Abstract
Mature human dendritic cells can be generated in substantial numbers from nonproliferating progenitors in human blood using a two-step protocol. T cell–depleted mononuclear cells are first cultured with granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) and then exposed to monocyte conditioned medium (MCM). The dendritic cells generated using this approach are rendered terminally mature and are the most potent antigen presenting cells identified to date in humans. We sought to characterize factors in MCM that induce the terminal differentiation of dendritic cells. MCM contained substantial, although varying, quantities of several factors including tumor necrosis factor-α, IL-1β, IL-6, and interferon-α. However, none of the four factors, individually or in various combinations, could fully substitute for the MCM to generate irreversibly differentiated dendritic cells. The yields, percentage of cells expressing the mature phase marker CD83, and mixed leukocyte reaction–stimulatory function were lower when defined cytokines were used in the place of MCM. Therefore, the full maturation of dendritic cells, because it entails changes in many known cell and molecular properties, requires a number of different cytokines that are released in tandem from appropriately stimulated monocytes. We propose that MCM-matured dendritic cells will be the most effective adjuvants for immunotherapy in vivo.
DENDRITIC CELLS are specialized antigen presenting cells (APCs) for the initiation of primary T cell immune responses.1-4 Mature terminally differentiated dendritic cells are relatively scarce in human blood, comprising less than 1% of the mononuclear cell population.5,6 The difficulty in obtaining large numbers of blood dendritic cells has been obviated by the recent development of methods that yield up to 4 × 106 cells from only 40 mL of blood.7,8 The protocol uses human plasma in the place of fetal calf serum (FCS) and involves two steps. The first is a priming phase, in which T cell–depleted mononuclear cells are cultured for 7 days in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The second step is a maturation or differentiation phase and requires exposure to monocyte conditioned medium (MCM) that is produced by culturing monocytes on immobilized human γ-globulin. The dendritic cells generated have a typical stellate morphology, are nonadherent, and have potent T cell–stimulating capacity, inducing strong mixed leukocyte reactions (MLRs) at stimulator to responder ratios of 1,000:1 or less. Several markers are expressed during maturation including CD83, p55, CD25, perinuclear CD68, and higher levels of HLA and costimulatory molecules.7,8 MCM appears to be a critical component of the maturation process. If it is omitted from the second culture step, GM-CSF– and IL-4–primed cells revert to a more adherent form, take on macrophage characteristics (CD14 and FcR expression), do not express CD83 or p55, and become substantially less stimulatory for T cells.7 8
The goal of this study was to identify cytokines in MCM that might account for the differentiating effect on dendritic cells. MCM was analyzed for candidate factors including tumor necrosis factor-α (TNF-α) and IL-1β, which are of particular interest because they induce the maturation of GM-CSF/IL-4–primed dendritic cells that are generated in medium containing FCS.9-11 Dendritic cells generated in MCM versus specific cytokine combinations were compared with respect to features associated with terminal maturation, including phenotypic stability and potent T cell–stimulatory capacity.
MATERIALS AND METHODS
Culture Medium
RPMI 1640 supplemented with 20 μg/mL of gentamicin, 10 mmol/L HEPES, and either 1% autologous plasma (heparinized) or 5% single donor human serum.
Cytokines
We were generously supplied with recombinant human (rh) GM-CSF (specific activity [SA] 1 × 108 U/mg) by Kirin Brewery Co, Maebashi, Gunma, Japan; and rhIL-4 by Immunex Corp, Seattle, WA (SA 5 × 107 U/mg) and Schering-Plough Corp, Union, NJ (SA 2.865 × 107 U/mg). The following cytokines were purchased: TNF-α (Endogen, Cambridge, MA), IL-1β and IL-6 (R and D Corp, Minneapolis, MN), interferon-α (IFN-α; Pestka Biomedical Labs, Inc, West Caldwell, NJ). ELISA kits were obtained from Pestka Biomedical Labs, Inc (IFN-α) and R and D Corp (TNF-α, IL-1β, IL-6).
Generation of Mononuclear Cell Subsets
Peripheral blood was obtained from normal donors in heparinized syringes, and peripheral blood mononuclear cells (PBMC) isolated by sedimentation in Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). T cell–enriched (ER+ ) and T cell–depleted (ER− ) populations were prepared by rosetting with neuraminidase-treated sheep red blood cells as previously described.5
T cells. T cells were purified from ER+ cells by removal of monocytes, natural killer (NK) cells, and major histocompatibility complex (MHC) class II+ cells as described.12 13
Dendritic cells. A total of 2 × 106 ER− cells were plated in 3-mL volumes in 6-well tissue culture dishes (Falcon, Lincoln Park, NJ) in complete medium containing 1% human plasma. GM-CSF and IL-4 were added at final concentrations of 1,000 U/mL on the initial day of culture. Cytokines were replenished every other day (days 2, 4, and 6) by removing 0.3 mL of the medium and adding back 0.5 mL fresh medium with 1× cytokines. On day 7 nonadherent cells were collected by moderately vigorous aspiration and transferred to new 6-well plates. The cultures were supplemented with MCM (final concentration 50% vol/vol) or various combinations of cytokines (detailed in Results) and harvested on days 10 and 11. In some experiments, cells were washed out of supplemental cytokines or MCM at day 7 or day 11 before use in phenotypic or functional assays.
MCM
Ig coated bacteriologic plates (100 mm, Falcon) were prepared immediately before use by the addition of 4 mL of human γ-globulin (10 mg/mL, Cappel Labs, Organon Teknika, West Chester, PA) for 1 minute. The plates were washed three times with cation-free phosphate buffered saline (PD) before use. T cell–depleted ER− cells (5 × 107 ) were layered onto the Ig-coated bacteriologic plates for 1 hour in 7-mL volumes.14 Nonadherent cells were washed away and discarded. The Ig-adherent cells were incubated in fresh complete medium with 1% autologous plasma at 37°C for no more than 24 hours. The medium was collected and frozen at −20°C before use.
Monoclonal Antibodies and Cytofluorography
Monoclonal antibodies (MoAbs) to the following antigens were used: HLA-DR, CD14, CD32, CD1a, CD25, CD45RO (Becton Dickinson, Mountainview, CA); CD80 and CD86 (IgG1, fluorescein isothiocyanate [FITC] conjugate; Pharmingen); CD83, which is detected on mature blood dendritic cells15 (IgG1, phycoerythrin [PE] conjugate; Coulter Corp, Miami, FL); CD68 (Dako, Carpinteria, CA); CD40 (Serotec); and p55 (an actin-bundling protein,16 K-2 clone; a gift from Dr E. Langhoff). Secondary antibody was PE-conjugated F[ab′]2 goat anti-mouse IgG (gamma and light chain; Tago, Burlingame, CA). Cell populations were phenotyped with the panel of MoAbs listed above and analyzed on a FACScan (Becton Dickinson). Dead cells and contaminating lymphocytes were excluded by forward and side scatter properties.
Allogeneic MLR
To test for T cell–stimulatory function, the APCs were added in graded doses as stimulators for 2 × 105 purified, allogeneic T cells in 96-well flat bottomed plates (Costar, Cambridge, MA). Proliferation was determined on days 4 to 6 with the addition of 4 μCi/mL of [3H]TdR for 10 to 16 hours to triplicate wells (mean counts per minute).
RESULTS
Cytokine Concentrations in MCM
MCM substantially increases the yield (3- to 10-fold), enrichment (5- to 15-fold), and immunostimulatory (5- to 15-fold) capacity of dendritic cells generated from GM-CSF/IL-4–treated precursor cells in blood.7,8 The MCM-generated cells are phenotypically and functionally stable in that they retain dendritic cell features for several days when cultured in the absence of cytokines, characteristics of full maturation.7 8 We therefore analyzed several MCM preparations for the presence of cytokines that could be candidates for maturation factors, such as TNF-α, IL-1β, IL-6, and IFN-α. Using commercially available ELISA kits, all four cytokines were detected, but in widely varying concentrations (Table 1). In some instances the concentrations were high (up to 1 μg/mL for IL-6). Notably, each of the MCM preparations analyzed was active in inducing dendritic cell maturation at a concentration of 50% vol/vol.
Concentrations of Cytokines in MCM
| . | TNF-α . | IL-1β . | IL-6 . | IFN-α . |
|---|---|---|---|---|
| Range | 9-182 | 1.5-92 | 6-1,000 | .02-.1 |
| Mean ± SD | 41 ± 58 | 27.7 ± 37 | 584.5 ± 420 | .04 ± .02 |
| No. | 7 | 5 | 4 | 17 |
| . | TNF-α . | IL-1β . | IL-6 . | IFN-α . |
|---|---|---|---|---|
| Range | 9-182 | 1.5-92 | 6-1,000 | .02-.1 |
| Mean ± SD | 41 ± 58 | 27.7 ± 37 | 584.5 ± 420 | .04 ± .02 |
| No. | 7 | 5 | 4 | 17 |
Several MCM preparations were evaluated for the presence of TNF-α, IL-1β, IL-6, and IFN-α using standard ELISA kits. The range of concentrations and the mean ± SD (ng/mL) for 4 to 17 samples are given. Concentrations are given in ng/mL.
Contribution of TNF-α in the Maturation of Dendritic Cells
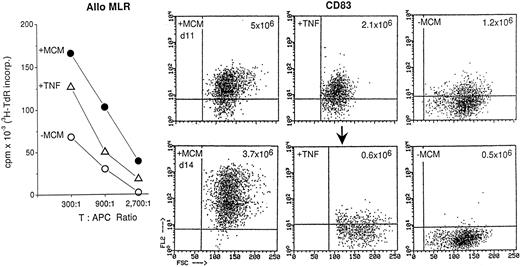
TNF-α, in conjunction with other cytokines, is critical for the development of dendritic cells from CD34+ progenitor cells in cord blood and bone marrow.17-21 It also mediates the maturation of GM-CSF/IL-4–primed blood progenitor cells that are cultured in medium with FCS.9-11 Because substantial quantities of TNF-α are present in MCM, we evaluated its role in the maturation of dendritic cells generated in medium containing 1% plasma. TNF-α [50 ng/mL] was added to GM-CSF/IL-4–treated ER− cells every other day on days 0, 2, 4, 6, and 8. After 11 days of culture, the cells were collected and assayed for immunostimulatory activity in the allo MLR and for CD83 expression (Fig 1). Residual cells were washed, returned to culture in the absence of additional cytokines, and reanalyzed 3 days later (day 14) to determine whether the cells had irreversibly matured. Compared with GM-CSF/IL-4 treatment alone, TNF-α did generate cells that expressed CD83 (Fig 1, upper panel), moderate levels of MHC molecules, adhesins, and costimulators (eg, CD80 and CD86, not shown) and that were potent stimulators of allogeneic T cells as previously described.11 However, they were less immunostimulatory than MCM-treated cells, and 60% fewer cells were obtained (2.1 × 106v 5 × 106 for MCM-treated cells on day 11).
Contribution of TNF-α in the maturation of dendritic cells. T cell–depleted blood mononuclear cells were cultured for 7 days in GM-CSF/IL-4 as described in Materials and Methods. From day 7 to 11 they were cultured in conditioned medium (MCM) or no additional supplementation (−MCM). TNF-α (50 ng/mL) was added every other day from days 0 to 8. On day 11, the cells were analyzed for CD83 expression. Residual cells were washed twice and returned to culture for 3 more days in RPMI containing 1% plasma. On day 14 the cells were tested for T cell–stimulatory capacity in the allo MLR (left panel) and reevaluated for CD83 expression. The recovered cell yields from a starting volume of 40 mL of blood are given in the upper right hand corner of each FACS panel. The results are representative of two experiments.
Contribution of TNF-α in the maturation of dendritic cells. T cell–depleted blood mononuclear cells were cultured for 7 days in GM-CSF/IL-4 as described in Materials and Methods. From day 7 to 11 they were cultured in conditioned medium (MCM) or no additional supplementation (−MCM). TNF-α (50 ng/mL) was added every other day from days 0 to 8. On day 11, the cells were analyzed for CD83 expression. Residual cells were washed twice and returned to culture for 3 more days in RPMI containing 1% plasma. On day 14 the cells were tested for T cell–stimulatory capacity in the allo MLR (left panel) and reevaluated for CD83 expression. The recovered cell yields from a starting volume of 40 mL of blood are given in the upper right hand corner of each FACS panel. The results are representative of two experiments.
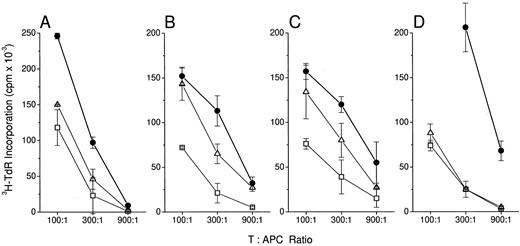
MCM is more effective than a combination of cytokines in inducing dendritic cell maturation. T cell–depleted blood mononuclear cells (ER− cells) were cultured for 7 days in GM-CSF/IL-4. They were washed twice, transferred to fresh 6-well plates, and cultured in MCM (•), a combination of cytokines (▵), or no additional supplementation (−MCM, □). On day 11 of culture, (A) and (B), the cells were evaluated for T cell–stimulatory activity in an allogenic MLR. In (C) and (D), dendritic cells were washed twice and returned to culture for 1 (C) or 3 (D) more days in RPMI supplemented with 1% autologous human plasma. (A) and (B), cytokines were IL-1 (20 ng/mL), IL-6 (20 ng/mL), IFN-α (0.02 ng/mL), and TNF-α (20 ng/mL). In (C) and (D), the cocktail of cytokines mimicked the concentrations measured by ELISA in the corresponding MCM. (C) IL-1 (92 ng/mL), IL-6 (1 μg/mL), IFN-α (0.02 ng/mL) and TNF-α (182 ng/mL); (D) IL-1 (20 ng/mL), IL-6 (734 ng/mL), IFN-α (0.08 ng/mL), and TNF-α (24 ng/mL). The values represent the averages of triplicates ± SD.
MCM is more effective than a combination of cytokines in inducing dendritic cell maturation. T cell–depleted blood mononuclear cells (ER− cells) were cultured for 7 days in GM-CSF/IL-4. They were washed twice, transferred to fresh 6-well plates, and cultured in MCM (•), a combination of cytokines (▵), or no additional supplementation (−MCM, □). On day 11 of culture, (A) and (B), the cells were evaluated for T cell–stimulatory activity in an allogenic MLR. In (C) and (D), dendritic cells were washed twice and returned to culture for 1 (C) or 3 (D) more days in RPMI supplemented with 1% autologous human plasma. (A) and (B), cytokines were IL-1 (20 ng/mL), IL-6 (20 ng/mL), IFN-α (0.02 ng/mL), and TNF-α (20 ng/mL). In (C) and (D), the cocktail of cytokines mimicked the concentrations measured by ELISA in the corresponding MCM. (C) IL-1 (92 ng/mL), IL-6 (1 μg/mL), IFN-α (0.02 ng/mL) and TNF-α (182 ng/mL); (D) IL-1 (20 ng/mL), IL-6 (734 ng/mL), IFN-α (0.08 ng/mL), and TNF-α (24 ng/mL). The values represent the averages of triplicates ± SD.
By day 14, a significant proportion of TNF-α–treated cells readhered to plastic or died, as reflected in the reduced yields (0.6 × 106 ). Furthermore, there was substantial downregulation of CD83 (Fig 1, lower panel) and MHC molecules (not shown) and reexpression of CD14 (data not shown). TNF-α–treated cells also failed to express the activation marker CD25. Similar results were obtained when different concentrations of TNF-α (10 to 100 ng/mL) were used or if it was added only on day 7.
In contrast to TNF-α, MCM treatment induced stable CD83 expression, high levels of MHC and costimulator molecules, and recovery of substantially higher cell numbers (3.7 × 106; Fig 1, lower panel). Cells cultured in the absence of either MCM or TNF-α had the least immunostimulatory activity, generated the fewest number of cells (0.5 × 106 ), and had low to no CD83 expression. Therefore, TNF-α alone does not account for the terminal maturational effects induced by MCM.
Several Cytokines That Are in MCM Do Not Reconstitute the Full Effect of MCM
We next ascertained whether a combination of cytokines could substitute for MCM. TNF-α, IL-1β, IL-6, and IFN-α were added in combination to GM-CSF/IL-4–treated ER− cells on day 7. After 11 to 14 days in culture the cells were enumerated and analyzed for stimulatory capacity (Fig 2) and CD83 expression (Fig 3). In all experiments cells were washed on days 7 and 11 before analysis to remove residual cytokines. In Fig 2C and D, the cytokines were added back in concentrations equivalent to those measured by ELISA in the corresponding MCM. The cytokine combination enhanced the immunostimulatory function of GM-CSF/IL-4–treated cells in the allo MLR and in some cases was similar to that seen with MCM (Fig 2B). However, in other experiments (Fig 2A and D), the cytokines did not fully substitute for MCM. This combination of cytokines is nevertheless effective to some degree because cells upregulated and retained CD83 expression for at least 3 days after cytokine removal (Fig 3).
Maturation of dendritic cells induced by MCM is irreversible. GM-CSF/IL-4–treated ER− cells were cultured for 4 days in medium alone [−MCM], two different MCM preparations (MCM A and B), or a combination of cytokines equal to that present in the MCM. On day 11, the cells were washed and analyzed for CD83 expression. Residual cells were returned to culture for 3 more days (day 14) in the absence of further supplementation and analyzed for the retention of CD83. MCM contained the following cytokines: (A) IL-1 (92 ng/mL), IL-6 (1 μg/mL), IFN-α (0.02 ng/mL), and TNF-α (182 ng/mL); (B) IL-1 (20 ng/mL), IL-6 (734 ng/mL), IFN-α (.08 ng/mL), and TNF-α (24 ng/mL).
Maturation of dendritic cells induced by MCM is irreversible. GM-CSF/IL-4–treated ER− cells were cultured for 4 days in medium alone [−MCM], two different MCM preparations (MCM A and B), or a combination of cytokines equal to that present in the MCM. On day 11, the cells were washed and analyzed for CD83 expression. Residual cells were returned to culture for 3 more days (day 14) in the absence of further supplementation and analyzed for the retention of CD83. MCM contained the following cytokines: (A) IL-1 (92 ng/mL), IL-6 (1 μg/mL), IFN-α (0.02 ng/mL), and TNF-α (182 ng/mL); (B) IL-1 (20 ng/mL), IL-6 (734 ng/mL), IFN-α (.08 ng/mL), and TNF-α (24 ng/mL).
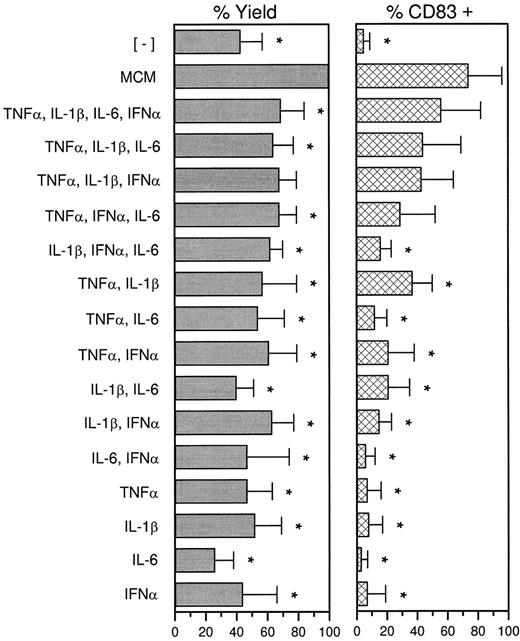
To identify which cytokines, if any, comprised an effective combination, all possible mixtures were tested. MCM consistently generated the greatest stimulatory activity on a per-cell basis (above and data not shown), highest yields, and CD83 expression (Fig 4) and was therefore considered the standard against which other culture conditions should be assessed. For example, in the absence of MCM, on average of only 42% of the maximal cell yield was obtained (of which few are actually mature dendritic cells). Cytokine cocktails increased the yield up to or as much as 69%. To evaluate stable CD83 expression, cells treated with various combinations of cytokines were washed on day 11 and recultured for 3 more days in the absence of additional supplements. CD83 could be induced on variable numbers of cells by several combinations of cytokines but not by individual cytokines. MCM generated the highest number of CD83+ cells. Collectively, the data suggest that the effects of MCM are complex and probably caused by the combined effect of several cytokines, including those evaluated here.
Treatment with MCM is superior to cytokines. ER− cells were cultured for 7 days in GM-CSF and IL-4, and recultured in the presence of MCM, cytokine combinations, or no additional supplementation (−MCM) for 4 more days. On day 11 cells were collected and counted. The percent yields reflect the number of cells obtained relative to treatment with MCM (100%). The cells were then washed to remove residual cytokines, cultured for 3 days in RPMI with 1% plasma in the absence of additional supplements, and analyzed for CD83 expression (day 14). Values are the mean ± SD and are representative of 5 to 7 experiments. *P < .05, paired Student's t-test.
Treatment with MCM is superior to cytokines. ER− cells were cultured for 7 days in GM-CSF and IL-4, and recultured in the presence of MCM, cytokine combinations, or no additional supplementation (−MCM) for 4 more days. On day 11 cells were collected and counted. The percent yields reflect the number of cells obtained relative to treatment with MCM (100%). The cells were then washed to remove residual cytokines, cultured for 3 days in RPMI with 1% plasma in the absence of additional supplements, and analyzed for CD83 expression (day 14). Values are the mean ± SD and are representative of 5 to 7 experiments. *P < .05, paired Student's t-test.
DISCUSSION
The ability of dendritic cells to process antigens and prime naive T cells develops sequentially, a process commonly referred to as maturation. We have developed an in vitro culture system whereby dendritic cells are induced to undergo irreversible maturation. The method uses a CD34−, CD14+–enriched precursor population in blood and entails two stages. The first is a priming phase by GM-CSF and IL-4 that yields cells that actively internalize particulate antigens and that are highly efficient at priming resting T cells to native soluble antigens.7-9 Phenotypically, these immature dendritic cells are characterized by moderate levels of MHC and costimulator molecules, and variable levels of the macrophage associated molecules CD14 and CD32 [FcR].7-9
The second or maturation step in dendritic cell development requires exposure to MCM. We define maturation as the irreversible acquisition of several properties: typical stellate morphology, nonadherence to plastic, upregulation of MHC and costimulator molecules, and expression of the two dendritic cell–restricted molecules CD8315 and p55.16 Maturation is typically marked by a coordinate series of additional changes that include downregulation of macropinocytosis, expression of activation molecules (CD25, CD95, CD45RO) and perinuclear CD68, and the loss of CD1a, CD32, CD115 and CD14.7,8 The mature dendritic cells are less efficient at processing soluble antigens but highly efficient at presenting peptide antigens to T cells.8
We found that MCM was required to ensure the development of fully differentiated dendritic cells from GM-CSF– and IL-4–primed progenitor cell cultures. MCM reproducibly generated the highest yields and T cell–stimulating capacity compared with any other cytokine or combination of cytokines evaluated. The resulting dendritic cells are the most powerful immunostimulatory APCs identified to date, stimulating strong allogeneic T cells at T to APC ratios of 1,000:1 or less.
TNF-α was a prime candidate for a dendritic cell maturation factor because it matures dendritic cells grown in GM-CSF/IL-4 in the presence of FCS.9-11 However, our results differ in two significant respects from these studies. First, we found that the effects of TNF-α on dendritic cell development were transient when 1% plasma was used in the place of FCS. Removal of the cytokine resulted in a substantial decrease in cell number. The cells acquired macrophage-like properties (adherence to plastic and CD14 expression) and CD83 expression was downregulated. This fact was not previously appreciated because dendritic cells are generally studied without reculturing them in the absence of cytokines.9-11 Second, dendritic cells generated in GM-CSF/IL-4 and TNF-α in FCS express CD1a and low levels of CD25.11 The loss of CD1a and upregulation of CD25 are associated with the terminal maturation of both blood- and skin-derived dendritic cells, which are CD83+, CD1a− and CD25+.7 8
Other stimuli aside from TNF-α that have been reported to induce dendritic cell maturation from GM-CSF/IL-4–primed progenitors include IL-1, LPS, and CD40-L.9,10 However, as with TNF-α, these studies used cells generated in FCS, which could provide additional growth inducing factors. We have studied IL-1β and Pansorbin (SAC) in our culture system. IL-1β alone or in combination with other cytokines was significantly less effective than MCM. Conditioned medium that is generated by stimulating monocytes with Pansorbin for 24 hours can substitute for MCM, probably by inducing the production of relevant cytokines.7 LPS contaminants are unlikely to explain the effects of MCM because we did not detect physiologically significant levels in several preparations by the Limulus amebocyte lysate assay (data not shown).
The responsible factor(s) in MCM probably include some combination of TNF-α, IL-1β, IL-6, and IFN-α, because these could partially substitute for MCM. Previous attempts to neutralize TNF-α, IL-1β, and IL-6 in MCM preparations with antibodies were unsuccessful.7 However, it is equally likely that other components, not yet defined, are also critical. For example, MCM contains the chemokines MIP-1α and Rantes (data not shown). IL-12, a factor that augments dendritic cell–dependent T cell–stimulatory capacity22-24 is also present in MCM, but neutralization of this factor with MoAbs does not affect MCM activity.7 Possibly a new factor, or even production of factors by the maturing dendritic cells themselves, are responsible for optimal development. Transforming growth factor (TGF)-β1 is one candidate. This cytokine appears to be essential for Langerhans cell development or epidermal localization.25 Future studies will be required to determine whether TGF-β1 is present in MCM and whether or not it participates in dendritic cell development. The complexity and diversity of MCM makes it difficult, if not impossible, to identify every relevant component. Nevertheless, the significance of MCM is underscored by our earlier observations that MCM is vital for the maturation of a subset of circulating blood dendritic cells.5 6 This effect also cannot be duplicated by individual cytokines.
Evidence for a physiological counterpart for MCM comes from studies showing that dendritic cells are rapidly induced to migrate into tissues (lung,26 synovial fluid27-29 ), and from organs (gut, heart, kidney, skin30-33 ) into lymph or lymphoid tissue in response to certain stimuli, eg, lipopolysaccharide, infection, chronic inflammation. Migration parallels maturation as determined by the downregulation of antigen processing capacity and upregulation of T cell–stimulatory function.34 The local production of cytokines (eg, TNF-α, IL-1, IL-6, IFN-α) by tissue macrophages in response to infectious or inflammatory stimuli may therefore regulate dendritic cell maturation and consequently antigen presentation.
The identification of an MCM-dependent step in dendritic cell maturation will facilitate functional and molecular characterization of this cell. From a clinical point of view, one now has easy and rapid access to sizeable numbers of irreversibly mature cells for immunotherapeutic purposes, eg, in malignancy and chronic viral infections. In this regard we have shown that dendritic cells generated using this MCM-dependent approach are potent inducers of specific antiviral CD8+ cytolytic T-cell responses in vitro, when pulsed with nonreplicating influenza virus or immunodominant peptides of the influenza matrix protein.7 8
ACKNOWLEDGMENT
We thank Ralph M. Steinman for helpful discussion and advice, and Judy Adams, Frank Isdell, and Jyotsna Rao for assistance with the graphics.
Supported by Grant Nos. AR-42557 and AI 39516 from the National Institutes of Health and by the New York Community Trust (to N.B.).
Address reprint requests to Nina Bhardwaj, MD, PhD, The Rockefeller University, 1230 York Ave, New York, NY, 10021.

![Fig. 3. Maturation of dendritic cells induced by MCM is irreversible. GM-CSF/IL-4–treated ER− cells were cultured for 4 days in medium alone [−MCM], two different MCM preparations (MCM A and B), or a combination of cytokines equal to that present in the MCM. On day 11, the cells were washed and analyzed for CD83 expression. Residual cells were returned to culture for 3 more days (day 14) in the absence of further supplementation and analyzed for the retention of CD83. MCM contained the following cytokines: (A) IL-1 (92 ng/mL), IL-6 (1 μg/mL), IFN-α (0.02 ng/mL), and TNF-α (182 ng/mL); (B) IL-1 (20 ng/mL), IL-6 (734 ng/mL), IFN-α (.08 ng/mL), and TNF-α (24 ng/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3640/4/m_bl_0044f3.jpeg?Expires=1769170514&Signature=Q-YBa7SnbQvO3j6VgbVu~1jum3iZDLbWLZOn5CHFtLCKoWjz20kkz57txBaO-DA5X4FtpmzmE7AOEy4Jmnut0xB3pK9XyMMhXfuZ5cpzolniXUc5TW0njP7H3HhUIz7NErxs3llXZi4XfwCL3bN4TrSBSYmg6Qy3-QdowyaGO2CkITjtOuchaKqO0WRFKoSsQvjkq1HezDuCGmmPCYzKWFGVaopK99jtp4wwhGEC-bhiMDavMnXzh8GgOeU8OIIyEHin0dzaxB5V2khaFZkvrA-utEC2hTYsCsqlhN6sVezcokc3MrSAUQMtkit1RST7F8vqmIuoSognrin1BV5lCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal