Abstract
The disease spectrum of natural killer (NK) cell leukemias and lymphomas has recently been expanding with the continuing evolution in diagnostic concepts. We describe here seven cases of acute leukemia of conceivable myeloid and NK cell precursor phenotype in six men and one woman varying from 19 to 59 years of age (median, 46 years). Striking extramedullary involvement was evident at initial presentation, with peripheral lymphadenopathy and/or mediastinal masses. Two lacked any leukemic cells in the bone marrow at diagnosis. Using cytochemical myeloperoxidase staining, less than 3% of the leukemic cells showed positive reactivity. However, expression of CD7, CD33, CD34, CD56, and frequently HLA-DR, but not other NK, T-cell, and B-cell markers was observed. Cytoplasmic CD3 was detected in three of the cases by flow cytometry and in six by Northern blotting, suggesting an origin from common progenitors between the NK cell and myeloid lineages. All but one presented germline configurations of the T-cell receptor β and γ chain genes and Ig heavy chain gene. With regard to morphology, the cells were generally L2-shaped, with variation in cell size, round to moderately irregular nuclei and prominent nucleoli, pale cytoplasm, and a lack of azurophilic granules. Histopathologic examination of biopsied specimens of extramedullary tumors showed a lymphoblast-like morphology, implying the differential diagnostic problem from lymphoblastic lymphomas, especially in cases lacking bone marrow involvement. Three patients were successfully treated with chemotherapy for acute myeloid leukemia (AML), whereas three other patients proved refractory to chemotherapeutic regimens for lymphoid malignancies, although two responded to subsequent AML chemotherapy. However, despite intensive chemotherapy, including allogeneic bone marrow transplantation, most persued fatal courses within 41 months. These data suggested that the CD7+ and CD56+ myeloid/NK cell precursor acute leukemia might constitute a distinct biologic and clinical disease entity. Its recognition appears to be particularly important for the clinicopathologic evaluation of CD56+ hematolymphoid malignancies and the development of therapeutic approaches to such disease.
CD56 IS A 200- TO 220-kD glycoprotein expressed predominantly on human natural killer (NK) cells and a minor subset of T cells mediating major histocompatability complex (MHC)-nonrestricted cytotoxicity.1-4 Its expression in leukemias and lymphomas has been extensively investigated over the past decade, and our knowledge of the CD56+ hematolymphoid malignancies has expanded dramatically.
The expression of CD56 is a rare phenomenon in lymphoid malignancies, mostly confined to those of T/NK cell origin,5-8 generally characterized by unique extranodal involvement, with lesions in the nasal-paranasal region, skin, lung, and stomach, and often display the angiocentric growth pattern. Because the CD56 antigen has been found to correspond to a neural cell adhesion molecule with homophilic binding characteristics,9,10 its expression in leukemia and lymphoma cells is thought to play an important role in their extranodal localization and behavior.5,11 12
In acute myeloid leukemia (AML), CD56 expression has been identified in approximately 20% of the cases examined, generally in association with a monocytic morphology and cytogenetic abnormalities t(8; 21) or trisomy 8.13 Because the incidence of extramedullary tumor formation is identical in both CD56+ and CD56− cases, the clinical significance of CD56 expression remains to be elucidated. Recently, Scott et al14 proposed a myeloid/NK cell acute leukemia disease entity presenting the HLA-DR−, CD33+, CD56+, and CD16− phenotype, although CD7 was not covered in their series. A mature myeloid morphology was manifested with deeply invaginated nuclear membrane, scant cytoplasm with fine azurophilic granules, and finely granular Sudan black B and/or myeloperoxidase (MPO) cytochemical reactivity. In their series, extramedullary disease was noted in only 5 (25%) of the 20 cases.
We have identified seven cases of a unique form of acute leukemia of conceivable myeloid/NK cell precursor phenotype (ie, CD7+, CD33+, CD34+, CD56+, and frequently HLA-DR+ type) suggested to be immature in terms of morphology, phenotype, and genotype compared with those described by Scott et al.14 These seven cases were all characterized by extramedullary disease, represented by peripheral lymphadenopathy and/or mediastinal bulky masses, with a lymphoblastoid appearance and a lack of azurophilic granules in the cytoplasm. Clinically, most of them responded to chemotherapeutic regimens for AML. However, in all, except for one case that died of graft-versus-host disease, recurrence of leukemia occurred and they persued fatal clinical courses within 41 months, thus appearing to be more aggressive than those of Scott et al.14 We believe that the present cases provide fundamental data for understanding the disease spectrum of myeloid/NK cell hematolymphoid malignancies.
MATERIALS AND METHODS
Patients.During the 8-year period between 1988 and 1995, seven cases of CD7+ and CD56+ myeloid/NK cell precursor acute leukemias were encountered in the Aichi Cancer Center Hospital (Nagoya, Japan). These cases were identified from more than 600 cases of fully immunophenotyped acute leukemias or malignant lymphomas. The clinical records and pathologic materials of these seven cases were reviewed.
Morphology.Patients' bone marrow or peripheral blood cells underwent May-Grünwald-Giemsa (MG), MPO, α naphthol AS-D chloroacetate esterase, α naphthyl butyrate (NB) esterase, and periodic acid-Schiff (PAS) staining. Biopsied tissues were fixed in 10% formaldehyde and embedded in paraffin. Sections were cut at 5 mm and stained with hematoxylin and eosin, Elastica-van Gieson, silver impregnation, PAS, MG, and methyl green-pyronin. Imprint smears of the surgically resected specimen were stained with MG stain.
Pretreatment Clinical Characteristics of Myeloid/NK Cell Precursor Acute Leukemia Cases
| Case No. . | Age (yr)/Sex . | WBC . | % Blasts . | Hb . | PLT . | BM Involvement . | Site of Involvement . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | (×109/L) . | . | (g/dL) . | (×109/L) . | Diagnosis (relapse) . | LN . | Liver . | Spleen . | Mediastinum . |
| 1 | 34/M | 8.8 | 0% | 17.0 | 381 | 0% (81.0%) | Neck, axillary, inguinal | − | − | − |
| 2 | 46/M | 51.0 | 87% | 14.4 | 39 | 86.5% | Neck | + | + | + |
| 3 | 54/M | 2.1 | 29% | 15.2 | 86 | 89.5% | Neck, axillary, tonsil | − | − | − |
| 4 | 29/F | 1.1 | 5% | 12.6 | 76 | 91.5% | Submandible, neck | − | − | − |
| 5 | 48/M | 5.9 | 0% | 15.7 | 217 | 0% (56.0%) | — | − | − | + |
| 6 | 19/M | 1.3 | 11% | 14.0 | 258 | 78.0% | Neck, inguinal | − | − | − |
| 7 | 59/M | 2.7 | 5% | 13.0 | 199 | 19.5% (84.0%) | Neck, axillary, inguinal | − | − | − |
| Case No. . | Age (yr)/Sex . | WBC . | % Blasts . | Hb . | PLT . | BM Involvement . | Site of Involvement . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | (×109/L) . | . | (g/dL) . | (×109/L) . | Diagnosis (relapse) . | LN . | Liver . | Spleen . | Mediastinum . |
| 1 | 34/M | 8.8 | 0% | 17.0 | 381 | 0% (81.0%) | Neck, axillary, inguinal | − | − | − |
| 2 | 46/M | 51.0 | 87% | 14.4 | 39 | 86.5% | Neck | + | + | + |
| 3 | 54/M | 2.1 | 29% | 15.2 | 86 | 89.5% | Neck, axillary, tonsil | − | − | − |
| 4 | 29/F | 1.1 | 5% | 12.6 | 76 | 91.5% | Submandible, neck | − | − | − |
| 5 | 48/M | 5.9 | 0% | 15.7 | 217 | 0% (56.0%) | — | − | − | + |
| 6 | 19/M | 1.3 | 11% | 14.0 | 258 | 78.0% | Neck, inguinal | − | − | − |
| 7 | 59/M | 2.7 | 5% | 13.0 | 199 | 19.5% (84.0%) | Neck, axillary, inguinal | − | − | − |
Abbreviations: LN, lymph node; BM, bone marrow; WBC, white blood cell count; Hb, hemoglobin level; PLT, platelet count.
Immunophenotyping.Surface immunophenotyping of the tumor cells was performed by flow cytometry using a broad panel of monoclonal antibodies (MoAbs). Mononuclear cells were separated from heparinized bone marrow samples using Ficoll-Hypaque centrifugation. Fresh biopsied specimens were also made into single-cell suspensions, and the tumor cells were analyzed on FACStar and FACScan analyzers (Becton Dickinson, Mountain View, CA) for fluorescent intensity using fluorescein isothiocyanate (FITC)- or phycoerythin (PE)-conjugated antibodies or unconjugated antibodies followed by fluorescein-conjugated antimouse second antibodies. The MoAbs used were OKT6 (CD1), OKT11 (CD2), OKM1 (CD11b), OKB7 (CD21), OKB22 (CD22), OKM5 (CD36), OKT10 (CD38), and OKT9 (CD71) (Ortho Diagnostics, Rarian, NJ); Leu4 (CD3), Leu3a (CD4), Leu1 (CD5), Leu2 (CD8), LeuM5 (CD11c), LeuM1 (CD15), Leu11 (CD16), Leu12 (CD19), Leu16 (CD20), HPCA-1 (CD34), HPCA-2 (CD34), Leu7 (CD57), TCR1 (TCRαβ), and anti-TCRγδ (Becton Dickinson); Tp120 (CD6) and Tp40 (CD7) (established in our laboratory15 ); TP82 (CD9), MCS-2 (CD13), KOLT2 (CD28), (CD41b), and Mik-β1 (CD122; Nichirei, Tokyo, Japan); J5 (CD10), My4 (CD14), My9 (CD33), and NKH-1 (CD56; Coulter, Hialeah, FL); Tac (CD25; kindly provided by Dr T. Uchiyama, Kyoto, Japan); and anti-CD11a, anti–HLA-DR, anti-IgA, anti-IgG, anti-IgM, anti-IgD, anti-κ, anti-λ, anti-MPO, and anti-terminal deoxynucleotidyl transferase (TdT; DAKO, Carpenteria, CA). Cytoplasmic antigens and TdT were analyzed as described elsewhere,16 with a modification on fixation from 0.5% methanol-free formaldehyde to 50% ethanol with 1% paraformaldehyde. Multiparameter analysis of gated cell populations on cell suspension studies was used to provide more definitive immunophenotyping information (Consort 30 and Lysis II computer softwares; Becton Dickinson).
Cytogenetic analysis.Cytogenetic analysis was performed as described previously.17 In brief, pretreatment bone marrow leukemic cells or single-suspended lymph node cells were cultured in RPMI1640 supplemented with 20% fetal calf serum without mitogens for 72 hours and incubated with colcemid at a final concentration of 0.02 μg/mL for 1.5 hours at 37°C. Cells were then exposed to 50 mmol/L KCl solution for 20 minutes at room temperature and fixed with methanol-acetic acid (3:1). Chromosomes were banded using the trypsin-Giemsa method.
Southern blot analysis.High molecular weight DNA was extracted from fresh or frozen stored leukemic cells or lymph nodes and digested with the restriction enzymes (BamHI, EcoRI, and Xba I), and genotypic blot hybridization was performed as described previously17 using the human T-cell receptor (TCR) β chain gene probes, CTβ and Jβ2 (provided by Dr T.W. Mak [Ontario Cancer Institute, Toronto, Canada] and Dr Y. Kurosawa [Institute for Comprehensive Medical Science, Fujita Health University School of Medicine, Aichi, Japan], respectively); the TCR γ chain gene probe, Jγ1 (provided by Dr T.H. Rabbitts, Medical Research Council Laboratory of Molecular Biology, Cambridge, UK); and the human Ig heavy chain (IgH) gene joining region probe JH (provided by Dr J.V. Ravetch, Laboratory of Molecular Genetics, National Institutes of Health, Bethesda, MD).
Northern blot analysis.Total RNA was extracted from mononuclear cells or frozen tumors, and Northern blot hybridization was performed as described previously.17 Probes used were the human MPO probe (provided by Dr K. Morishita, National Cancer Center Research Institute, Tokyo, Japan) and CD3δ and CD3ε chain probes (provided by Dr T.H. Rabbitts).
Epstein-Barr virus (EBV) study.Detection of EBV-encoded small RNAs (EBERs) by in situ hybridization (ISH) using EBER oligonucleotides was performed on formalin-fixed paraffin-embedded sections, according to the method previously described.8 Presence of the EBV genome was also assessed by Southern blotting analysis using an EBV terminal sequence-specific Eco I probe (provided by Dr K. Takada, Cancer Institute, Hokkaido University School of Medicine, Sapporo, Japan).
RESULTS
Clinical features.The clinical features of the seven patients are summarized in Table 1. Six were men and one was a woman; their ages ranged from 19 to 59 years, with a median of 46 years. At the time of initial presentation, circulating leukemic cells were detected in five patients (nos. 2, 3, 4, 6, and 7), and thrombocytopenia was observed in three (nos. 2, 3, and 4). No patients were anemic. Initial bone marrow involvement was detected in five cases (nos. 2, 3, 4, 6, and 7) to various degrees, whereas two (nos. 1 and 5) showed bone marrow involvement at relapse. Although patient no. 7 presented with 19.5% of leukemic blasts in bone marrow, a rather low percentage for the diagnosis of acute leukemia, his bone marrow examination at relapse showed hypercellular marrow with 84.0% leukemic blasts. Peripheral lymphadenopathy and/or mediastinal bulky masses were common findings, although none presented B symptoms. Four patients (nos. 3, 4, 6, and 7) initially presented with localized lymphadenopathy, occurring mainly in the cervical region, and bone marrow involvement. Two patients (nos. 2 and 5) clinically manifested a mediastinal bulky mass associated superior vena cava syndrome, further accompanied in one case (no. 2) by cervical lymphadenopathy, hepatosplenomegaly, and bone marrow involvement. Testicular and meningeal involvement was encountered at relapse in patients nos. 5 and 7, respectively. Serum obtained from all patients did not show antibodies to human T-cell leukemia/lymphoma virus type 1 or human immunodeficiency virus using an enzyme-linked immunosorbent assay.
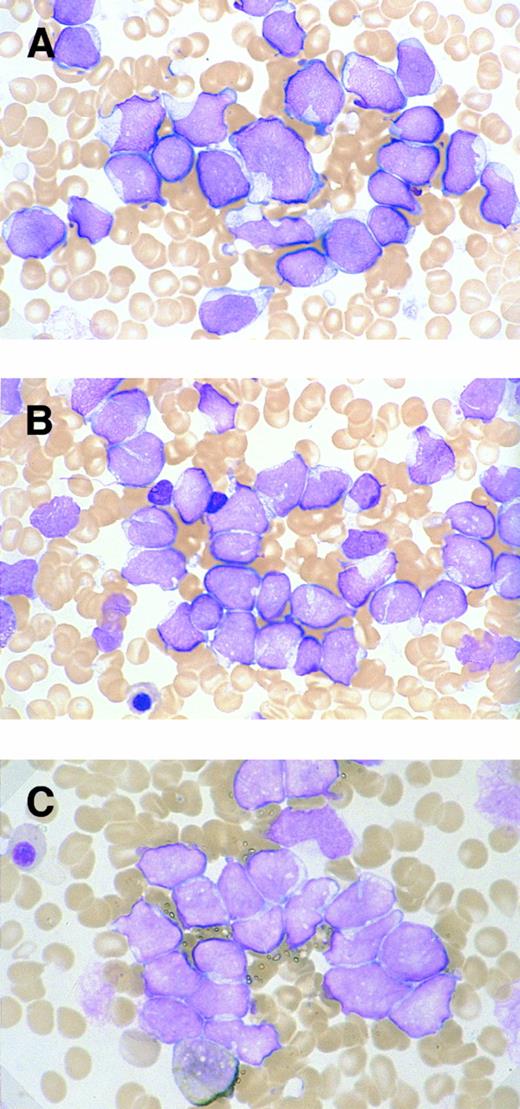
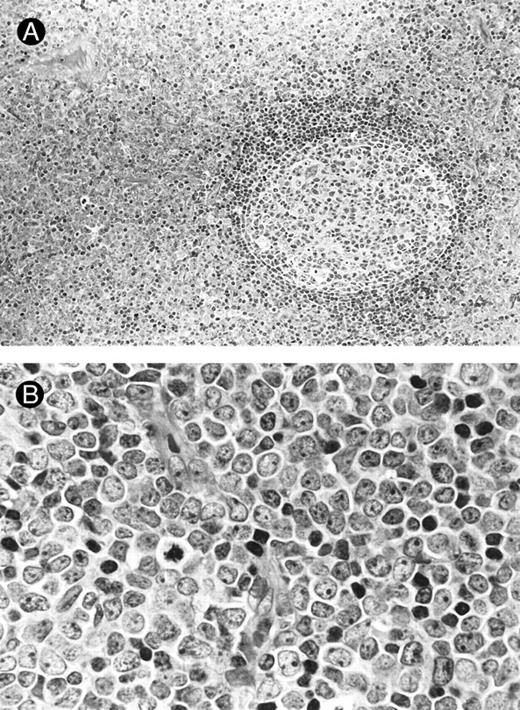
Morphology.The bone marrow and peripheral blood smears of all patients (nos. 1 and 5 at relapse) showed L2-shaped leukemic cells of various sizes, with moderately irregular nuclei, prominent nucleoli, and pale cytoplasm (Fig 1). Cytoplasmic granules or Auer's rods were not recognized in bone marrow smears and in touch imprints of biopsied specimens. Less than 3% of leukemic cells showed cytochemical MPO reactivity in all cases. The leukemic cells were histochemically negative for α-naphthol AS-D chloroacetate esterase, α-NB esterase, and PAS. Lymph node biopsies was obtained from six patients (nos. 1 through 4, 6, and 7) and needle biopsy specimen of a mediastinal tumor was available for patient no. 5. All these biopsied specimens morphologically showed diffuse infiltration by monotonous proliferation of immature mononuclear cells (Fig 2). In most of the affected lymph nodes, such infiltrates showed a T-zone distribution with sparing of follicles. The component cells had a similar blastic morphology to those seen in bone marrow or peripheral blood. These findings seriously pose the differential diagnostic problem from lymphoblastic lymphoma (LBL); indeed, a diagnosis of LBL was tentatively made for four of the patients (nos. 1, 2, 5, and 6).
Morphologic and cytochemical features of myeloid/NK cell precursor acute leukemia. MG-stained bone marrow smear of patients no. 4 (A) and no. 3 (B). (A) The leukemic cells show a L2-shaped morphology, with varying cell size, irregular nuclei with prominent nucleoli, and pale cytoplasm. Azurophilic granules are not apparent in the cytoplasm (original magnification × 1,200). (B) The leukemic cells present with unremarkable nucleoli and some with deeply invaginated nuclear membrane (original magnification × 1,200). (C) MPO-stained bone marrow smear of patient no. 3. Less than 3% of leukemic cells are positive for MPO staining (original magnification × 1,200).
Morphologic and cytochemical features of myeloid/NK cell precursor acute leukemia. MG-stained bone marrow smear of patients no. 4 (A) and no. 3 (B). (A) The leukemic cells show a L2-shaped morphology, with varying cell size, irregular nuclei with prominent nucleoli, and pale cytoplasm. Azurophilic granules are not apparent in the cytoplasm (original magnification × 1,200). (B) The leukemic cells present with unremarkable nucleoli and some with deeply invaginated nuclear membrane (original magnification × 1,200). (C) MPO-stained bone marrow smear of patient no. 3. Less than 3% of leukemic cells are positive for MPO staining (original magnification × 1,200).
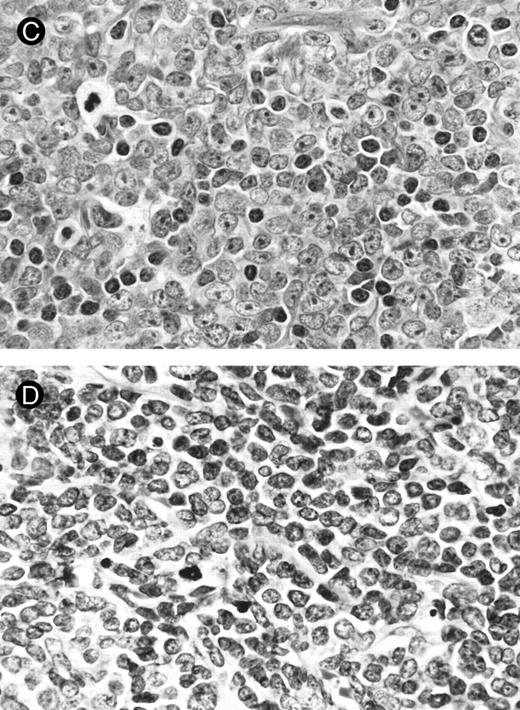
Lymph node biopsies of patients no. 1 (A and B), no. 3 (C), and no. 4 (D). (A) Diffuse infiltration of tumor cells is noted in a T-zone area with sparing of follicles (original magnification × 100). (B and C) The component cells show blastic morphology with round to moderately irregular nuclei, small to intermediate-sized nucleoli, and scant cytoplasm (original magnification × 600). (D) The tumor cells show irregular nuclei with inconspicuous nucleoli.
Lymph node biopsies of patients no. 1 (A and B), no. 3 (C), and no. 4 (D). (A) Diffuse infiltration of tumor cells is noted in a T-zone area with sparing of follicles (original magnification × 100). (B and C) The component cells show blastic morphology with round to moderately irregular nuclei, small to intermediate-sized nucleoli, and scant cytoplasm (original magnification × 600). (D) The tumor cells show irregular nuclei with inconspicuous nucleoli.
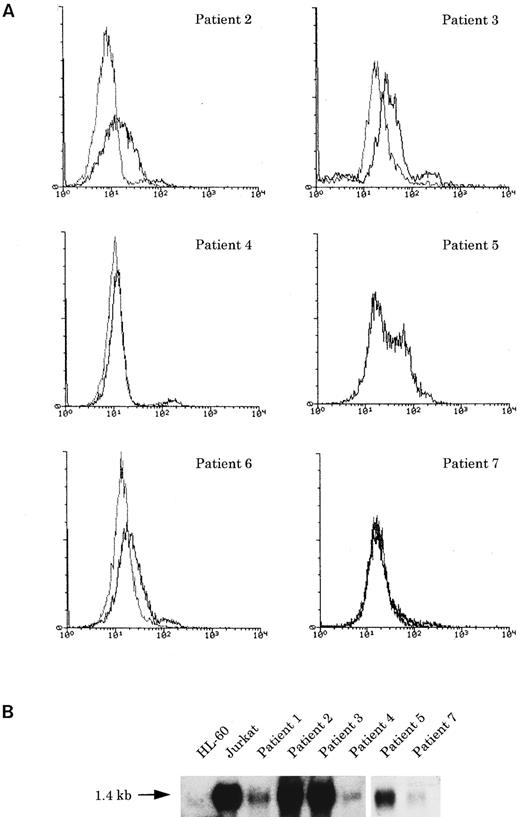
Immunophenotyping.The immunophenotypes of all seven cases are listed in Table 2. All cases expressed the NK cell-associated CD56 antigen, but neither CD16 nor CD57. All cases also expressed CD7, CD33, and CD34, together with the other myeloid/monocyte-associated antigens, CD11b or CD13, to varying degrees. HLA-DR–related (Ia) antigen was positive in five (nos. 1 through 4 and 7) of the seven cases. One case (no. 3) was CD2+, and three (nos. 2, 3, and 6) of six cases examined exhibited faint cytoplasmic CD3 (cyCD3; Fig 3), in contrast to high expression of cyCD33 or cyMPO, although all were negative for other B- or T-cell antigens. TdT was negative in all six cases examined.
Phenotypic Analysis of Myeloid/NK Cell Precursor Acute Leukemia Cases
| . | Case No. . | ||||||
|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5* . | 6 . | 7 . |
| . | LN . | PB . | BM . | BM . | BM . | BM . | BM . |
| CD1 | 10 | 1 | 1 | 0 | 0 | 0 | 1 |
| CD2 | 17 | 7 | 28 | 6 | 6 | 9 | 7 |
| CD3 | 13 | 5 | 4 | 5 | 5 | 4 | 5 |
| CD4 | 12 | 5 | 2 | 3 | 2 | 3 | 19 |
| CD5 | 14 | 6 | 13 | 9 | 19 | 6 | 23 |
| CD6 | 44 | 12 | 8 | 60 | 6 | 24 | 29 |
| CD7 | 89 | 94 | 96 | 89 | 94 | 84 | 64 |
| CD8 | 5 | 7 | 4 | 2 | 3 | 4 | 4 |
| CD9 | 90 | 87 | 21 | 42 | 26 | 10 | 37 |
| CD10 | 1 | 1 | 1 | 0 | 0 | 4 | 3 |
| CD11a | ND | 34 | 98 | 91 | 55 | 78 | 74 |
| CD11b | 72 | 82 | 56 | 24 | 88 | 74 | 85 |
| CD11c | ND | 26 | 40 | 10 | 4 | 27 | 37 |
| CD13 | 19 | 4 | 5 | 65 | 0 | 13 | 21 |
| CD14 | 5 | 3 | 1 | 0 | 0 | 3 | 17 |
| CD15 | 10 | 5 | 2 | 5 | 1 | 10 | 22 |
| CD16 | 1 | 5 | 0 | 1 | 1 | 2 | 8 |
| CD19 | 2 | 11 | 3 | 1 | 0 | 8 | 9 |
| CD20 | 2 | 3 | 3 | 2 | 0 | 6 | 2 |
| CD21 | 1 | 2 | 2 | 1 | 1 | 1 | 2 |
| CD25 | 4 | 7 | 1 | 1 | 0 | 2 | 26 |
| CD28 | 8 | 3 | 25 | 4 | 3 | 4 | 3 |
| CD33 | 80 | 49 | 76 | 43 | 74 | 63 | 92 |
| CD34 | 74 | 67 | 30 | 60 | 78 | 50 | 43 |
| CD36 | 3 | 4 | 9 | 3 | 4 | 6 | 17 |
| CD38 | 42 | 81 | 96 | 81 | 93 | 88 | 94 |
| CD41b | 4 | 3 | 3 | 0 | 1 | 3 | 1 |
| CD56 | 78 | 86 | 77 | 77 | 46 | 41 | 66 |
| CD57 | 1 | 3 | 3 | 2 | 3 | 1 | 2 |
| CD71 | 64 | 88 | 66 | 89 | 64 | 36 | 59 |
| CD122 | ND | 2 | 2 | 1 | 1 | 3 | 28 |
| HLA-DR | 86 | 86 | 61 | 92 | 9 | 19 | 66 |
| TCRαβ | 14 | 4 | 2 | 4 | 4 | 3 | 5 |
| TCRγδ | ND | 1 | 2 | 0 | 1 | ND | 1 |
| cyCD3 | ND | 18† | 12† | 5 | 4 | 15† | 6 |
| cyCD22 | ND | 3 | 1 | 1 | ND | 3 | 0 |
| cyCD33 | ND | 12 | 37 | 41 | 22 | 70 | 57 |
| cyIgM | ND | ND | 10 | 2 | ND | ND | 4 |
| cyMPO | ND | 35 | 65 | 28 | 19 | 1 | 82 |
| TdT | ND | 2 | 1 | 2 | 12 | 4 | 5 |
| . | Case No. . | ||||||
|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5* . | 6 . | 7 . |
| . | LN . | PB . | BM . | BM . | BM . | BM . | BM . |
| CD1 | 10 | 1 | 1 | 0 | 0 | 0 | 1 |
| CD2 | 17 | 7 | 28 | 6 | 6 | 9 | 7 |
| CD3 | 13 | 5 | 4 | 5 | 5 | 4 | 5 |
| CD4 | 12 | 5 | 2 | 3 | 2 | 3 | 19 |
| CD5 | 14 | 6 | 13 | 9 | 19 | 6 | 23 |
| CD6 | 44 | 12 | 8 | 60 | 6 | 24 | 29 |
| CD7 | 89 | 94 | 96 | 89 | 94 | 84 | 64 |
| CD8 | 5 | 7 | 4 | 2 | 3 | 4 | 4 |
| CD9 | 90 | 87 | 21 | 42 | 26 | 10 | 37 |
| CD10 | 1 | 1 | 1 | 0 | 0 | 4 | 3 |
| CD11a | ND | 34 | 98 | 91 | 55 | 78 | 74 |
| CD11b | 72 | 82 | 56 | 24 | 88 | 74 | 85 |
| CD11c | ND | 26 | 40 | 10 | 4 | 27 | 37 |
| CD13 | 19 | 4 | 5 | 65 | 0 | 13 | 21 |
| CD14 | 5 | 3 | 1 | 0 | 0 | 3 | 17 |
| CD15 | 10 | 5 | 2 | 5 | 1 | 10 | 22 |
| CD16 | 1 | 5 | 0 | 1 | 1 | 2 | 8 |
| CD19 | 2 | 11 | 3 | 1 | 0 | 8 | 9 |
| CD20 | 2 | 3 | 3 | 2 | 0 | 6 | 2 |
| CD21 | 1 | 2 | 2 | 1 | 1 | 1 | 2 |
| CD25 | 4 | 7 | 1 | 1 | 0 | 2 | 26 |
| CD28 | 8 | 3 | 25 | 4 | 3 | 4 | 3 |
| CD33 | 80 | 49 | 76 | 43 | 74 | 63 | 92 |
| CD34 | 74 | 67 | 30 | 60 | 78 | 50 | 43 |
| CD36 | 3 | 4 | 9 | 3 | 4 | 6 | 17 |
| CD38 | 42 | 81 | 96 | 81 | 93 | 88 | 94 |
| CD41b | 4 | 3 | 3 | 0 | 1 | 3 | 1 |
| CD56 | 78 | 86 | 77 | 77 | 46 | 41 | 66 |
| CD57 | 1 | 3 | 3 | 2 | 3 | 1 | 2 |
| CD71 | 64 | 88 | 66 | 89 | 64 | 36 | 59 |
| CD122 | ND | 2 | 2 | 1 | 1 | 3 | 28 |
| HLA-DR | 86 | 86 | 61 | 92 | 9 | 19 | 66 |
| TCRαβ | 14 | 4 | 2 | 4 | 4 | 3 | 5 |
| TCRγδ | ND | 1 | 2 | 0 | 1 | ND | 1 |
| cyCD3 | ND | 18† | 12† | 5 | 4 | 15† | 6 |
| cyCD22 | ND | 3 | 1 | 1 | ND | 3 | 0 |
| cyCD33 | ND | 12 | 37 | 41 | 22 | 70 | 57 |
| cyIgM | ND | ND | 10 | 2 | ND | ND | 4 |
| cyMPO | ND | 35 | 65 | 28 | 19 | 1 | 82 |
| TdT | ND | 2 | 1 | 2 | 12 | 4 | 5 |
Values are expressed as percentages of cells positive for each marker. Leukemic cells considered to be positive for each marker are underlined.
Abbreviation: ND, not determined.
Patient no. 5 was immunophenotyped at the time of relapse.
Dimly positive, as described in Fig 3.
(A) Flow cytometric analysis of cyCD3 in myeloid/NK cell precursor leukemia cases. The x-axis represents fluorescence intensity (4-decade log scale) and the y-axis the relative cell number. The thick line indicates the histogram for leukemic cells stained with cyCD3 and the thin line indicates the histogram for leukemic cells stained with cyCD22 (negative control). The right shift of the histogram wave observed in patients nos. 2, 3, and 6 indicates that the leukemic cells are cyCD3 dimly positive. In patient no. 2, the fluorescence intensity of 18% of the leukemic cells was calculated to exceed the cut-off level (maximum intensity of negative control), but most (approximately more than 80% to 90%) of them are dimly positive. In patient no. 5, the cyCD22 analysis was not available. (B) CD3ε expression by Northern blotting analysis. HL-60 was used as a negative control and Jurkat was used as a positive control. All six cases expressed CD3ε mRNA to varying extents.
(A) Flow cytometric analysis of cyCD3 in myeloid/NK cell precursor leukemia cases. The x-axis represents fluorescence intensity (4-decade log scale) and the y-axis the relative cell number. The thick line indicates the histogram for leukemic cells stained with cyCD3 and the thin line indicates the histogram for leukemic cells stained with cyCD22 (negative control). The right shift of the histogram wave observed in patients nos. 2, 3, and 6 indicates that the leukemic cells are cyCD3 dimly positive. In patient no. 2, the fluorescence intensity of 18% of the leukemic cells was calculated to exceed the cut-off level (maximum intensity of negative control), but most (approximately more than 80% to 90%) of them are dimly positive. In patient no. 5, the cyCD22 analysis was not available. (B) CD3ε expression by Northern blotting analysis. HL-60 was used as a negative control and Jurkat was used as a positive control. All six cases expressed CD3ε mRNA to varying extents.
Genotypic analysis.The results are listed in Table 3. In all but one case (no. 4), no clonal rearrangement of the TCR β and γ chain genes or the IgH gene was detected with all three restriction enzymes used. Case no. 4 exhibited clonal rearrangement of all these genes.
Genotypic Features of Myeloid/NK Cell Precursor Acute Leukemia Cases
| . | Case No. . | ||||||
|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
| Southern blot analysis | |||||||
| IgH | G/G | G/G | G/G | R/G | G/G | G/G | G/G |
| TCR β | G/G | G/G | G/G | R/G | G/G | G/G | G/G |
| TCR γ | G/G | G/G | G/G | R/R | G/G | G/G | G/G Northern blot analysis |
| MPO | + | − | − | + | + | ND | + |
| CD3δ | − | − | + | + | ND | ND | ND |
| CD3ε3-150 | + | + | + | + | + | ND | + |
| . | Case No. . | ||||||
|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
| Southern blot analysis | |||||||
| IgH | G/G | G/G | G/G | R/G | G/G | G/G | G/G |
| TCR β | G/G | G/G | G/G | R/G | G/G | G/G | G/G |
| TCR γ | G/G | G/G | G/G | R/R | G/G | G/G | G/G Northern blot analysis |
| MPO | + | − | − | + | + | ND | + |
| CD3δ | − | − | + | + | ND | ND | ND |
| CD3ε3-150 | + | + | + | + | + | ND | + |
Abbreviations: IgH, Ig heavy chain; G, germline; R, rearranged; ND, not determined.
The result of Northern blot analysis is shown in Fig 3B.
Of six cases examined by Northern blot analysis, four (nos. 1, 4, 5, and 7) expressed the MPO gene, two (nos. 3 and 4) the CD3δ chain gene, and six (nos. 1 through 5 and 7) the CD3ε chain gene.
Cytogenetic analysis.The results are listed in Table 4. One case (no. 3) presented a normal karyotype, 46,XY, but the other six cases had various chromosomal abnormalities. Although these varied from case to case, four showed chromosome 7 translocation. No t(9; 22) or 11q23 abnormalities were detected.
Cytogenetic Characteristics of Myeloid/NK Cell Precursor Acute Leukemia Cases
| Case No. . | Karyotype . |
|---|---|
| 1 | 45,XY,+del(3)(p21),−4,t(7;?)(p21;?),−10,−20,+mar |
| 2 | 45,XY,−7,t(7; 11)(q11;p11) |
| 3 | 46,XY |
| 4 | 46,XX,t(1; 3)(p36;p21),−16,−17,−22,+3mar |
| 5 | 46,XY,add(1)(q44),t(7; 14)(p15;q32),del(9)(p13) |
| 6 | 46,XY,del(6)(q23),−12,t(12; 17)(q11;p13) |
| 7 | 46,XY,t(3; 7)(p25;p15) |
| Case No. . | Karyotype . |
|---|---|
| 1 | 45,XY,+del(3)(p21),−4,t(7;?)(p21;?),−10,−20,+mar |
| 2 | 45,XY,−7,t(7; 11)(q11;p11) |
| 3 | 46,XY |
| 4 | 46,XX,t(1; 3)(p36;p21),−16,−17,−22,+3mar |
| 5 | 46,XY,add(1)(q44),t(7; 14)(p15;q32),del(9)(p13) |
| 6 | 46,XY,del(6)(q23),−12,t(12; 17)(q11;p13) |
| 7 | 46,XY,t(3; 7)(p25;p15) |
EBV study.ISH did not detect EBV transcripts in paraffin sections of any of the six cases examined (nos. 1 through 5 and 7). Southern blot analysis showed all seven cases to be negative for EBV genome in leukemic cells.
Therapy and clinical course.The initial therapies, responses, and clinical course of myeloid/NK cell precursor leukemias are summarized in Table 5. Three patients (nos. 3, 4, and 7) were treated with chemotherapy including daunorubicin and cytosine arabinoside on diagnosis of AML and three with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) -based regimens because of initial diagnosis of LBL/acute lymphoblastic leukemia (ALL). One patient (no. 5) with a sole mediastinal bulky mass underwent thoracic irradiation and subsequent chemotherapy for LBL. All those who received the initial regimens for AML achieved complete remission (CR), although patient no. 4 required three courses of induction chemotherapy. None of the patients treated with CHOP-based chemotherapy achieved CR; one (no. 6) showed a partial response (PR) and two (nos. 1 and 2) proved refractory. Two (nos. 1 and 6) of them attained CR with subsequent AML chemotherapy. Patient no. 5 also achieved CR after irradiation. Patient no. 4 underwent allogeneic bone marrow transplantation (BMT) from a human leukocyte antigen (HLA) genotypically identical sibling, but died of chronic graft-versus-host disease 12 months after BMT without evidence of relapse. Patient no. 6 received allogeneic BMT from the HLA phenotypically identical father, but relapsed 9 months after BMT. Four patients treated with consolidation and maintenance chemotherapy relapsed 3, 6, 23, and 27 months after remission, respectively. All but one patient (no. 4) had died of leukemia and/or related complications within 41 months. The median survival was 19 months.
Therapeutic Responses and Clinical Courses of Myeloid/NK Cell Precursor Acute Leukemia Cases
| Case No. . | Age (yr)/Sex . | Initial Therapy . | Response . | Subsequent Therapy and Clinical Course . | Survival (mo) . |
|---|---|---|---|---|---|
| 1 | 34/M | CHOP-B | PD | ||
| Received DCVP and attained CR. Relapsed in BM 6 mo after CR, and DOD. | 17 | ||||
| 2 | 46/M | Radiation, CHOP-B | NR | Received DCVP but no response. DOD. | 4 |
| 3 | 54/M | DCMP | CR | Relapsed in BM 27 mo after CR, no response to DCMP. DOD. | 30 |
| 4 | 29/F | DCMP | CR | Received allo BMT from HLA identical sibling, and died of GVHD in CR. | 19 |
| 5 | 48/M | Radiation | CR | Received chemotherapy for LBL. Relapsed in BM and testis 23 mo after CR. Attained short 2nd CR by Ara-C + ACR. DOD. | 41 |
| 6 | 19/M | CHOP-L | PR | Received DCVP and attained CR. Received allo BMT from phenotypically identical father in 1st CR. Relapsed in BM at 13 mo after CR. DOD. | 19 |
| 7 | 59/M | LDCA, DCVP | CR | Relapsed in CNS 6 mo after CR, with subsequent BM relapse. No response to high-dose Ara-C and DOD. | 11 |
| Case No. . | Age (yr)/Sex . | Initial Therapy . | Response . | Subsequent Therapy and Clinical Course . | Survival (mo) . |
|---|---|---|---|---|---|
| 1 | 34/M | CHOP-B | PD | ||
| Received DCVP and attained CR. Relapsed in BM 6 mo after CR, and DOD. | 17 | ||||
| 2 | 46/M | Radiation, CHOP-B | NR | Received DCVP but no response. DOD. | 4 |
| 3 | 54/M | DCMP | CR | Relapsed in BM 27 mo after CR, no response to DCMP. DOD. | 30 |
| 4 | 29/F | DCMP | CR | Received allo BMT from HLA identical sibling, and died of GVHD in CR. | 19 |
| 5 | 48/M | Radiation | CR | Received chemotherapy for LBL. Relapsed in BM and testis 23 mo after CR. Attained short 2nd CR by Ara-C + ACR. DOD. | 41 |
| 6 | 19/M | CHOP-L | PR | Received DCVP and attained CR. Received allo BMT from phenotypically identical father in 1st CR. Relapsed in BM at 13 mo after CR. DOD. | 19 |
| 7 | 59/M | LDCA, DCVP | CR | Relapsed in CNS 6 mo after CR, with subsequent BM relapse. No response to high-dose Ara-C and DOD. | 11 |
Abbreviations: CHOP-B, cyclophosphamide, doxorubicin, vincristine, prednisolone, and bleomycin; DCMP, daunorubicin, cytosine arabinoside, 6-mercaptopurine, and prednisolone; CHOP-L, CHOP and L-asparaginase; LDCA, low-dose cytosine arbinoside; DCVP, daunorubicin, cytosine arabinoside, vindesine, and prednisolone; CR, complete remission; PR, partial response; NR, no response; PD, progressive disease; DOD, died of disease; allo BMT, allogeneic bone marrow transplantation; HLA, human leukocyte antigen; GVHD, graft-versus-host disease; LBL, lymphoblastic lymphoma; ACR, aclarubicin.
DISCUSSION
In the present study, we have described seven cases of acute leukemia characterized by distinct morphologic, immunophenotypic, genotypic, and clinical features. Leukemic cells showed immature morphologic appearance without MPO reactivity and coexpression of CD7, CD33, CD34, CD56, and frequently HLA-DR. Although one case expressed CD2, the present cases exclusively lacked the other T- or B-cell–specific antigens and, of note, the genotypical germline configurations of TCR β and γ chain genes and IgH gene except for only one case. Although case no. 4 showed gene rearrangements in the TCR β, TCR γ, and IgH genes, this does not necessarily mean commitment to T- or B-cell lineages, as shown previously for AML cases.18 19 These data suggested that the leukemic cells of the present series might be phenotypically those of myeloid/NK cell precursors. One of the clinical characteristics of this myeloid/NK cell precursor acute leukemia was the presence of extramedullary involvement, superficial lymphadenopathy, and/or mediastinal mass. Two cases lacked the bone marrow involvement at diagnosis, which provided some difficulties in differential diagnosis from LBL. Etiologically, EBV was not detected in myeloid/NK cell precursor acute leukemia using Southern blot analysis and ISH. These findings of the present cases showed that they appeared to constitute a distinct clinicopathologic entity of myeloid/NK cell precursor acute leukemia, hitherto undescribed in the literature.
NK cells differentiate from immature thymocytes under appropriate conditions in vitro and in vivo and share cytotoxic activity and some surface antigens with T cells, all of which suggest a thymocyte origin.20-24 On the other hand, they arise in athymic mice25 and are present in severe combined immunodeficient (SCID) mice26 and human SCID patients.27 Recently, NK cells were proven to develop from a population of CD34+, CD33+, CD56− cells in vitro, which indicates a close relationship with the myeloid lineage.24,28 NK cell progenitors are now considered to have a CD34+, CD33+, CD7+, CD2+/−, CD56−, cyCD3+ phenotype, whereas mature NK cells exhibit CD34−, CD33−, CD7+, CD2+/−, CD56+. Our series of seven leukemias (CD34+, CD33+, CD7+, CD2−/+, CD56+, cyCD3dim), therefore showed a phenotype midway between these two. NK cells contain truncated CD3ε mRNA, which is expressed as cyCD3, causing some confusion with the surface CD3 (sCD3) that distinguishes NK cells from NK-like T cells.29,30 In the present series, although sCD3 was negative in all cases, the fact that cyCD3 was dimly detected in three cases by flow cytometry and in six cases by Northern blotting showed their NK cell lineage, although the clinical behavior and therapeutic response were rather more indicative of immature myeloid leukemia than lymphoblastic leukemia/lymphoma. In our study, all cases were CD16−, using Leu11 MoAb. However, because some cases of chronic LGL leukemia have been reported to be negative with Leu11 but positive with other anti-CD16 MoAbs,31 we cannot be certain from the present study whether these myeloid/NK cell precursor acute leukemias expressed IgG Fc receptors.
Recently, Scott et al14 also proposed a myeloid/NK cell acute leukemia entity characterized by mature myeloid morphology, scant cytoplasm with fine azurophilic granules, and MPO cytochemical reactivity. Their cases were described to be exclusively negative for HLA-DR, and less commonly for CD34, indicating a more mature state than found for our series. CD7, which is expressed in immature NK cell progenitors, was a feature of our myeloid/NK cell precursor acute leukemia, not described in the Scott's type. There were also some morphologic differences between the two series, presumably reflecting the different developmental stage of leukemic cells. Although the therapeutic schedules were not uniform, the prognosis differed considerably, with their myeloid/NK cell acute leukemias, mainly treated according to SWOG protocol, showing a relatively fair prognosis (9 of 20 alive after a median of 30 months). In our series of seven myeloid/NK cell precursor acute leukemias, although six attained CR with chemotherapy including daunorubicin and cytosine arabinoside, the remission duration was relatively short with a fatal outcome, despite multiagent intensive chemotherapy including BMT.
Leukemia of NK cell origin has historically been considered to fall within the large granular lymphocyte (LGL) leukemia category, now divided into two main groups, T-LGL leukemia and NK-LGL leukemia.32 The first presents an indolent and chronic clinical course, an sCD3+ phenotype, and rearrangement of the TCR gene, suggesting a T-cell origin.33-35 The seven cases of myeloid/NK cell precursor acute leukemias in the present study were found to be quite different from T-LGL leukemias in their clinical, morphologic, phenotypical, and genotypic characteristics. An aggressive variant of CD3+ CD56+ T-LGL leukemia,36 which is now considered to be the leukemic form of NK-like T-cell lymphoma,37,38 has also been reported, with the TCR gene analysis confirming its T-cell origin. NK-LGL leukemia exhibits an aggressive clinical course, a lack of sCD3 but a cyCD3ε+ phenotype, and a germline configuration of TCR genes, indicative of a true NK cell origin.39-44 Although the expression of myeloid and stem cell-associated antigens has not been described for most of these LGL malignancies, CD56+ aggressive NK cell leukemias reported by Imamura et al43 were CD13− and CD33−. Patients with aggressive NK cell leukemia have a tendency to present B symptoms and marked splenomegaly, although lymphadenopathy is not common (3 of 11 cases [27%]).32 Some investigators have also emphasized the EBV integration seen in NK-LGL leukemia.42 44 The morphologic, phenotypical, clinical, and virologic differences between myeloid/NK cell precursor acute leukemia and NK-LGL leukemia thus suggest that they were distinct disease entities, despite sharing NK cell characteristics and an aggressive clinical course.
Malignant lymphomas of conceivable NK cell phenotype were first described as angiocentric immunoproliferative lesions45 and are known to predominantly show an extranodal presentation with a broad morphologic spectrum.5-7,11,46-50 Such CD56+ lymphomas are prevalent in Asian populations, and an increasing number of reports have described nasal and nasal-type T/NK cell lymphomas, with pleomorphic/polymorphous morphology, a frequent mucosal presentation (most commonly nasopharyngeal region), and EBV positivity.51 The tumor cells of both nasal and nasal-type lymphomas also often show an LGL appearance and a CD2+, sCD3−, cyCD3+, CD5−, CD7+, CD56+, and myeloid marker negative phenotype, suggesting a mature NK cell nature, distinct from the present myeloid/NK cell precursor acute leukemia. Recently, we also described a distinct entity designated the blastic NK cell lymphoma, characterized by a lymphoblastoid appearance often with azurophilic granules; a proclivity to involve the skin, lymph nodes, and bone marrow; and EBV negativity.8 The lymphoma cells of this entity were usually CD2−, sCD3−, cyCD3+/−, CD5−, CD7+/−, CD33−, CD34−, CD56+, and TdT+/− (data unpublished). Some of these features were shared by the present myeloid/NK cell precursor acute leukemias, and only one exceptional case of CD33+ and CD34+ phenotype in our previous reports giving this case no. 4 was recategorized into myeloid/NK cell precursor acute leukemia as case no. 2 in the present study. Although these two entities might lie on a continuous spectrum with overlapping and borderline cases, the clinicopathologic profile of the blastic NK cell lymphoma appeared to correspond to an intermediate position between myeloid/NK cell precursor acute leukemia and nasal and nasal-type T/NK cell lymphoma. Other types of malignant lymphoma, such as NK-like T-cell lymphoma,11,37 hepatosplenic γδ T-cell lymphoma52,53 and S-100+ T-cell lymphoproliferative disorder54 have also been described to be CD56+. NK-like T-cell lymphoma, which is considered to be lymphomatous form of aggressive CD3+, CD56+ LGL leukemia,36 shows CD3 and TCR expression, rearrangement of TCR genes, and an LGL-like morphology. The hepatosplenic γδ T-cell lymphoma and S-100+ T-cell lymphoproliferative disorder are characteristically also positive for CD3 and TCR and for TCR gene rearrangement. With this background, these CD56+ lymphomas are suggested to have T-cell nature and thus to be distinct from myeloid/NK cell precursor acute leukemias.
Because the biopsied specimens of myeloid/NK cell precursor acute leukemias were characterized by diffuse proliferation of lymphoblasts and negative esterase staining, with phenotypic and genotypic studies confirming their non–T- and non–B-cell lineage. These finding pose a differential diagnostic problem between myeloid/NK cell precursor acute leukemia and LBLs unless flowcytometric analysis is performed. However, LBLs virtually all express TdT, and the majority occur in children or young adults. Although there are some reports of LBL cases with NK cell marker expression,55,56 these were positive for CD57 (Leu7) and T-cell markers (CD2, CD5, or CD8), but no information was available for the expression of CD56 or myeloid antigens. T-ALL with the same phenotype have also been reported.57,58 CD57+ T-LBL/ALL may thus constitute a definite disease entity, but again this is different from the myeloid/NK cell precursor acute leukemia. Very recently, Ichinohasama et al59 reported a case of LBL with note of the CD56, myeloid antigen, CD7 and cyCD3 expression, and germline configurations of TCR and Ig genes. The mediastinal mass without bone marrow involvement found at the initial diagnosis and the leukemic conversion at relapse were similar to the clinical course of our patient no. 5. An LBL diagnosis was made on the basis of histopathologic features of biopsied specimens, but it could be included in a single nosological entity of myeloid/NK cell precursor acute leukemia.
The particular morphologic, phenotypic, genotypic, and clinical characteristics of the present cases included features of AML M060 or minimally differentiated AML.61 The AML M0 is characterized by an immature lymphoblastoid morphology without cytochemical MPO reactivity, expression of myeloid antigens, lack of T- or B-cell antigens, a complex karyotype, and a poor prognosis.60-65 CD7 was found to be expressed in 31 of 73 (42%) cases,62,65 66 whereas CD56 expression has not been described in the literature. By definition, M0 is cyCD3−, whereas some of our myeloid/NK cell precursor acute leukemias were dimly positive. The possibility that some M0 cases might be classifiable as myeloid/NK cell precursor acute leukemias on the basis of CD56 expression needs further investigation.
CD7+, CD4−, CD8− acute leukemias67 or CD7+ stem cell leukemias68-70 also share some clinical, morphologic, and phenotypic features with myeloid/NK cell precursor acute leukemias. Kurtzberg et al67 described acute leukemia cases with frequent extramedullary involvement, MPO and TdT nonreactivities, CD7 expression, and a poor prognosis. Although they did not mention expression of myeloid antigens, other investigators have identified myeloid antigen-positive cases.68-70 Because CD56 expression was not investigated, the possibility of overlap between CD7+ stem cell leukemia and myeloid/NK cell precursor acute leukemia remains open.
Because the expression of CD7 and cyCD3 observed in our series of leukemias has historically been considered as a characteristic of T cells, some cases of the literarily described mixed lineage leukemia of myeloid and T-cell might be reclassifiable into the myeloid/NK cell precursor acute leukemia category. However, mixed-lineage leukemias sometimes present Philadelphia chromosome or 11q23 abnormalities,71-76 whereas none of our cases had such aberrations. In our seven cases, four cases showed abnormality of chromosome 7 (3 for 7p and 1 for 7q) and three showed abnormality of chromosome 3p. Although the T-cell receptor γ chain gene is located in 7p15 locus, none of the present cases carrying 7p abnormality exhibited the rearrangement of TCR γ gene. Further investigation is required for the understanding the mechanism of neoplastic transformation of myeloid/NK cell precursor acute leukemia.
These seven myeloid/NK cell precursor acute leukemia cases exhibited extramedullary presentation, including two of them with mediastinal mass, and some of them lacked bone marrow involvement at initial presentation. Because the CD56 antigen is a cell adhesion molecule, its expression on tumor cells is believed to play an important role in their localization and CD56+ lymphomas5,8,77 or leukemias12,78 with unusual sites of involvement have been reported. In a recent review of extramedullary myeloid cell tumors, CD56 was described as a possible risk factor in extramedullary leukemia.79 On the other hand, studies of large populations for AML showed no correlation between CD56 expression and an extramedullary localization,13,80 81 suggesting that cell adhesion molecules other than CD56 might determine the sites of involvement. Further exploration of this area is needed to interpret the contradictory results.
In this series, three cases proved to be refractory to CHOP-based chemotherapeutic regimens for lymphoid malignancies; in contrast, five of six cases were successfully treated with regimens for AML including daunorubicin and cytosine arabinoside. These data also support the clinicopathologic concept of a myeloid/NK cell precursor acute leukemia. Although the number of cases was limited, the fact that 5 of the 6 remission cases eventually relapsed within 3 years after remission, despite intensive chemotherapy including allogeneic BMT, suggests the need for more intensive therapeutic approaches.
ACKNOWLEDGMENT
The authors are grateful to K. Koike, Y. Okada, M. Andoh, and T. Kobayashi for technical assistance.
Supported in part by a Grant-in-aid for the 2nd-Term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health and Welfare; a Grant-in-aid for Science on Primary Areas (Cancer Research) from the Ministry of Education, Science and Culture, Japan; and Bristol-Myers Squibb Unrestricted Biomedical Research Grants Program.
Address reprint requests to Ritsuro Suzuki, MD, Department of Hematology and Chemotherapy, Aichi Cancer Center Hospital, Kanokoden 1-1, Chikusa-ku, Nagoya, Japan 464.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal