Abstract
During vascular injury, such as observed in atherosclerosis, restenosis, vasculitides, transplantation, or sepsis, vascular smooth muscle cells (SMC) can be exposed to platelets or platelet products. Under these conditions proliferation or cytokine production of SMC stimulated by platelets or platelet products may contribute to regulation of vascular pathogenesis. Thus, we investigated interleukin-6 (IL-6) and IL-8 production as well as proliferation of SMC in response to platelets or platelet lysates. Platelets not already preactivated by thrombin induced IL-6 (10- to 50-fold) or IL-8 production of unstimulated SMC in a cell number dependent fashion. Preactivation of platelets with thrombin potently increased the platelet-mediated IL-6 (50- to 1,000-fold) and IL-8 production of SMC. Hirudin specifically inhibited the activation of platelets with thrombin. Isolated platelets cultured in the absence of SMC did not contain detectable IL-6 or IL-8. Prestimulation (4 hours) of SMC with pathophysiologically relevant substances (lipopolysaccharide [LPS], tumor necrosis factor-α [TNF-α], or IL-1α) further increased the platelet-induced cytokine production. The platelet-derived SMC stimulatory activity was IL-1, since IL-1 receptor antagonist (IL-1-Ra) inhibited the platelet-induced cytokine production of SMC. Anti-platelet-derived growth factor (PDGF)-antibody did not further reduce this activity. Thrombin itself stimulated expression of IL-6 and IL-8 to some degree and induced IL-6 production of SMC synergistically with IL-1. Platelets also induced proliferation of SMC, however, anti-PDGF antibodies, rather than IL-1-Ra blocked this response. These data show that platelet-derived IL-1 stimulates cytokine production of vascular smooth muscle cells, indicating that platelet-derived IL-1 may contribute to regulation of local pathogenesis in the vessel wall by activation of the cytokine regulatory network.
STUDIES IN THE last decade provided evidence that vascular cells, such as endothelial cells (EC) or smooth muscle cells (SMC), have the capacity to contribute to regulation of local pathogenesis by expression of adhesion molecules or by production of and response to cytokines. Several independent laboratories have shown that EC or SMC produce cytokines including interleukin-1 (IL-1),1-6 IL-6,7-10 or IL-8.11,12These cytokines are considered to be involved in regulation of inflammatory responses. Thus, in the vessel wall these cytokines may contribute to establishment of local chemotactic gradients (ie, IL-8), activation of invading leukocytes (ie, IL-1 or IL-6), or stimulation of proliferation of vascular SMC (ie, IL-1 or PDGF). Among the activators of vascular cytokine production are cytokines themself, such as IL-1 and tumor necrosis factor-α (TNF-α), or bacterial products, such as endotoxin (lipopolysaccharide [LPS]).13 IL-1 is a central mediator in the cytokine cascade and is known to be a potent activator of vascular cytokine production.7,8 This mediator also regulates other important cell functions of vascular cells, such as NO production,14 activation of soluble guanylate cyclase,15 or expression of adhesion molecules.16,17 Therefore, IL-1 may be of major importance for regulation of vascular pathogenesis. IL-1 was originally described in monocytes18 and a number of other cells can produce this cytokine.19 It has been shown previously that platelets can produce IL-1,20 and evidence has been provided that platelet-derived IL-1 can activate endothelial cells.21 22
Under pathological conditions, eg, during atherosclerosis, in certain vasculitides (ie, necrotizing angiitis), in sepsis, or other situations with injured endothelium, platelets gain direct contact to the subendothelial SMC. Under these conditions platelets may activate SMC and stimulate cytokine production or proliferation of these cells, contributing to local inflammation and/or medial thickening. However, only limited information exists regarding interaction of platelets with SMC. In a recent report Durante et al23showed that platelet lysates reduced the NO production of rat SMC stimulated by recombinant IL-1. They suggested that platelet-derived growth factor (PDGF) mediates the inhibition, since exogenous recombinant PDGF, but not fibroblast growth factor (FGF), inhibited the NO production of SMC stimulated by IL-1.24 It has also been reported earlier that rat SMC can use platelet-derived arachidonate and prostaglandin endoperoxide intermediates to form PG-I2.25 In vivo experiments using denuded rats made thrombocytopenic by anti-rat-platelet polyclonal antibody showed reduced lesion formation in the absence of platelets. However, the proliferation of subendothelial SMC was not different in thrombocytopenic and control rats.26 The reasons for these findings remained unclear, although mediator production by SMC or platelets was considered. In line with these findings is the notion that platelets may have a role in formation of intimal lesions during development of atherosclerosis, releasing growth factors at EC denudation.27 Interaction of platelets with vascular SMC may not only contribute to pathogenesis of atherosclerosis, but also to a variety of diseases characterized by vascular injury, as found in vasculitides, transplantation, or sepsis. Despite these data and suggestions, it was unclear if cellular interaction of platelets with SMC can result in activation, ie, cytokine production or proliferation of human SMC. Thus, we investigated the interaction of human platelets and human SMC in vitro in a coculture system. We report here that platelets can activate the IL-6 or IL-8 production of vascular smooth muscle cells as well as proliferation of SMC. Cytokine production of SMC is induced by platelet-derived IL-1. In contrast, IL-1 does not appear to have a major role for stimulation of proliferation of cultured SMC, since IL-1-Ra did not block platelet-induced proliferation. These data indicate that interaction of platelets with medial vascular smooth muscle cells during vascular injury may contribute to local vascular pathogenesis.
MATERIALS AND METHODS
Materials.
The following media and supplements were obtained from Biochrom (Berlin, Germany): Dulbecco's modified Eagle's medium (DMEM) (with 1 g glucose/L), DMEM (with 4.5 g glucose/L), RPMI 1640, antibiotics (penicillin and streptomycin; 10,000E/10,000 μg/mL), L-glutamine (200 mmol/L) and trypsin/EDTA (0.05%/0.02%). Fetal calf serum (FCS; endotoxin <50 pg/mL) was purchased at Serva Feinbiochemika (Heidelberg, Germany), bovine thrombin and heparin were from Sigma (Deisenhofen, Germany), hirudin, fibronectin, and endothelial cell growth factor were from Boehringer Mannheim (Mannheim, Germany). Dr H. Gallati (Hoffmann La Roche, Basel, Switzerland) kindly provided human recombinant tumor necrosis factor-α (TNF-α), IL-1α, IL-1β, and PDGF. IL-1 receptor antagonist (IL-1-Ra) was a kind gift of Dr J. Vannice (Synergen, Boulder, CO). LPS of Salmonella friedenau was kindly provided by Prof Dr H. Brade (Forschungszentrum Borstel, Borstel, Germany). Recombinant IL-6 was purchased at Boehringer Mannheim. Anti-α-SMC-actin antibody (HHF35) was obtained from Dako Diagnostica (Hamburg, Germany), polyclonal anti-PDGF antibody from Biermann (Bad Nauheim, Germany), and peroxidase-conjugated goat-anti-rabbit antibody from Dianova (Hamburg, Germany). Polyclonal anti-IL-854-72 antiserum was prepared by immunization of rabbits with a synthetic peptide (IL-854-72).
Isolation of human vascular SMC and measurement of SMC proliferation.
SMC were isolated by an explant technique28 from unused portions of human saphenous veins obtained following bypass surgery. The explants were cultured in petri dishes in DMEM (1 g glucose/L) containing FCS (10%), antibiotics (1%) and L-glutamine (1%) for 10 to 14 days until outgrowing SMC formed confluent monolayers. Subsequently the tissue pieces were removed and the remaining cells subcultured by trypsin/EDTA treatment. The cells were then cultured in 75- or 150-cm2 culture flasks. These isolated SMC were characterized by their typical “hill and valley” growth pattern and by staining with SMC-specific anti-α-SMC-actin antibody HHF35.
The proliferation of SMC cultured in 24-well plates was measured by uptake of radiolabeled [3H]-thymidine (37 kBq/well, 74 GBq/mmol; TRA 310, Amersham, Braunschweig, Germany). For this purpose cell cultures were grown to confluency, washed, and incubated in medium containing insulin (1 μmol/L) and transferrin (5 μg/mL), but lacking FCS (IT-medium) for 24 hours. The stimuli and/or platelets were added for 72 hours and [3H]-thymidine was added for further 24 hours. The cells were then harvested by trypsin/EDTA treatment and the thymidine incorporation measured in a liquid scintillation counter (1205 Betaplate; LKB-Wallace, Turku, Finland).
Isolation of platelets.
Platelets were isolated from human citrated blood (0.38%) by two centrifugation steps. Briefly, platelet-rich plasma was obtained by centrifugation at 500g for 10 minutes at room temperature. To this preparation three volumes of GSE-buffer (0.6% glucose, 0.7% sodium chloride, 0.35% sodium citrate, 0.38% EDTA) were added. Platelets were then separated by a second centrifugation at 1,400g for 20 minutes, washed in GSE-buffer, and resuspended in RPMI 1640. For preparation of platelet lysates, platelets were isolated and incubated for 1 hour (37°C) with RPMI 1640 or RPMI 1640 containing thrombin (1 U/mL). Platelets were harvested (1,400g,20 minutes), washed, resuspended in RPMI 1640 (3 to 10 × 109 cells/mL), and lysed by sonification (Branson Sonifire 250; Schwäbisch Gmünd, Germany).
Coincubation of vascular cells with platelets.
SMC were cultured in 24-well culture plates at a cell density of 5,000 cells/cm2. After reaching confluency the cells were washed (RPMI 1640) and incubated with or without different stimuli (prestimulation) as indicated in the figure legends. Isolated platelets were then added to the SMC in coincubation medium (RPMI 1640 with antibiotics [1%] and L-glutamine [1%]). After addition of platelets the culture plates were centrifuged (500g, 10 minutes). Subsequently medium with or without thrombin was added (1 U/mL) and the cocultures incubated for 24 hours. The cultures were centrifuged (1,500g, 5 minutes), the supernatants harvested and stored at −20°C in the presence of 2% FCS. IL-6 or IL-8 present in these preparations was measured in the test systems described below. All experiments were performed with SMC during passages 2 to 6.
Detection of IL-6 activity in 7TD1-assay.
Cells of the murine B-cell line 7TD129 were routinely cultured in IL-6 containing medium. For the assay30 samples were diluted in various threefold steps in medium lacking recIL-6 in 96-well culture plates. Cells were centrifuged twice in medium lacking recIL-6, adjusted to 40,000 cells/mL and 50 μL of this cell suspension was added to the diluted samples. The cultures were incubated for 72 hours and MTT (2-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; 0.5 mg/mL in phosphate-buffered saline (PBS); 10 μL/well) was added for 4 hours. Then stop-solution (5% sodium dodecyl sulfate [SDS], 50% dimethylformamide; 100 μL/well) was added for 2 hours and the absorption was measured at 550 nm in an enzyme-linked immunosorbent assay reader (Dynatech MR700; Denkendorf, Germany). The IL-6 activity of samples was calculated with respect to an IL-6 standard (10 ng recIL-6/mL). The data are derived from triplicate cultures and given as mean ± SD. They are representative for the number of experiments indicated in the legends.
Detection of IL-8 in Western blot analysis.
Supernatants of cells were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions31 in 15% gels. The proteins were transferred to polyvinylidene difluoride membranes (Immobilon P; Millipore, GmbH, Eschborn, Germany) for 30 minutes at a constant current of 0.8 mA/cm2 in a semi dry blotting device. The blots were incubated (1 hour) with rabbit anti-IL-8-antibody (IL-854-72, 1:1,000), washed with Tris buffer (10 mmol/L Tris, 0.05% Tween-20, 0.01% thimerosal), peroxidase-conjugated goat-anti-rabbit antibody (IgG; 1:2,000) was added for 1 hour, and the blots were washed. Finally, diaminobenzidine (400 μg/mL; Sigma) in substrate buffer (17 mmol/L citric acid, 65 mmol/L NaH2PO4, 0.01% thimerosal) was added, and incubated with the membranes for 3 minutes.
Reverse transcriptase-polymerase chain reaction (RT-PCR) of platelet mRNA.
Platelets were isolated as described above. The total mRNA of these platelets or leukocytes was prepared by the method of Chomczynski et al.32 The leukocyte isolation, primer sequences and conditions used for RT-PCR were as described previously.12The expected sizes of the PCR products were 810, 816, and 589 bp, for IL-1α, IL-1β, and IL-8 receptor, respectively.
RESULTS
Isolated platelets stimulate the IL-6 production of human vascular SMC in a cell number dependent fashion.
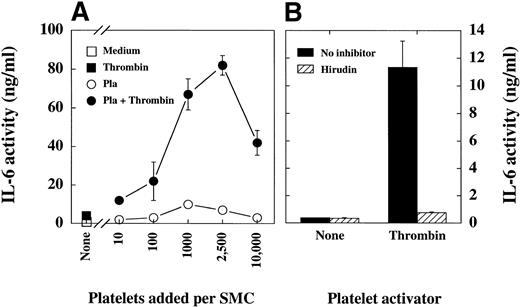
Platelets may contribute to regulation of pathogenesis during vascular injury by cytokine-mediated activation of human vascular SMC. Thus, we investigated whether platelets can induce IL-6 production of human vascular smooth muscle cells. SMC cultured for 24 hours with medium or thrombin in the absence of platelets released little IL-6 activity (Fig1A; squares). SMC incubated with unactivated platelets or thrombin-activated platelets produced higher amounts of IL-6 activity (Fig 1A, circles). Both, unactivated platelets, as well as thrombin-activated platelets induced the IL-6 production of SMC depending on the platelet to SMC ratio. The optimal platelet to SMC ratio was 1,000 to 2,500 platelets per SMC. Thrombin-activated platelets stimulated the SMC much more potently than unactivated platelets. Summarizing a number of additional experiments, unactivated platelets increased the IL-6 production of SMC 10- to 50-fold (compared to basal IL-6 production of SMC), whereas thrombin-activated platelets increased the IL-6 production of SMC 50- to 1,000-fold, depending on the SMC and platelet isolate. Neither supernatants nor cell lysates of unstimulated or thrombin-activated platelets contained detectable IL-6 activity.
(A) Platelets stimulate the IL-6 production of SMC in a cell number dependent fashion. Human vascular SMC were incubated in 24-well plates until they reached confluency. The cultures were washed (RPMI 1640) and medium (squares) or different numbers of isolated platelets (Pla; circles) added. After centrifugation of plates (500g, 10 minutes), medium (open symbols), or medium containing thrombin (1 U/mL; closed symbols) was added. Supernatants were harvested after 24 hours and centrifuged (1,500g, 5 minutes). IL-6 activity in supernatants was measured in 7TD1-assay. Similar results were obtained in 5 experiments. (B) The activation of platelets with thrombin is specific. In a separate experiment SMC were incubated in 24-well plates until they reached confluency, washed, and medium (▪) or medium containing hirudin (20 U/mL; ▨) was added. Subsequently platelets at a ratio of 1,000 platelets per SMC and finally, medium (None) or medium containing thrombin (Thrombin; 1 U/mL) was added. After the incubation (24 hours) supernatants were harvested and IL-6 was measured in 7TD1-assay. Two additional experiments showed similar results.
(A) Platelets stimulate the IL-6 production of SMC in a cell number dependent fashion. Human vascular SMC were incubated in 24-well plates until they reached confluency. The cultures were washed (RPMI 1640) and medium (squares) or different numbers of isolated platelets (Pla; circles) added. After centrifugation of plates (500g, 10 minutes), medium (open symbols), or medium containing thrombin (1 U/mL; closed symbols) was added. Supernatants were harvested after 24 hours and centrifuged (1,500g, 5 minutes). IL-6 activity in supernatants was measured in 7TD1-assay. Similar results were obtained in 5 experiments. (B) The activation of platelets with thrombin is specific. In a separate experiment SMC were incubated in 24-well plates until they reached confluency, washed, and medium (▪) or medium containing hirudin (20 U/mL; ▨) was added. Subsequently platelets at a ratio of 1,000 platelets per SMC and finally, medium (None) or medium containing thrombin (Thrombin; 1 U/mL) was added. After the incubation (24 hours) supernatants were harvested and IL-6 was measured in 7TD1-assay. Two additional experiments showed similar results.
To determine the specificity of platelet activation by thrombin, we performed inhibition experiments with the thrombin inhibitor hirudin (Fig 1B). In these experiments hirudin blocked the stimulation of platelets by thrombin by more than 95%, as shown in Fig 1B by reduction of platelet-mediated IL-6 production in SMC. Hirudin did not reduce the IL-6 production of SMC incubated with unactivated platelets (None). Furthermore, we show in this manuscript that in the absence of platelets thrombin can activate SMC directly (compare Fig 1A; closed squares), a finding which is in line with previous reports. Similar to the activation of platelets by thrombin, as presented above, this thrombin-mediated activation of SMC was specific, since hirudin blocked the thrombin-mediated IL-6 production of SMC (data not shown).
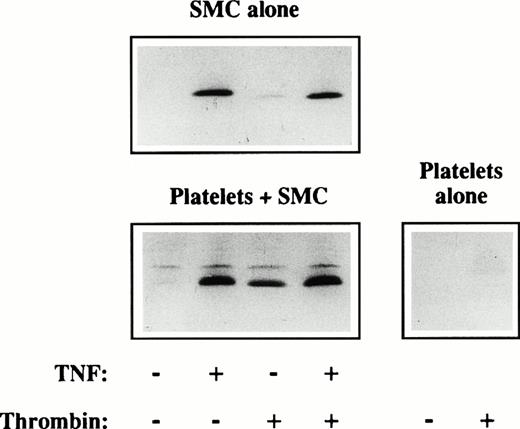
Platelets induce IL-8 production of SMC. SMC were incubated in 24-well plates until they reached confluency. The cultures were washed and the SMC prestimulated with (+) or without (−) recombinant TNF-α (TNF, 50 ng/mL). Untreated (−) platelets or platelets in the presence of thrombin (+; 1 U/mL) were added at a ratio of 1,000 platelets per SMC. The supernatants were harvested after 24 hours, run on a reducing SDS-PAGE, and blotted to Immobilon P. A rabbit polyclonal antibody (IL-854-72; 1:1,000) was added (1 hour) and the blot stained with peroxidase-conjugated goat-anti-rabbit (1 hour) and subsequent addition of DAB. Similar results were obtained in three experiments.
Platelets induce IL-8 production of SMC. SMC were incubated in 24-well plates until they reached confluency. The cultures were washed and the SMC prestimulated with (+) or without (−) recombinant TNF-α (TNF, 50 ng/mL). Untreated (−) platelets or platelets in the presence of thrombin (+; 1 U/mL) were added at a ratio of 1,000 platelets per SMC. The supernatants were harvested after 24 hours, run on a reducing SDS-PAGE, and blotted to Immobilon P. A rabbit polyclonal antibody (IL-854-72; 1:1,000) was added (1 hour) and the blot stained with peroxidase-conjugated goat-anti-rabbit (1 hour) and subsequent addition of DAB. Similar results were obtained in three experiments.
Platelets and thrombin stimulate the IL-8 production of SMC.
Besides IL-6 other cytokines may be involved in regulation of vascular response to injury. IL-8 is a potent chemoattractant for granulocytes and possibly involved in the recruitment of inflammatory cells into the injured vessel wall. Thus, we investigated the production of IL-8 in response to platelets and/or thrombin. Neither unstimulated nor thrombin-stimulated platelets alone expressed measurable amounts of IL-8 (Fig 2). Unstimulated SMC alone also did not release IL-8, whereas SMC stimulated with TNF for 4 hours potently produced this chemokine. SMC incubated with thrombin released IL-8, although much less than induced by TNF. Similar to the data described above for IL-6, unstimulated platelets (−/−; TNF/thrombin) also activated the SMC to produce some IL-8. Incubation of thrombin-activated platelets with unstimulated SMC (−/+) resulted in an enhanced release of IL-8, as compared to unstimulated conditions. Prestimulation of SMC with TNF and subsequent incubation with either unstimulated (+/−) or trombin-stimulated (+/+) platelets triggered a maximal release of IL-8 from the SMC.
Platelets activate the proliferation of cocultured SMC.
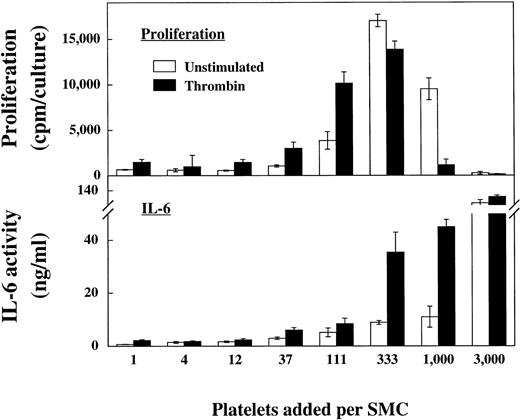
Platelets may also affect medial proliferation of SMC in injury-associated pathogenesis. As shown in Fig3 low numbers of platelets initiated proliferation of SMC (12 to 111 platelets per SMC). At higher platelet to SMC ratios (>333) the stimulatory effect of thrombin-activated platelets is no longer observed, and the stimulation becomes suboptimal. The IL-6 production of parallel cultured cells was optimal at a ratio of 1,000 platelets per SMC (compare Fig 1).
Platelets induce proliferation of SMC. SMC were cultured in 24-well culture plates until they reached confluency, washed with serum-free medium, and incubated for 24 hours in serum-free medium containing insulin and transferrin (IT-medium). The cultures were then washed, platelets at the denoted ratios added, and the cocultures incubated with or without the respective stimuli (72 hours). Subsequently radiolabeled thymidine was added for 24 hours and thymidine incorporation of cells measured in a scintillation counter (Proliferation). For IL-6 measurement parallel cultures were washed and incubated with the stimuli for 24 hours (IL-6). Two additional experiments showed comparable results.
Platelets induce proliferation of SMC. SMC were cultured in 24-well culture plates until they reached confluency, washed with serum-free medium, and incubated for 24 hours in serum-free medium containing insulin and transferrin (IT-medium). The cultures were then washed, platelets at the denoted ratios added, and the cocultures incubated with or without the respective stimuli (72 hours). Subsequently radiolabeled thymidine was added for 24 hours and thymidine incorporation of cells measured in a scintillation counter (Proliferation). For IL-6 measurement parallel cultures were washed and incubated with the stimuli for 24 hours (IL-6). Two additional experiments showed comparable results.
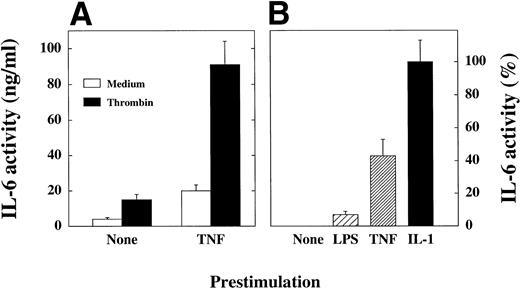
(A) TNF prestimulation of SMC enhances the response to platelets. SMC were incubated in 24-well plates until they reached confluency, washed, and medium (None) or recombinant TNF (50 ng/mL) was added. After 4 hours SMC were washed and 1,000 platelets per SMC were added. Plates were centrifuged (500g, 10 minutes), and medium lacking thrombin (Medium) or medium containing thrombin (1 U/mL; Thrombin) was added for a further 24 hours. IL-6 activity in supernatants was measured in 7TD1-assay. Four similar experiments were performed. (B) Prestimulation of SMC with LPS, TNF, or IL-1. In a separate experiment SMC were cultured and prestimulated for 4 hours with LPS (1 μg/mL), TNF-α (TNF, 50 ng/mL), or IL-1α (IL-1, 10 ng/mL) as described in (panel A) and thrombin-activated platelets were added. The IL-6 production of SMC not prestimulated but incubated with thrombin-activated platelets (None) was determined 0% and the maximal IL-6 production after IL-1 prestimulation determined 100%. Two similar experiments were performed.
(A) TNF prestimulation of SMC enhances the response to platelets. SMC were incubated in 24-well plates until they reached confluency, washed, and medium (None) or recombinant TNF (50 ng/mL) was added. After 4 hours SMC were washed and 1,000 platelets per SMC were added. Plates were centrifuged (500g, 10 minutes), and medium lacking thrombin (Medium) or medium containing thrombin (1 U/mL; Thrombin) was added for a further 24 hours. IL-6 activity in supernatants was measured in 7TD1-assay. Four similar experiments were performed. (B) Prestimulation of SMC with LPS, TNF, or IL-1. In a separate experiment SMC were cultured and prestimulated for 4 hours with LPS (1 μg/mL), TNF-α (TNF, 50 ng/mL), or IL-1α (IL-1, 10 ng/mL) as described in (panel A) and thrombin-activated platelets were added. The IL-6 production of SMC not prestimulated but incubated with thrombin-activated platelets (None) was determined 0% and the maximal IL-6 production after IL-1 prestimulation determined 100%. Two similar experiments were performed.
Prestimulation of SMC enhances platelet-induced IL-6 production of SMC.
Under pathological conditions SMC may be preactivated before contact with platelets. Thus, we investigated the influence of prestimulation with TNF and other pathophysiologically relevant substances on platelet-induced IL-6 production of SMC. Prestimulation of SMC with TNF potently (up to 10-fold) enhanced the IL-6 production of SMC cocultured with untreated as well as thrombin-activated platelets (Fig4A). Time course experiments showed that 4 hours of TNF-prestimulation were optimal (data not shown). The data presented in Fig 4B show, that besides TNF, prestimulation by LPS or recIL-1α also enhanced the response of SMC to platelets.
Identification of platelet-derived IL-1 as the inducer of IL-6 production of SMC.
To characterize the platelet-derived activity responsible for stimulation of cytokine production or proliferation of SMC we performed inhibition experiments with IL-1-Ra and anti-PDGF-antibody (Table1). For this purpose we incubated untreated or TNF-prestimulated SMC with medium, or medium containing thrombin, as well as unactivated platelets, or thrombin-activated platelets. As shown above thrombin increased the IL-6 production of SMC to some degree. As expected, neither IL-1-Ra nor anti-PDGF antibody reduced the amount of IL-6 induced by thrombin. Also thrombin-activated platelets induced IL-6 production in SMC more potently than unactivated platelets. This response was largely reduced following addition of IL-1-Ra. The inhibition was not complete, thus we also studied inhibition in the presence of additional anti-PDGF antibodies. However, the IL-1-Ra–mediated inhibition was not further extended by addition of anti-PDGF-antibody. Additional experiments with IL-1-Ra showed that IL-1-Ra also blocked the cytokine production by supernatants or washed lysates of both unstimulated or stimulated platelets, and that IL-1-Ra blocked stimulation of IL-6 production, but had no effect on proliferation (data not shown).
IL-1-Ra, But Not Anti-PDGF Antibodies Reduce Platelet-Stimulated IL-6 Production of SMC
| . | Not Prestimulated* . | TNF-Prestimulated . | ||||
|---|---|---|---|---|---|---|
| . | None . | IL-1-Ra† . | IL-1-Ra + Ab† . | None . | IL-1-Ra . | IL-1-Ra + Ab . |
| Addition to SMC . | IL-6 Activity (ng/mL) . | IL-6 Activity (ng/mL) . | ||||
| Medium | 0.180 ± 0.025 | 0.180 ± 0.000 | 0.180 ± 0.000 | 1.000 ± 0.250 | 0.970 ± 0.085 | 1.100 ± 0.056 |
| Medium + thrombin | 0.400 ± 0.055 | 0.600 ± 0.075 | 0.800 ± 0.000 | 3.800 ± 0.250 | 3.900 ± 0.600 | 3.600 ± 0.540 |
| Platelets | 2.000 ± 0.350 | 1.000 ± 0.000 | 1.100 ± 0.000 | 21.000 ± 3.600 | 2.100 ± 0.300 | 2.100 ± 0.300 |
| Platelets + thrombin | 33.000 ± 0.400 | 5.200 ± 0.690 | 5.200 ± 1.800 | 139.000 ± 12.000 | 5.800 ± 0.450 | 7.700 ± 1.450 |
| . | Not Prestimulated* . | TNF-Prestimulated . | ||||
|---|---|---|---|---|---|---|
| . | None . | IL-1-Ra† . | IL-1-Ra + Ab† . | None . | IL-1-Ra . | IL-1-Ra + Ab . |
| Addition to SMC . | IL-6 Activity (ng/mL) . | IL-6 Activity (ng/mL) . | ||||
| Medium | 0.180 ± 0.025 | 0.180 ± 0.000 | 0.180 ± 0.000 | 1.000 ± 0.250 | 0.970 ± 0.085 | 1.100 ± 0.056 |
| Medium + thrombin | 0.400 ± 0.055 | 0.600 ± 0.075 | 0.800 ± 0.000 | 3.800 ± 0.250 | 3.900 ± 0.600 | 3.600 ± 0.540 |
| Platelets | 2.000 ± 0.350 | 1.000 ± 0.000 | 1.100 ± 0.000 | 21.000 ± 3.600 | 2.100 ± 0.300 | 2.100 ± 0.300 |
| Platelets + thrombin | 33.000 ± 0.400 | 5.200 ± 0.690 | 5.200 ± 1.800 | 139.000 ± 12.000 | 5.800 ± 0.450 | 7.700 ± 1.450 |
*SMC were incubated in 24-well culture plates until they reached confluency, washed and prestimulated with medium (Not prestimulated) or TNF-α (50 ng/mL; TNF-prestimulated) for 4 hours. Subsequently the cells were washed and medium (None), IL-1-Ra, or IL-1-Ra and anti-PDGF antibody (IL-1-Ra + Ab) were added. Then medium, medium containing thrombin (1 U/mL), platelets, or platelets with thrombin were added (24 hours). Platelets were added at a ratio of 1,000 platelets per SMC. IL-6 activity was measured in 7TD1-assay. Three similar experiments were performed.
IL-1-Ra, 2 μg/mL; Ab, anti-PDGF antibody, 1:200.
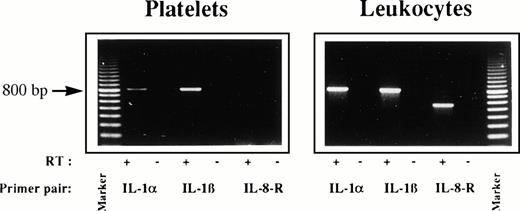
Platelet preparations contain IL-1 mRNA.
To provide further evidence for the presence of IL-1 in the used platelets we performed RT-PCR with total mRNA isolated from platelets or leukocyte preparations (Fig 5). The used primer pairs readily detected IL-1α, IL-1β, or IL-8 receptor type 1 (IL-8-R) mRNA in the leukocyte preparations used for control. In contrast, mRNA of platelets did not contain IL-8-R mRNA, indicating that the preparations were not contaminated by detectable leukocyte-derived mRNA. PCR samples without prior RT reaction (−) did not express specific bands, indicating absence of endogenous genomic DNA in the samples. However, platelets contained mRNA specific for IL-1α and IL-1β.
Platelets contain IL-1 mRNA. The total mRNA of platelets or leukocytes was isolated. These preparations were incubated with (+) or without (−) reverse transcriptase for 1 hour and PCR was performed (35 cycles) for IL-1α, IL-1β, and IL-8 receptor type I (IL-8-R). Samples were run on a 1.3% agarose gel and visualized by UV-transillumination. The expected size of the PCR products are 810, 816, and 589 bp, respectively. A 100-bp ladder was included as marker. Three experiments showed similar results.
Platelets contain IL-1 mRNA. The total mRNA of platelets or leukocytes was isolated. These preparations were incubated with (+) or without (−) reverse transcriptase for 1 hour and PCR was performed (35 cycles) for IL-1α, IL-1β, and IL-8 receptor type I (IL-8-R). Samples were run on a 1.3% agarose gel and visualized by UV-transillumination. The expected size of the PCR products are 810, 816, and 589 bp, respectively. A 100-bp ladder was included as marker. Three experiments showed similar results.
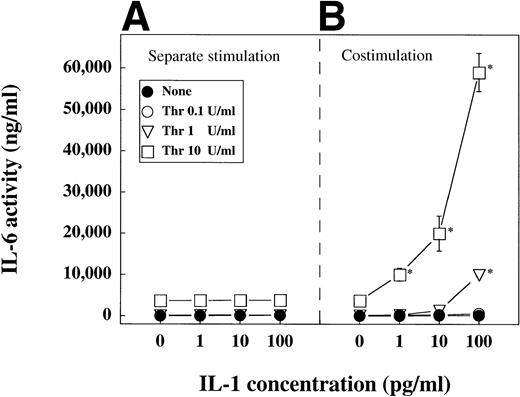
Thrombin and recombinant IL-1α induce IL-6 production of SMC synergistically.
The above data showed, that thrombin-activated platelets are stronger activators of SMC than unactivated platelets, and that platelet-derived stimulatory activity is IL-1. Thus, we investigated whether IL-1 may interfere with thrombin in stimulation of IL-6 production of SMC. To investigate this question, we stimulated SMC with combinations of IL-1α and thrombin at different concentrations (Fig6). For comparison, we also incubated the SMC with the same concentrations of stimuli separately. Figure 6A shows calculated results of SMC stimulated separately with IL-1 or thrombin, upon adding up the separate IL-6 activities of these individual supernatants. However, as shown in Fig 6B, the costimulation of SMC with 1 pg/mL IL-1α and 10 U/mL thrombin and other combinations of IL-1 and thrombin, as indicated by the asterisks, synergistically stimulated the IL-6 production of these cells.
Thrombin and IL-1 synergistically induce IL-6 production of SMC. SMC were incubated in 24-well plates until they reached confluency, washed and stimulated separately (A) with IL-1α (1, 10 or 100 pg/mL) or thrombin (Thr; 0.1, 1, 10 U/mL), or with combinations (B) of IL-1α and thrombin. IL-6 activities detected in supernatants of SMC costimulated with combinations of IL-1α and thrombin are shown in (B). For estimation of the synergistic effect IL-6 activities in supernatants of SMC stimulated separately with IL-1α or thrombin are added up and presented in (A). IL-6 activity in supernatants was measured in 7TD1-assay. Four independent experiments showed similar results. The asterisks indicate costimulation cocultures expressing a synergistic effect.
Thrombin and IL-1 synergistically induce IL-6 production of SMC. SMC were incubated in 24-well plates until they reached confluency, washed and stimulated separately (A) with IL-1α (1, 10 or 100 pg/mL) or thrombin (Thr; 0.1, 1, 10 U/mL), or with combinations (B) of IL-1α and thrombin. IL-6 activities detected in supernatants of SMC costimulated with combinations of IL-1α and thrombin are shown in (B). For estimation of the synergistic effect IL-6 activities in supernatants of SMC stimulated separately with IL-1α or thrombin are added up and presented in (A). IL-6 activity in supernatants was measured in 7TD1-assay. Four independent experiments showed similar results. The asterisks indicate costimulation cocultures expressing a synergistic effect.
DISCUSSION
Under physiological conditions the vascular endothelium maintains normal hemostasis and blood platelets do not attach in large numbers to the vessel wall and form thrombi. Platelets may even bind to the endothelium33,34 without clot formation. However, the endothelial layer may be injured under a variety of conditions including sepsis, restenosis, transplantation, thrombosis, or atherosclerosis. It has been suggested that platelets themselves or platelet products may contribute to endothelial injury.35During sepsis or bacterial infections endotoxin (LPS) of gram-negative bacteria may also initiate injury of the endothelial layer36 or render the endothelium more susceptible to injury.37 Other cells of the vessel lumen, such as polymorphonuclear cells (PMN) are possibly involved in development of vascular injury by enzyme release.38,39However, upon injury of a normal vessel wall platelets constitute the first line of host defense.40 Platelets may not only contribute to injury, but also to activation of subendothelial cells. In an injured vessel wall platelets can access the subendothelium and activate proliferation of subendothelial human vascular SMC or enhance the inflammatory response by stimulation of cytokine production of SMC. Although experimental data have been provided recently that platelet products23 (ie, platelet lysates) can reduce the NO production of SMC induced by recombinant IL-1, no data exist about platelet-induced cytokine production of SMC. In the present report we show that platelets can activate the cytokine production, but not the proliferation of SMC by an IL-1–dependent mechanism. The platelet activator thrombin activated cytokine production of SMC, however, compared to thrombin-activated platelets it stimulated the cytokine production of SMC only to a low degree. These observations indicate that during vascular injury platelet-derived IL-1 may activate subendothelial cells, such as SMC, thereby contributing to regulation of local pathogenesis by induction of vascular cytokine production.
The induction of SMC cytokine production by stimulated or unstimulated platelets was depending on the platelet concentration. Already 10 thrombin-activated platelets per SMC induced the IL-6 production of SMC as compared to unactivated platelets. The maximal activation was achieved at a ratio of 1,000 platelets per SMC. This range may reflect physiological ratios. In the presented experiments usually the platelet stimulus thrombin was used. This activator was the most potent compound; however, collagen- or adenosine diphosphate-treated platelets also induced the cytokine production of SMC. The activation of the platelets by thrombin was specific because addition of the thrombin inhibitor hirudin blocked the activation of the SMC by platelets. Our experiments also showed that thrombin by itself activated the IL-6 and IL-8 production of SMC, although to a much lower degree than thrombin-activated platelets. This observation is in line with previous reports showing that thrombin stimulated the production of IL-6 or monocyte chemotactic protein [MCP-1].41,42It has also been shown that thrombin activates proliferation43 and PDGF-A gene44 expression, however, delayed in comparison to PDGF.45 Other authors reported that thrombin inhibits IL-1–induced NO production46 of SMC.
We showed that thrombin stimulated the IL-6 or IL-8 production of SMC to some degree; however, platelet products are also involved in activation of SMC. Previous reports showed that platelets express IL-120 and that this cytokine can activate EC.21,22 IL-1 is a central mediator involved in various diseases.19 Thus, we investigated the role of this cytokine in platelet-mediated activation of SMC. Experiments with the IL-1-Ra showed that IL-1-Ra inhibited the platelet-induced SMC activation almost completely, indicating a major role of platelet-derived IL-1. The remaining platelet-derived stimulatory activity may result from other platelet-products. Platelets contain in their alpha granules mediators such as PDGF, β-thromboglobulin, transforming growth factor-β, platelet factor-4 or thrombospondin, which may interfere with activation of SMC. However, the role of these mediators has not yet been evaluated, although other reports showed that recombinant PDGF inhibited IL-1–induced NO production of rat SMC.23 However, PDGF is not involved importantly in the cytokine induction reported in this report, as indicated by inhibition experiments employing anti-PDGF antibodies.
Thrombin and IL-1 synergistically activated IL-6 production of SMC. The mechanism of this synergism has not been defined yet, however, thrombin may regulate IL-1 receptor expression, or IL-1 may interfere with thrombin receptor expression or activation. Although there has no evidence been reported for these hypotheses, some indications for interactions of cytokines and thrombin receptors or thrombin with cytokine receptors have been published.47-49
Under the conditions used SMC are cultured in the presence of thrombin, IL-1, and/or platelet-mediators. To limit the SMC activation to platelet-derived mediators we prepared lysates of washed and sonified platelets. These preparations also activated SMC and IL-1-Ra inhibited the SMC activation to the same extent as detected in the coincubation experiments with thrombin-activated platelets, indicating that platelets express IL-1 activity which readily can activate SMC. The mechanism of IL-1 expression by platelets remains unclear. Platelets do not possess a potent protein synthesis apparatus. Thus, it appears possible that preformed IL-1 in platelets may become accessible after activation of platelets, by release from the canalicular system. This suggestion is supported by our observation that both lysates and supernatants of stimulated platelets contained IL-1-Ra inhibitable SMC stimulatory activity. RT-PCR experiments provided some evidence that platelets, or their precursor cells, actively produced the detected IL-1. The low amounts of IL-1α and IL-1β mRNA detected in the platelet preparations may reflect residual megakariocyte mRNA. It is unlikely that the detected IL-1 mRNA is derived from a leukocyte contamination, since the RT-PCR for IL-8 receptor was negative, indicating the absence of leukocyte- or monocyte-derived mRNA.
In pathological situations with injured endothelium the underlaying subendothelium may be already activated by additional mediators before activation by platelets. Activation by TNF may reflect such a preactivation. The evidence provided in this report showed that preactivation of SMC indeed enhanced the response to platelets. The preactivation of SMC by TNF may increase the expression of thrombin or IL-1 receptors, thereby enhancing the response. The costimulation experiments with exogenous thrombin and IL-1 provide evidence for the suggested mechanism, since costimulation resulted in a synergistic rather than in an additive response, as expected for independent activation. On the other hand it appears possible that the produced cytokines in turn may activate platelets to produce additional SMC activators, since platelets express cytokine receptors.50 51 The data presented in this report indicate that IL-1–mediated interaction of platelets and subendothelial cells, such as vascular smooth muscle cells may contribute to local pathogenesis during vascular injury.
ACKNOWLEDGMENT
The expert technical assistance of Gina Tillmann is gratefully acknowledged. We thank Drs H. Brade (Forschungszentrum Borstel, Borstel, Germany), H. Gallati (Hoffman LaRoche, Basel, Switzerland), and J. Vannice (Synergen, Boulder, CO) for providing us with LPS, recombinant cytokines, and IL-1-Ra, respectively.
Supported by grant Lo 385/4-1 of the Deutsche Forschungsgemeinschaft (H.L.), Bonn, Germany, by SFB 367 project C4 (E.B. and H.-D.F.), Bonn, Germany, and by grant 01 ZZ 95120/06 of the BMBF (K.W.), Bonn, Germany.
Address reprint requests to Harald Loppnow, PhD, Martin-Luther-Universität Halle-Wittenberg, Lehrstuhl für Kardiologische Intensivmedizin, Forschungslabor, Magdeburger Str. 21, 06097 Halle/Saale, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal