.24 nmol/L), or LPS (1 μg/mL). (A) The cells were pelletted and frozen until processed for RT-PCR analysis of MCP-1 mRNA compared with the levels of expression of the housekeeping gene GAPDH. (B) Cell culture supernatants were harvested and frozen at −80°C until tested for MCP-1 protein content by ELISA. Similar results were obtained with PBMCs from two additional HIV-negative and two HIV-seropositive individuals cultured under the same conditions for 24 hours. (□) Unstimulated; (▪) IL-6 (0.24 nmol/L); (▨) LPS (1 μg/mL).
Induction of MCP-1 mRNA and protein by IL-6 and LPS in PBMCs. One HIV-negative and one HIV-positive individual are shown. PBMCs were cultivated for 24 hours with medium alone (control), IL-6 (0.24 nmol/L), or LPS (1 μg/mL). (A) The cells were pelletted and frozen until processed for RT-PCR analysis of MCP-1 mRNA compared with the levels of expression of the housekeeping gene GAPDH. (B) Cell culture supernatants were harvested and frozen at −80°C until tested for MCP-1 protein content by ELISA. Similar results were obtained with PBMCs from two additional HIV-negative and two HIV-seropositive individuals cultured under the same conditions for 24 hours. (□) Unstimulated; (▪) IL-6 (0.24 nmol/L); (▨) LPS (1 μg/mL).
Thus, no significant differences were observed between HIV-negative and HIV-positive individuals, indicating that this viral infection does not influence either basal production of MCP-1 or leukocyte responsiveness to IL-6 and LPS, at least in the peripheral blood compartment. In support of this interpretation, no substantial levels of MCP-1 were detected in the plasma of AIDS patients with CMV encephalitis, which is in sharp contrast to the substantial accumulation of this chemokine in their CSF.4
IL-6 induces MCP-1 expression in U937 and in U937-derived chronically HIV-infected U1 cell lines.
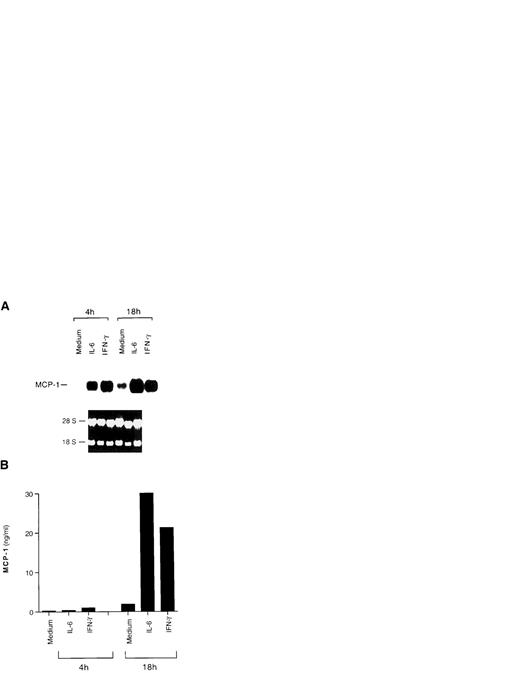
Among immune cells, monocytic cells are known to be the main producers of MCP-1.13 Furthermore, the IL-6–related molecule LIF was shown to induce MCP-1 in human monocytes.15 Therefore, we tested whether IL-6 was capable of activating MCP-1 expression in the promonocytic cell lines U937 and U1. IL-6 induced substantial levels of MCP-1 in U937 cells; in addition, TNF-α and IFN-γ, which were previously described to upregulate MCP-1 expression,10-12,15 40-43 also induced secretion of this chemokine (Fig 3). Basal expression of MCP-1 was shown in HIV-infected U1 cells using Northern blot analysis (Fig 4A), whereas stimulation with either IL-6 or IFN-γ caused an increased accumulation of both mRNA (Fig 4A) and release of MCP-1 in culture supernatants (Fig 4B), as observed in uninfected U937 cells. In experiments in which different concentrations of TNF-α were compared, a dose-response of MCP-1 secretion from U1 cells was observed (with 0.3, 12.6, 23.9, and 27.7 ng/mL of MCP-1 secreted from cells that were unstimulated or were stimulated with 0.02, 0.2, or 2 nmol/L of TNF-α, respectively; data not shown).
Kinetics of chemokine secretion by U937 cells. U937 cells were cultivated with (○) medium alone, (•) IL-6 (0.24 nmol/L), (▪) IFN-γ (1.5 nmol/L), or (▴) TNF-α (0.02 nmol/L) for 72 hours. Supernatants were harvested daily, aliquoted, and frozen at −80°C until tested for the presence of MCP-1.
Kinetics of chemokine secretion by U937 cells. U937 cells were cultivated with (○) medium alone, (•) IL-6 (0.24 nmol/L), (▪) IFN-γ (1.5 nmol/L), or (▴) TNF-α (0.02 nmol/L) for 72 hours. Supernatants were harvested daily, aliquoted, and frozen at −80°C until tested for the presence of MCP-1.
Induction of MCP-1 expression by IL-6 and IFN-γ in HIV-infected U1 cells. (A) Northern blot analysis of MCP-1 expression after 4 and 18 hours of culture with medium alone, IL-6 (0.24 nmol/L), or IFN-γ (1.5 nmol/L). The results of one representative experiment of three independently performed are shown. Intermediate (11 hours) and later (36 hours) time points were evaluated in the additional two experiments, showing that baseline and cytokine-induced MCP-1 message decreases after 36 hours of culture. Ethidium bromide staining of the 18S and 28S ribosomal RNA is shown as a control for RNA loading. (B) MCP-1 secretion in supernatants harvested after 4 and 18 hours of culture from the same cells of the experiment shown in (A). Low, but detectable levels of MCP-1 were already present in the supernatants 4 hours after stimulation.
Induction of MCP-1 expression by IL-6 and IFN-γ in HIV-infected U1 cells. (A) Northern blot analysis of MCP-1 expression after 4 and 18 hours of culture with medium alone, IL-6 (0.24 nmol/L), or IFN-γ (1.5 nmol/L). The results of one representative experiment of three independently performed are shown. Intermediate (11 hours) and later (36 hours) time points were evaluated in the additional two experiments, showing that baseline and cytokine-induced MCP-1 message decreases after 36 hours of culture. Ethidium bromide staining of the 18S and 28S ribosomal RNA is shown as a control for RNA loading. (B) MCP-1 secretion in supernatants harvested after 4 and 18 hours of culture from the same cells of the experiment shown in (A). Low, but detectable levels of MCP-1 were already present in the supernatants 4 hours after stimulation.
IL-6 induced a twofold upregulation of MCP-1 secretion over low but detectable constitutive levels also in the myelomonocytic cell line HL-60 (data not shown).
Selective MCP-1 induction by IL-6 and TNF-α in U1 cells.
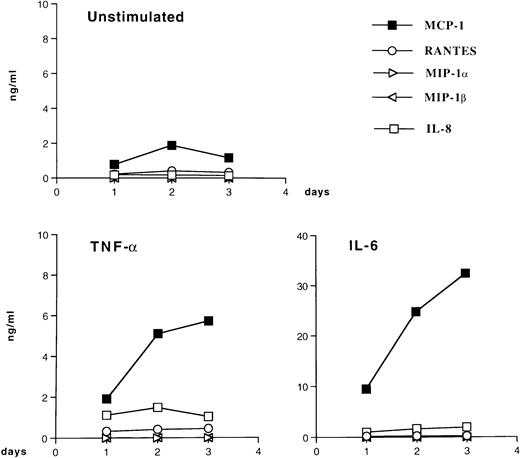
Stimulation of U937 and U1 cells with PMA resulted in the increased expression of the CC chemokines MCP-1, RANTES, MIP-1α, and MIP-1β and of the CXC chemokine IL-8 (data not shown). In contrast, both IL-6 and TNF-α induced MCP-1 but not the other tested chemokines in U937 (data not shown) and U1 cells (Fig 5). In this latter cell line, IL-6 and TNF-α also induced comparable levels of virus production in culture supernatants (Table1), as previously reported.37Therefore, we attempted to investigate whether endogenous MCP-1 played a role in cytokine-induced virus expression from U1 cells by adding the anti–MCP-1 monoclonal antibody 5D3-F734 to both unstimulated and stimulated U1 cells. Overall results were inconclusive, although a partial (up to 50%) inhibition of HIV production was observed in some experiments (data not shown). Because this as well as other anti–MCP-1 monoclonal antibodies can only partially neutralize the biologic effects of the chemokine, the possibility that MCP-1 may influence HIV replication remains to be thoroughly investigated.
Patterns of chemokine secretion by HIV-infected U1 cells in unstimulated conditions and in response to TNF-α and IL-6. U1 cells were cultured with medium alone, TNF-α (0.02 nmol/L), or IL-6 (0.24 nmol/L) for 72 hours; supernatants were harvested daily and stored at −80°C until tested. The data shown are from one representative experiment of four independently performed. Four CC chemokines (MCP-1, RANTES, MIP-1α, and MIP-1β) and one CXC chemokine (IL-8) were measured in the supernatants of unstimulated and stimulated cultures. Low (MCP-1, RANTES, and IL-8) to undetectable (MIP-1α and MIP-1β) levels of chemokines were present in supernatants of unstimulated U1 cells. Both IL-6 and TNF-α upregulated MCP-1 secretion; a modest enhancement of IL-8 production was also noted in U1 cells stimulated with TNF-α and IL-6.
Patterns of chemokine secretion by HIV-infected U1 cells in unstimulated conditions and in response to TNF-α and IL-6. U1 cells were cultured with medium alone, TNF-α (0.02 nmol/L), or IL-6 (0.24 nmol/L) for 72 hours; supernatants were harvested daily and stored at −80°C until tested. The data shown are from one representative experiment of four independently performed. Four CC chemokines (MCP-1, RANTES, MIP-1α, and MIP-1β) and one CXC chemokine (IL-8) were measured in the supernatants of unstimulated and stimulated cultures. Low (MCP-1, RANTES, and IL-8) to undetectable (MIP-1α and MIP-1β) levels of chemokines were present in supernatants of unstimulated U1 cells. Both IL-6 and TNF-α upregulated MCP-1 secretion; a modest enhancement of IL-8 production was also noted in U1 cells stimulated with TNF-α and IL-6.
Comparable HIV Expression Induced by TNF-α and IL-6 in U1 Cells
| Days . | Unstimulated . | TNF-α . | IL-6 . |
|---|---|---|---|
| 1 | 87 | 92 | 77 |
| 2 | 81 | 616 | 404 |
| 3 | 127 | 1,496 | 2,224 |
| Days . | Unstimulated . | TNF-α . | IL-6 . |
|---|---|---|---|
| 1 | 87 | 92 | 77 |
| 2 | 81 | 616 | 404 |
| 3 | 127 | 1,496 | 2,224 |
HIV expression was monitored by measuring viral RT activity (counts per minute per microliter) in culture supernatants harvested at the indicated time points and frozen at −80°C until tested. Data are mean values from duplicate cultures. TNF-α (0.02 nmol/L) and IL-6 (0.24 nmol/L) induced comparable levels of RT activity in U1 cells, as reported.37
Hydrocortisone inhibition of IL-6–induced MCP-1 in U1 cells.
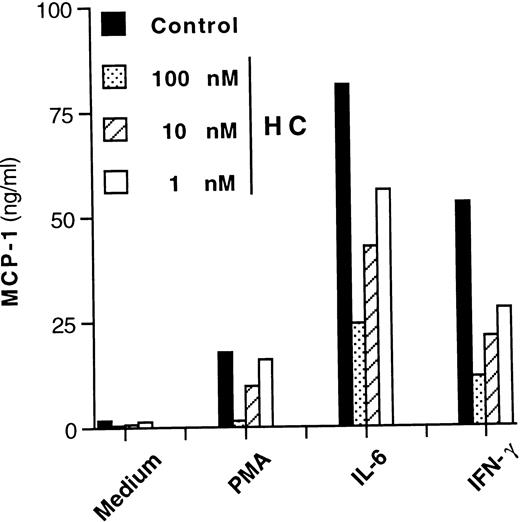
It has been reported that induction of MCP-1 by stimuli other than IL-6 can be inhibited by anti-inflammatory agents such as glucocorticoid hormones.44 On the other hand, glucocorticoids potentiate certain IL-6–mediated effects on hepatocytes and other cell types,45-47 including HIV expression in U1 cells.48 We therefore tested whether hydrocortisone could modulate IL-6–dependent MCP-1 secretion in this cell line. A concentration-dependent inhibition of MCP-1 secretion was observed when hydrocortisone was added simultaneously (cotreatment) to U1 cell cultures stimulated with PMA, IL-6, or IFN-γ (Fig6). One hour of pretreatment or posttreatment with hydrocortisone (10 nmol/L) versus the time of addition of the stimuli showed no significant differences in terms of inhibition of MCP-1 secretion as compared with cultures that were cotreated with the hormone (data not shown).
Concentration-dependent inhibitory effect of hydrocortisone (HC) on MCP-1 secretion from U1 cells stimulated by different agents. Cells cultivated with medium alone, PMA (10 nmol/L), IL-6 (0.24 nmol/L), and IFN-γ (1.5 nmol/L) were coincubated with decreasing concentrations (ranging from 100 to 1 nmol/L) of hydrocortisone. After 72 hours, culture supernatants were harvested and stored at −80°C until tested for MCP-1 content. Mean values of duplicate cultures from one of three independent experiments are shown. Intersample variability was ≤10%.
Concentration-dependent inhibitory effect of hydrocortisone (HC) on MCP-1 secretion from U1 cells stimulated by different agents. Cells cultivated with medium alone, PMA (10 nmol/L), IL-6 (0.24 nmol/L), and IFN-γ (1.5 nmol/L) were coincubated with decreasing concentrations (ranging from 100 to 1 nmol/L) of hydrocortisone. After 72 hours, culture supernatants were harvested and stored at −80°C until tested for MCP-1 content. Mean values of duplicate cultures from one of three independent experiments are shown. Intersample variability was ≤10%.
DISCUSSION
In the present study, we have observed that IL-6 is a potent inducer of MCP-1 expression and secretion in primary PBMCs obtained from both healthy seronegative and HIV-seropositive individuals. Further, IL-6 selectively induced MCP-1, but not other chemokines, including RANTES, MIP-1α, MIP-1β, or IL-8 in the U937 cell line and in its HIV-infected counterpart, U1. Finally, hydrocortisone partially inhibited MCP-1 expression induced by IL-6, IFN-γ, or PMA in U1 cells.
MCP-1, which is predominantly produced by monocytes, EC, and fibroblasts,9-15,49 is an important molecule in the regulation of leukocyte trafficking by eliciting the directional migration of mononuclear phagocytes.1-3 Several proinflammatory and immunoregulatory cytokines, including IL-1β, TNF-α, IFN-γ, and IL-4, have been shown to induce synthesis and secretion of MCP-1 by human EC.9-12,13,41 In this regard, it has recently been shown that soluble IL-6 receptor together with exogenous IL-6 is required for induction of MCP-1 expression from ECs that constitutively express the gp130 transducing chain in IL-6−/− mice.50 The IL-6–related molecule LIF induced both MCP-1 and IL-815 in purified human monocytes, suggesting that the ability of inducing chemokines may be a common feature among cytokines using gp130 as the common transducer for signaling.16
In vivo, a selective increase in MCP-1 has recently been found in the CSF of AIDS patients with CMV encephalitis, but not in HIV seronegative controls without neurologic symptoms or in AIDS patients with other OI of the CNS.4 CMV has been reported to induce IL-6 from macrophages and microglial cells in the CNS,5 suggesting the possibility that a concomitant expression of IL-6 and/or TNF-α and MCP-1 may occur in the CNS of AIDS patients, a hypothesis that we are currently investigating in our laboratory. It is of interest that CMV encodes a homologue of a CC chemokine receptor gene (US28) that is capable of binding MCP-1.51 The observation that this chemokine accumulates in the CSF of individuals with CMV encephalitis suggests that autocrine/paracrine pathways may be triggered by CMV aimed at recruiting monocytes from the bloodstream to the brain, which may affect the ability of the virus to persist and replicate in this organ. Furthermore, MCP-1 has also been shown to induce expression of cytokines, including IL-6, in human monocytes,52 suggesting an alternative or complementary loop of amplification of inflammatory responses. It is of interest that we have also recently observed a concomitant accumulation of MCP-1 and HIV in the CSF of AIDS patients with HIV encephalitis (Cinque et al, manuscript submitted), suggesting the possibility that, in addition to CMV, HIV can also induce MCP-1 expression. However, in the present study, we have failed to show substantial differences between uninfected and chronically HIV-infected U937 cells in the expression of several chemokines, including MCP-1. Whether acute HIV replication in vitro may affect the expression of MCP-1 or other chemokines is under investigation in our laboratory.
Increased levels of mRNA of two other CC chemokines, MIP-1α and MIP-1β, have also been found expressed by microglia and astrocytes in brain tissues from HIV-infected patients with dementia.53Thus, the localized overexpression of certain chemokines in the CNS or other tissues and organs may play an important pathogenetic role via leukocyte recruitment and either their direct activation or their priming to the regulatory effects of proinflammatory cytokines.
IL-6 is a multifunctional cytokine that acts on B cells, T cells, hematopoietic progenitor cells, and hepatocytes, thus influencing the regulation of the immune response and of hematopoiesis, contributing to the inflammatory process, and triggering the acute-phase reaction.16,20-27 In this regard, IL-6–deficient mice have shown impaired immune and acute-phase responses.25-27Increased levels of IL-6 are found together with those of IL-1β and TNF-α during sepsis and septic shock,22-24 although IL-6 accumulation usually occurs later than that of other proinflammatory cytokines, and its increase may be prevented by antibodies neutralizing TNF-α.24 Elevated levels of IL-6 have also been found in the plasma/serum or CSF of some HIV-infected individuals,28-31 whereas this cytokine can induce HIV replication in primary monocyte-derived macrophages and activation of virus expression from latently infected U1 cells.37 Whether endogenous MCP-1 is a mediator of the upregulating effect of IL-6 or other inflammatory cytokines on HIV replication remains to be further investigated.
IL-6 and IL-6–induced MCP-1 may also play a potential pathogenetic role in Kaposi's sarcoma (KS), a pathologic process characterized by a prominent leukocyte infiltrate54-56 and in B-cell lymphomas.56 In this regard, both IL-6 and Oncostatin M (also belonging to the family of gp130-related cytokines16) have been early recognized as growth factors for KS cells and lymphoma cells.54-56 Later, expression of MCP-1 has been observed in KS cells both in vitro and in vivo,57 whereas a new human herpesvirus, KSHV or HHV-8, has been recently involved in the etiopathogenesis of both endemic and AIDS-associated KS and body cavity lymphomas (BCL).58-60 Of particular interest is the fact that KSHV encodes viral homologues of a constitutively active chemokine receptor, an IFN-response gene, and IL-6 (v-IL-6).61 Thus, it is conceivable that v-IL-6 may directly induce MCP-1 expression and monocyte recruitment during the development and growth of KS and BCL.
In conclusion, the novel finding that IL-6 is a strong inducer of MCP-1 in PBMCs adds another important property to this multifunctional cytokine in terms of regulation of monocyte extravasation and tissue infiltration occurring during viral, inflammatory, and neoplastic diseases.
ACKNOWLEDGMENT
The authors thank Dr Elisa Vicenzi for helpful discussions and critical reading of the manuscript, Dr Fabrizio Veglia for statistical analyses, and Pietro Transidico for MCP-1 determinations.
Supported entirely by grants of the 1996 AIDS Project of the Istituto Superiore di Sanità, Rome, Italy.
Address reprint requests to Priscilla Biswas, PhD, P2-P3 Laboratories, DIBIT, Via Olgettina n. 58, 20132, Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal