Abstract
To investigate the fate of human megakaryocytes, CD34+ hematopoietic progenitor cells were purified from the peripheral blood or bone marrow of healthy donors and seeded in serum-free chemically defined suspension cultures. In the presence of thrombopoietin (TPO; 100 ng/mL), CD34-derived cells showed an eightfold numerical expansion and a progressive maturation along the megakaryocytic lineage. Megakaryocyte maturation was characterized ultrastructurally by the presence of a demarcation membrane system and phenotypically by a high surface expression of αIIbβ3 integrin. The number of mature megakaryocytes peaked at days 12 to 15 of culture. On the other hand, the number of platelets released in the culture supernatant by CD34-derived megakaryocytes peaked at days 18 to 21, when a high percentage of megakaryocytes showed the characteristic features of apoptosis, as evaluated by electron microscopy, terminal deoxynucleotidyl transferase (TdT)-mediated d-UTP-biotin nick end-labeling technique (TUNEL) and uptake of propidium iodide. In other experiments, primary αIIbβ3+ megakaryocytic cells were directly purified from the bone marrow aspirates of normal donors and seeded in serum-free suspension cultures. In the absence of cytokines, αIIbβ3+ megakaryocytes progressively underwent apoptotic cell death. The addition of TPO but not interleukin-3 or erythropoietin showed some protection of αIIbβ3+ cells from apoptosis at early culture times (days 2 to 4), but it did not show any significant effect at later time points. These findings suggest that the terminal phase of the megakaryocyte life span is characterized by the onset of apoptosis, which can be modulated only to a certain extent by TPO.
HUMAN MEGAKARYOCYTOPOIESIS in adults occurs predominantly in the extravascular compartment of the bone marrow (BM).1-3 Normal megakaryocyte development proceeds from hematopoietic stem cells and requires the generation of granules, the formation of platelet territories, and cytoplasmic fragmentation.4-6 Maturation of megakaryocytes is followed either by the formation of cytoplasmic processes that extend into sinusoids where they undergo fragmentation into platelets or by egress of whole cells into the venous circulation.7 At the conclusion of platelet release, senescent megakaryocytes sometimes consist of a large nucleus enveloped by a thin layer of cytoplasm, and this state has frequently been referred to as denuded megakaryocyte (DMK). Usually, BM biopsies taken from normal individuals contain only a very small number of these DMK. On the other hand, a common feature occurring in BM biopsies of patients with various pathologic conditions affecting the megakaryocytic lineage, such as human immunodeficiency virus-1 disease or chronic myeloproliferative disorders, is an increased occurrence of DMK.8-10
It has also been reported that macrophages are frequently located in the vicinity of degenerating megakaryocytes and extend processes that partially or completely engulf the dying cells.11 Occasionally, large bodies were observed within a macrophage, suggesting that fragmentation of the degenerating cell had taken place.
However, despite the relatively large size of the senescent megakaryocyte, a reflection of its ploidy, its fate has not been studied in depth. This was mainly due to the fact that the low number of megakaryocytes found in normal BM (∼0.05% of total mononuclear cells) has long restricted the study of megakaryocytopoiesis and only allowed the morphologic examination of megakaryocytic cells with different degrees of maturation in BM biopsies.
Only in recent years has progress in the separation procedures of megakaryocytes and the availability of in vitro culture systems for megakaryocyte progenitors offered more insight into megakaryocytopoiesis and its regulation.3,12 A real breakthrough came from the characterization of the thrombopoiesis-stimulating hormone thrombopoietin (TPO),13-17 which is able to drive full megakaryocyte development of hematopoietic progenitors seeded in serum-free liquid or semi-solid cultures.18,19 In fact, it has been shown by several groups of investigators that TPO acts on both the proliferative and maturative steps of megakaryocytopoiesis, stimulating the growth and differentiation of megakaryocyte progenitors as well as the release of functional platelets in vitro by mature megakaryocytes.18-22 The central role of TPO in thrombopoiesis is also provided by c-Mpl-null mice, which present a severe although nonlethal thrombocytopenia.23
The aim of this study was to investigate in vitro the fate of normal senescent megakaryocytes. For this purpose, we used (1) primary CD34+ hematopoietic progenitor cells, induced to differentiate along the megakaryocytic lineage in liquid suspension cultures by the continuous addition of 100 ng/mL TPO, and (2) primary megakaryocytes directly purified from the BM of normal donors.
MATERIALS AND METHODS
Cell purifications.Informed consent to the study was obtained according to the Helsinki declaration of 1975 for human rights from 11 healthy BM and 18 blood donors.
For CD34+ cell selection, mononuclear cells were isolated from BM aspirates or leukapheresis units by Ficoll-Paque (d = 1.077 g/mL; Pharmacia, Uppsala, Sweden), rinsed, and adherence-depleted overnight. After removal of adherent cells, CD34+ cells were isolated using a magnetic cell sorting program Mini-MACS (Miltenyi Biotec, Auburn, CA) and the CD34 isolation kit in accordance with the manufacturer's recommendations. The purity of CD34-selected cells was determined for each isolation by FACScan (Becton Dickinson, San Jose, CA) using a monoclonal antibody (MoAb) that recognizes a separate epitope of the CD34 molecule (HPCA-2; Becton Dickinson) directly conjugated to fluorescein. CD34+ cells averaged about 85% to 98%.
αIIbβ3+ megakaryocytic cells were purified as previously described.9 Briefly, BM samples were diluted 1:3 immediately after aspiration with a megakaryocyte collection buffer (calcium and magnesium-free Hank's balanced salt solution containing 10−3 mol/L adenosine, 2 × 10−3 mol/L theophylline, 3.8% sodium citrate, 3.5% bovine serum albumin, 5 × 10−5 mol/L lidocaine and 2 × 10−7 mol/L prostaglandin E1α). BM samples were then centrifuged twice at 200g for 10 minutes to eliminate platelet contamination. Cells were next diluted with 10 mL of megakaryocyte collection medium and supplemented with a 1:100 dilution of an anti-CD41a MoAb (Serotec, Oxford, UK) directed against the glycoproteic αIIbβ3 complex.24 After 1 hour in ice, cells were washed twice and 5 × 106 immunomagnetic beads, coated with goat antimouse IgG (MPC 450 Dynabeads; Dynal, Oslo, Norway), were added to the tube in a final volume of 20 mL of megakaryocyte collection medium for 30 minutes in ice under continuous agitation. αIIbβ3+ cells were then positively selected by a magnet (MPC1; Dynabeads). The purity of megakaryocytic cells was determined for each isolation by FACScan using an MoAb to CD41b (SZ22, mouse IgG1), which reacts against the αIIb subunit independently of the presence of β3 (Immunotech Inc, Westbrook, ME), followed by a goat antimouse IgG serum directly conjugated with fluorescein (GAM-Fl; Becton Dickinson). To exclude the contamination of monocytes with attached platelets, αIIbβ3+ cells were also routinely analyzed for the expression of CD14 antigen by using an anti-CD14 MoAb directly conjugated to fluorescein (Becton Dickinson).
Liquid suspension cultures and cell counts.To avoid the interference of serum, purified CD34+ cells (50,000/mL) were resuspended in a chemically defined serum-free medium: Iscove's modified Dulbecco's medium (GIBCO, Grand Island, NY) containing 10−4 mol/L bovine serum albumin (BSA)-adsorbed cholesterol, nucleosides (uridine, adenosine, deoxyadenosine, guanosine, deoxyguanosine, cytidine, deoxycytidine, and deoxythymidine at 10 μg/mL each), 0.5% BSA (fraction V Chon), 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, and 5 × 10−5 mol/L 2-β-mercaptoethanol (all purchased from Sigma, St Louis, MO) in the presence of 100 ng/mL of human recombinant TPO (Genzyme, Boston, MA). Every 3 days, viable cells were scored by Trypan blue dye exclusion and cultures were amplified with fresh serum-free medium, readjusting the cell density to 50,000 cells/mL. Each well was then supplemented with 100 ng/mL TPO.
In other experiments, 2 × 105 primary megakaryocytes were seeded into 48-multiwell culture plates (Costar Corp, Cambridge, MA) in 1 mL of the chemically defined serum-free medium described above in the absence or presence of predetermined optimal concentrations of the following cytokines: TPO (100 ng/mL), human recombinant interleukin-3 (IL-3; 10 ng/mL; Genzyme), and human recombinant erythropoietin (EPO; 10 ng/mL; Cilag, Milan, Italy).
Evaluation of nuclear ploidy and phenotype.To evaluate nuclear ploidy, 5 × 104 CD34-derived megakaryocytic cells were fixed in 1 mL cold 70% ethanol at 4°C for 1 hour. The cells were centrifuged, washed in phosphate-buffered saline (PBS), resuspended in 0.4 mL PBS, and treated with 0.5 μg RNAse (Type I-A; Sigma) for 1 hour at 37°C. Forty micrograms per milliliter of propidium iodide (PI; in PBS; Sigma) was next added to each sample and, after gentle mixing, samples were incubated in the dark at 4°C for 10 minutes and then analyzed. The PI fluorescence of individual nuclei was measured by FACScan (Lysis II program; Becton Dickinson) equipped with an Argon ion laser (Coherent Enterprise) tuned at 488 nm wavelength, 50 mW output.
The membrane phenotype of CD34-derived cells was analyzed by FACScan. Staining was performed on 5 × 104 cells in 200 μL of PBS containing 1% BSA, 0.1% azide, and 10 μg of CD41a MoAb directly conjugated to fluorescein (Serotec) at 4°C for 30 minutes. Autofluorescence was assessed using cells treated only with 20% normal mouse serum for 10 minutes to block potentially unoccupied binding sites. Nonspecific fluorescence was assessed by using an isotype-matched control (anti-CD8 directly conjugated to fluorescein; Becton Dickinson) for anti-CD41a.
Apoptotic assays.The presence of apoptosis was examined at different time cultures by various techniques: transmission electron microscopy, terminal deoxynucleotidyl transferase (TdT)-mediated d-UTP-biotin nick end-labeling (TUNEL), and PI uptake.
For the morphologic studies, CD34-derived megakaryocytes were fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer, postfixed with 1% osmium tetroxide, and embedded in araldite according to routine techniques. Semithin sections were stained with 1% toluidine blue and then observed at light microscopy. The thin sections were mounted on nickel grids and examined with a Philips CM10 electron microscope after staining with uranyl acetate and lead citrate.
The TUNEL technique was modified to stain the cells with 1 μg/mL fluorescein isothiocyanate (FITC)-avidin to monitor the DNA fragmentation in situ, as described previously.25 Fluorescent nuclei were scored by means of either flow cytometry (FACScan) or a MCR-1000 confocal microscopy (Bio-Rad Microscience Ltd, Hemel, Hempstead, UK) equipped with a krypton/argon ion laser emitting at 488 nm. The signal was achieved through an Epidetector filter (passing band 522/35 nm), analyzed by CoMOS software, and printed on Ektachrome 64T-Kodak film by a Focus Imagerecorder Plus (Focus Graphics, Inc, Foster City, CA).
PI uptake by nonfixed cells was performed as described previously.26 Briefly, nonpermeabilized cells (1 × 105) were incubated with 40 μg/mL PI for 1 hour at 37°C. The samples were analyzed by FACScan in their staining solution. Cells of higher size were gated on the basis of side scatter (SSC)/forward scatter (FSC) dot plot and apoptotic cells were next detected on a PI hystogram as a PIdim peak.
Platelet collection and counts.In some experiments, platelets released in the culture supernatants by CD34-derived megakaryocytes were separated by gel filtration27 and counted. For this purpose, culture medium was harvested by centrifugation at 700 rpm. Platelets were then separated from culture supernatants by gel filtration on Sepharose 2B (Pharmacia Fine Chemical, Uppsala, Sweden) using an elution gel filtration buffer that contained 137 mmol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2 , 5.6 mmol/L glucose, 1 mg/mL BSA, and 20 mmol/L HEPES, pH 7.4, and counted at light microscopy. Platelet sizing was performed using an electronic cell counter (Sysmex, Chicago, IL) according to Choi et al.28
Statistics.Data are expressed as means ± standard deviation (SD) of separate experiments performed in duplicate. Statistical analysis was performed using the two-tailed Student's t-test for paired data.
RESULTS
Characterization of the terminal differentiation and apoptotic death of cultured CD34-derived megakaryocytes.To explore megakaryocyte maturation, CD34+ hematopoietic progenitor cells were seeded in serum-free liquid cultures in the presence of 100 ng/mL TPO that was added in culture at day 0 and then every 3 days. At various time points, the total number of viable cells was counted by Trypan blue dye exclusion, and the following markers of megakaryocytic differentiation were examined: (1) quantitative surface expression of αIIbβ3 by indirect immunofluorescence and flow cytometry, (2) light microscopy examination after May-Grünwald staining, and (3) DNA content after PI staining by flow cytometry.
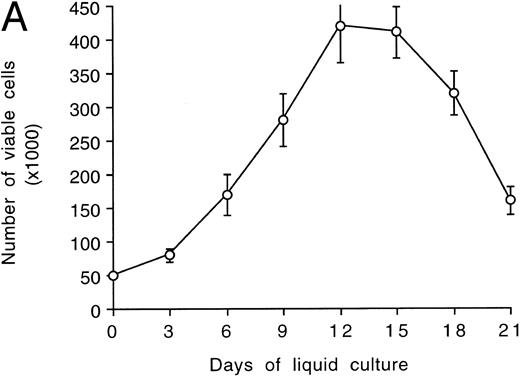
After an initial numerical expansion occurring during the first 12 to 15 days of liquid culture (8-fold increase with respect to day 0), proliferation ceased (Fig 1A) and polyploid megakaryocytes (Fig 1B) became readily detectable at morphologic analysis. The peak of maturation was reached between days 12 and 15 of culture, when more than 50% of the cell population showed a ploidy of 8 to 16 N or greater (Table 1). The remaining 40% to 45% of the cells showing a ploidy of 2 to 4 N was mainly represented by small megakaryoblasts, as judged by high-level expression of αIIbβ3 (Table 2) and by morphologic criteria. By using the same criteria, the fraction of contaminating lymphocytes and monocytes appeared constantly to be less than 5% to 6%.
Evaluation of TPO-induced proliferation and differentiation along the megakaryocytic lineage of CD34+ cells. In (A), viable cells were scored by Trypan blue dye exclusion after various days of liquid cultures supplemented with 100 ng/mL of TPO. Data are expressed as the means ± SD of three separate experiments performed in duplicate. In (B), cytospins were observed at light microscopy after May-Grünwald-Giemsa staining. Original magnification (OM) × 400.
Evaluation of TPO-induced proliferation and differentiation along the megakaryocytic lineage of CD34+ cells. In (A), viable cells were scored by Trypan blue dye exclusion after various days of liquid cultures supplemented with 100 ng/mL of TPO. Data are expressed as the means ± SD of three separate experiments performed in duplicate. In (B), cytospins were observed at light microscopy after May-Grünwald-Giemsa staining. Original magnification (OM) × 400.
Ploidy Distribution of CD34-Derived Megakaryocytic Cells
| . | d 12 . | d 15 . | d 18 . | d 21 . |
|---|---|---|---|---|
| 2N | 22 | 18 | 37 | 54 |
| 4N | 24 | 21 | 33 | 37 |
| 8N | 20 | 18 | 22 | 8 |
| 16N | 18 | 25 | 7 | 1 |
| >16N | 16 | 18 | 1 | 0 |
| . | d 12 . | d 15 . | d 18 . | d 21 . |
|---|---|---|---|---|
| 2N | 22 | 18 | 37 | 54 |
| 4N | 24 | 21 | 33 | 37 |
| 8N | 20 | 18 | 22 | 8 |
| 16N | 18 | 25 | 7 | 1 |
| >16N | 16 | 18 | 1 | 0 |
The DNA content was evaluated by flow cytometry after PI staining at different time points of liquid cultures supplemented with 100 ng/mL of TPO. Data represent the means of three separate experiments, each performed in duplicate starting from a different CD34+ cell sample.
αIIBβ3 Expression in CD34-Derived Megakaryocytic Cells
| Days of Culture . | % of αIIbβ3+ Cells . |
|---|---|
| 0 | 7 |
| 3 | 46 |
| 6 | 65 |
| 9 | 79 |
| 12 | 96 |
| 15 | 94 |
| 18 | 97 |
| 21 | 94 |
| Days of Culture . | % of αIIbβ3+ Cells . |
|---|---|
| 0 | 7 |
| 3 | 46 |
| 6 | 65 |
| 9 | 79 |
| 12 | 96 |
| 15 | 94 |
| 18 | 97 |
| 21 | 94 |
αIIbβ3 expression was evaluated by flow cytometry after staining with an anti-CD41a MoAb at different time points of liquid cultures supplemented with 100 ng/mL of TPO. Data represent the means of three separate experiments, each performed in duplicate starting from a different CD34+ cell sample.
However, from day 15 onwards, a progressive decline of viable cells was observed despite the continuous readdition of high (100 ng/mL) TPO concentrations (Fig 1A). No significant differences were observed in the kinetics of megakaryocyte maturation and cell death when using CD34+ cells purified from peripheral blood or BM.
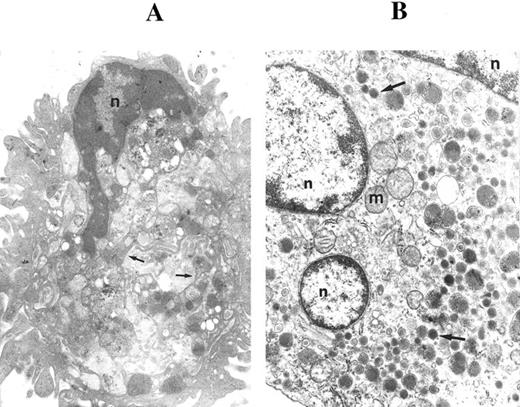
Because the crucial steps in megakaryocytopoiesis are characterized primarily by morphologic criteria,4,19 29 in the next experiments we used transmission electron microscopy to observe granule formation and the development of demarcation membranes. When cells were examined after 12 to 15 days of liquid culture in the presence of TPO, mature megakaryocytes were recognized for the presence of (1) demarcation membranes, which appeared as profiles of parallel membranes arising from invaginations of the plasma membrane (Fig 2A), and (2) dense core granules (Fig 2B). Only a minority of the cells showed apoptotic features at these time points.
Ultrastructural examination of CD34-derived megakaryocytes. In (A), a CD34-derived megakaryocyte illustrates demarcation membranes (arrows). n, nucleus. OM × 5,000. (B) shows a megakaryocyte with three sections of the nucleus (n) and various dense core granules (arrows). m, mitochondria. OM × 10,000.
Ultrastructural examination of CD34-derived megakaryocytes. In (A), a CD34-derived megakaryocyte illustrates demarcation membranes (arrows). n, nucleus. OM × 5,000. (B) shows a megakaryocyte with three sections of the nucleus (n) and various dense core granules (arrows). m, mitochondria. OM × 10,000.
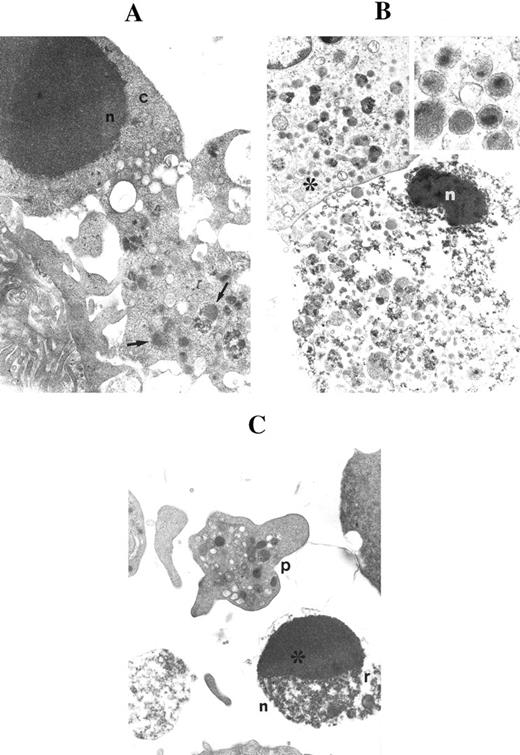
On the other hand, at days 17 to 18, many megakaryocytes had lost most of their cytoplasm and appeared as electron dense apoptotic nuclei surrounded by a layer of granulated cytoplasm (Fig 3A). These ultrastructural features were reminiscent of the DMK nuclei occasionally observed at the histomorphologic evaluation of BM biopsies.8-10 Figure 3A shows an apoptotic cell with dense core granules. In apoptotic megakaryocytes, the cytoplasm became progressively more vacuolated. Two types of granules, one more electron dense than the other, were often found in both normal and apoptotic cells (Fig 3B). In several fields, apoptotic cells were mixed to platelet-size cytoplasmic fragments (Fig 3C).
Ultrastructural examination of CD34-derived apoptotic megakaryocytes. (A) shows an apoptotic nucleus (n) surrounded by a short rim of cytoplasm (c) in continuity with a portion of granulated (arrows) cytoplasm. OM × 8,000. (B) shows a late apoptotic nucleus (n) surrounded by granulated cytoplasm. Also note that the apoptotic nucleus is in close proximity to a normal megakaryocyte (*) showing the typical dense core granules. OM × 6,000. Inset shows a higher magnification of dense core granules (OM × 60,000). (C) shows an apoptotic micronucleus (n) with the characteristic chromatin cap (*) and cytoplasmic remnants (r) in proximity to a mature platelet (p). OM × 8,000.
Ultrastructural examination of CD34-derived apoptotic megakaryocytes. (A) shows an apoptotic nucleus (n) surrounded by a short rim of cytoplasm (c) in continuity with a portion of granulated (arrows) cytoplasm. OM × 8,000. (B) shows a late apoptotic nucleus (n) surrounded by granulated cytoplasm. Also note that the apoptotic nucleus is in close proximity to a normal megakaryocyte (*) showing the typical dense core granules. OM × 6,000. Inset shows a higher magnification of dense core granules (OM × 60,000). (C) shows an apoptotic micronucleus (n) with the characteristic chromatin cap (*) and cytoplasmic remnants (r) in proximity to a mature platelet (p). OM × 8,000.
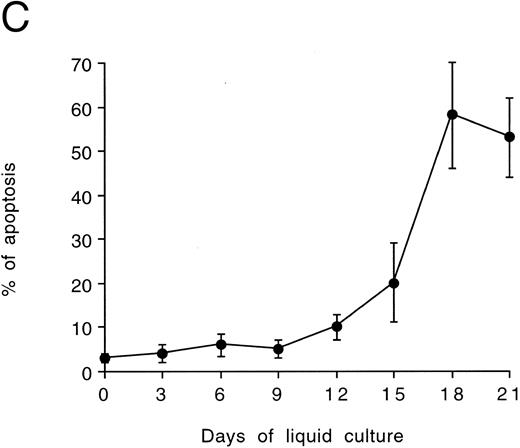
Quantitative evaluation of apoptosis in cultured CD34-derived megakaryocytes.In further experiments, the presence of apoptotic cells was quantitatively evaluated by using the TUNEL technique shown by flow cytometry (Fig 4A through C). Although the percentage of apoptosis showed background levels (<10%) until days 9 to 12, a progressive increase of apoptotic death occurred from day 15 onwards, with more than 50% of cells being in apoptosis at days 18 to 21 (Fig 4C). At these time points, the fraction of polyploid cells was significantly decreased with respect to days 12 or 15 (Table 1).
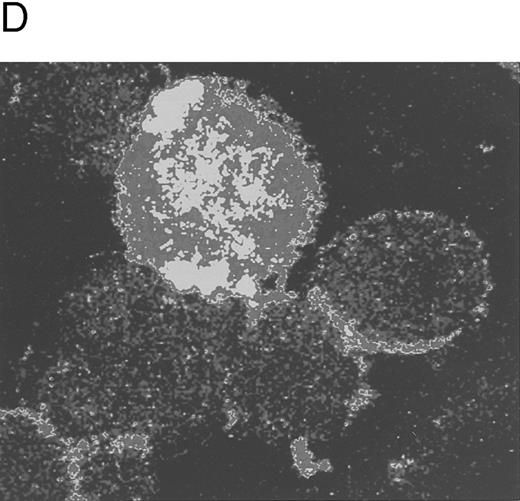
Quantitative evaluation of apoptosis in CD34-derived megakaryocytes by the TUNEL technique. In (A) and (B), the intensity of fluorescence in the whole cell population was shown by flow cytometry at day 18 of liquid culture. (A) represents a negative control: cells treated with avidin-FITC. (B) shows a typical double profile in which the left area represents viable cells overlapping the negative control and the right area represents apoptotic cells that incorporate the biotin-UTP FITC-avidin complex. The × axis shows the relative fluorescence intensity. The y axis shows the relative number of cells. (C) shows the percentage of apoptosis evaluated by TUNEL technique and shown by flow cytometry at different culture times. Data are expressed as the means ± SD of four separate experiments performed in duplicate. In (D), apoptotic megakaryocytes appeared as intensively yellow-green fluorescent cells at confocal microscopy.
Quantitative evaluation of apoptosis in CD34-derived megakaryocytes by the TUNEL technique. In (A) and (B), the intensity of fluorescence in the whole cell population was shown by flow cytometry at day 18 of liquid culture. (A) represents a negative control: cells treated with avidin-FITC. (B) shows a typical double profile in which the left area represents viable cells overlapping the negative control and the right area represents apoptotic cells that incorporate the biotin-UTP FITC-avidin complex. The × axis shows the relative fluorescence intensity. The y axis shows the relative number of cells. (C) shows the percentage of apoptosis evaluated by TUNEL technique and shown by flow cytometry at different culture times. Data are expressed as the means ± SD of four separate experiments performed in duplicate. In (D), apoptotic megakaryocytes appeared as intensively yellow-green fluorescent cells at confocal microscopy.
Because CD34-derived megakaryocytic cells grown in the presence of TPO represent an asynchronous population of cells at various stages of maturation and with different ploidy and size, we next investigated whether apoptosis occurred predominantly in polyploid megakaryocytes or in small megakaryoblasts. Two distinct approaches were used. In the first one, apoptotic cells were subdivided in two groups according to their size, by using the TUNEL technique shown by confocal microscopy (Fig 4D). The vast majority of apoptotic cells showed a diameter greater than 20 μm (Table 3).
Evaluation of Apoptosis in CD34-Derived Megakaryocytic Cells of Different Size
| Cell Diameter . | No. of Cells Examined . | % . |
|---|---|---|
| <20 μm | 24 | 8 |
| >20 μm | 289 | 92 |
| Cell Diameter . | No. of Cells Examined . | % . |
|---|---|---|
| <20 μm | 24 | 8 |
| >20 μm | 289 | 92 |
Apoptosis was evaluated by the TUNEL technique shown by confocal microscopy, analyzing randomly selected fluorescent cells. The samples examined (day 18 of liquid culture) derive from three separate experiments, each performed in duplicate starting from a different BM sample.
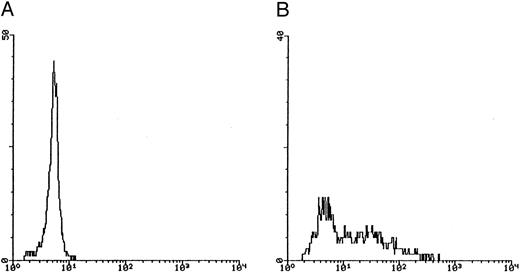
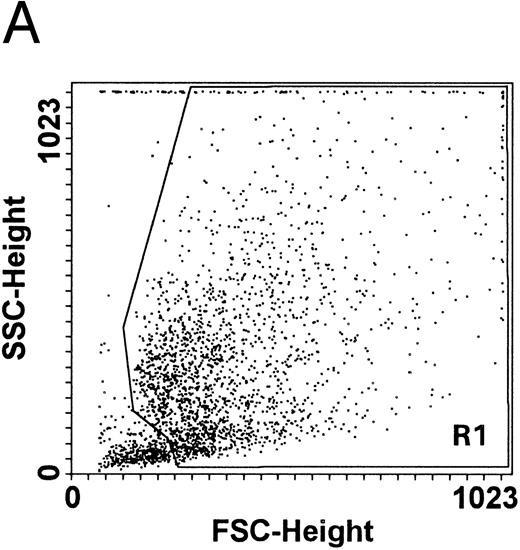
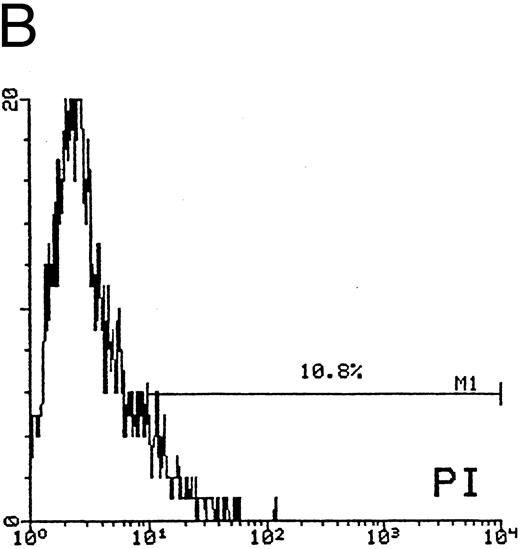
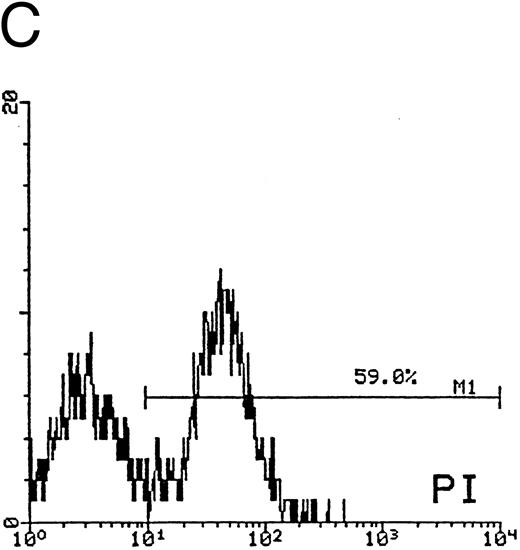
In the second approach, apoptosis was evaluated by using an additional technique26 based on the uptake of PI by nonfixed cells (Fig 5). Similar to the TUNEL technique, this assay also allows us to explore relatively early events in the apoptotic program. The cells of higher size, presumably mature megakaryocytes, were gated on SSC/FSC (Fig 5A) and examined at different time points. Whereas at day 12 of culture the fraction of apoptosis was constantly less than 15% (Fig 5B), at days 18 to 21 most megakaryocytes showed a dim PI uptake (Fig 5C), typical of apoptotic cells.26 Taken together, these findings confirmed that CD34-derived cells undergoing apoptotic cell death were mainly represented by polyploid megakaryocytes of higher size.
Quantitative evaluation of apoptosis in CD34-derived megakaryocytes by supravital PI incorporation. (A) SSC/FSC dot plot showing the gate region (R1) used to analyze apoptotic cells. Gated apoptotic cells were monitored after 12 (B) and 21 (C) days of liquid suspension culture in the presence of 100 ng/mL of TPO. Left areas represent viable cells, which do not incorporate PI, whereas the right area represents apoptotic cells, incorporating PI. M1 is the percentage of apoptotic cells. The × axis shows the relative fluorescence intensity. The y axis shows the relative number of cells. Results of one experiment representative of three separate experiments are shown.
Quantitative evaluation of apoptosis in CD34-derived megakaryocytes by supravital PI incorporation. (A) SSC/FSC dot plot showing the gate region (R1) used to analyze apoptotic cells. Gated apoptotic cells were monitored after 12 (B) and 21 (C) days of liquid suspension culture in the presence of 100 ng/mL of TPO. Left areas represent viable cells, which do not incorporate PI, whereas the right area represents apoptotic cells, incorporating PI. M1 is the percentage of apoptotic cells. The × axis shows the relative fluorescence intensity. The y axis shows the relative number of cells. Results of one experiment representative of three separate experiments are shown.
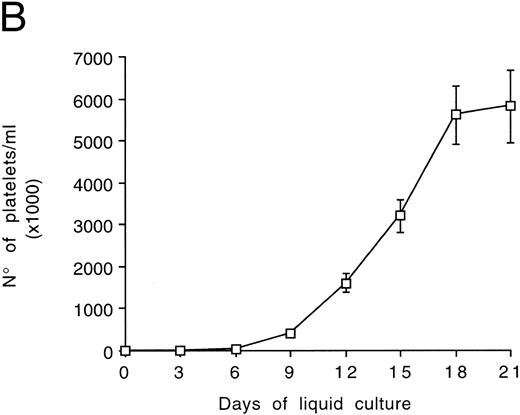
Kinetics of platelet release in culture by CD34-derived megakaryocytes.At light microscopy examination, samples obtained after 12 to 21 days of culture often showed the presence of clusters of platelet-size fragments mixed to viable and apoptotic cells (Fig 6A). In agreement with previous reports,28 we found that the mean platelet volume of the cultured platelets was similar to that of normal platelets (6 to 10 fL). The concentration of platelets released by CD34-derived megakaryocytes was next evaluated at different time points after gel filtration (Fig 6B). Of note, the number of cultured platelets peaked at days 18 to 21. Thus, the kinetics of appearance of platelets in culture differed significantly from that of megakaryocytes, whose number peaked at days 12 to 15 and then rapidly declined (Fig 1A).
Examination of morphology (A) and counts (B) of platelets released in the culture supernatants by CD34-derived megakaryocytes. (A) shows semithin sections with the presence of apoptotic (arrows) megakaryocytes surrounded by clusters of platelet-size fragments (*). OM × 40. In (B), gel-filtered platelets were counted at different culture times. Data are expressed as the means ± SD of three separate experiments performed in duplicate.
Examination of morphology (A) and counts (B) of platelets released in the culture supernatants by CD34-derived megakaryocytes. (A) shows semithin sections with the presence of apoptotic (arrows) megakaryocytes surrounded by clusters of platelet-size fragments (*). OM × 40. In (B), gel-filtered platelets were counted at different culture times. Data are expressed as the means ± SD of three separate experiments performed in duplicate.
Characterization and cytokine regulation of apoptosis in primary BM megakaryocytes.To rule out the possibility that apoptosis observed in CD34-derived megakaryocytes might be due to an abnormal in vitro maturation rather than reflecting a potentially physiologic event, in the next group of experiments primary megakaryocytes were isolated by immunomagnetic selection from normal BM aspirates, using an anti-CD41a MoAb.9 αIIbβ3+ cells morphologically comprised a high number of fully developed megakaryocytes together with smaller megakaryoblasts (data not shown).
By using the TUNEL technique shown by flow cytometry, we found that the fraction of αIIbβ3+ apoptotic cells rapidly increased in serum-free liquid cultures in the absence of growth factors (Table 4). Various cytokines, which are known to influence megakaryocytopoiesis,12 were also used to evaluate their effect on the survival of primary αIIbβ3+ cells. At early culture times (days 2 and 4), the addition of TPO (100 ng/mL) significantly (P < .05) reduced the percentage of apoptosis with respect to αIIbβ3+ cells left untreated. On the other hand, neither IL-3 (10 ng/mL) nor Epo (10 ng/mL) was able to significantly protect these cells from apoptosis. At later time points, no differences in the fraction of apoptotic cells could be detected between TPO-treated, IL-3–treated, Epo-treated, or untreated cells. Moreover, the combination of TPO plus IL-3 did not significantly increase the degree of protection from apoptosis observed in the presence of TPO alone.
Effect of Various Cytokines on the Percentage of Primary αIIbβ3+ BM Cells Undergoing Apoptosis in Liquid Cultures
| Cytokines . | Days of Culture . | |||
|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . |
| None | 9 ± 2.7 | 22 ± 4.5 | 36 ± 6 | 52 ± 7 |
| TPO (100 ng/mL) | 9 ± 2.7 | 11.5 ± 2 | 19 ± 4 | 46 ± 6 |
| EPO (10 ng/mL) | 9 ± 2.7 | 20 ± 4.8 | 31 ± 5 | 56 ± 8 |
| IL-3 (10 ng/mL) | 9 ± 2.7 | 16 ± 5 | 29 ± 7 | 49 ± 8 |
| TPO (100 ng/mL) + IL-3 (10 ng/mL) | 9 ± 2.7 | 10 ± 3 | 17 ± 5 | 43 ± 7.7 |
| Cytokines . | Days of Culture . | |||
|---|---|---|---|---|
| . | 0 . | 2 . | 4 . | 6 . |
| None | 9 ± 2.7 | 22 ± 4.5 | 36 ± 6 | 52 ± 7 |
| TPO (100 ng/mL) | 9 ± 2.7 | 11.5 ± 2 | 19 ± 4 | 46 ± 6 |
| EPO (10 ng/mL) | 9 ± 2.7 | 20 ± 4.8 | 31 ± 5 | 56 ± 8 |
| IL-3 (10 ng/mL) | 9 ± 2.7 | 16 ± 5 | 29 ± 7 | 49 ± 8 |
| TPO (100 ng/mL) + IL-3 (10 ng/mL) | 9 ± 2.7 | 10 ± 3 | 17 ± 5 | 43 ± 7.7 |
Apoptosis was evaluated by the TUNEL technique and shown by flow cytometry. Data are expressed as the means ± SD of four to seven separate experiments, each performed in duplicate starting from a different BM sample.
DISCUSSION
Megakaryocytopoiesis is a unique hematopoietic process that requires both mitotic cell division and endomitotic nuclear replication for the development of mature megakaryocytes from the hematopoietic progenitor cell pool.1-3 Mitosis and endomitosis occur nearly sequentially and they appear to be tightly regulated,30 because the size and extent of polyploidization achieved by a developing megakaryocyte is inversely related to the mitotic past history of its precursor cell. Despite this close relationship between nucleus and cytoplasm of maturating megakaryocytes, little is known of the fate of the polyploid megakaryocytic nucleus after maturation has been completed with the platelet release.
In this study, we have provided evidence suggesting that the natural fate of human megakaryocytes might be, at least in vitro, apoptotic cell death. This conclusion was drawn on the basis of multiple assays. Previous observations of the morphology of the senescent megakaryocytes were mainly focused on the appearance immediately after fragmentation of the cytoplasm into platelets.5,6,19 31-35 Following the ultrastructure of cultured CD34-derived megakaryocytes for various time points, we were able to unequivocally show the characteristic features of apoptotic death in senescent cells. Furthermore, primary αIIbβ3+ megakaryocytic cells, directly isolated from the BM of normal individuals, underwent apoptotic cell death in culture more rapidly than CD34-derived megakaryocytes. These findings allowed us to rule out the possibility that apoptosis observed in CD34-derived megakaryocytes was due to an abnormal in vitro megakaryocyte maturation and suggest that apoptosis might represent a physiologic event for end-stage megakaryocytes.
In BM biopsies, mature megakaryocytes appear typically located near the venous sinusoids throughout the marrow space-special capillary vessels serving cellular exchange between BM and blood.36 Because megakaryocytes break up the whole cytoplasm into platelets or somewhat larger particles,37 38 a peculiarity of apoptosis in these cells seems to be that it mainly involves the cell nucleus and a short rim of cytoplasm surrounding the nucleus.
Unfortunately, the identification of apoptotic megakaryocytes in BM tissue sections or biopsies remains extremely difficult owing to (1) the speed of the whole apoptotic process, (2) the low number of megakaryocytes, and (3) the presence in the BM of professional phagocytes (resident BM macrophages), which clear apoptotic bodies very rapidly.39 In assessing the fate of senescent megakaryocytes, we could overcome the last two difficulties mentioned above by using an in vitro liquid suspension culture system supplemented with TPO, which allowed us to obtain relatively homogeneous populations of CD34-derived megakaryocytic cells in the absence of phagocytic cells. However, the extension of these findings to the in vivo megakaryocytopoiesis must be considered with caution. In fact, cytokines other than TPO and complex cell-to-cell and cell-to-BM matrix interactions are known to play a primary role in the development of megakaryocytes and platelet production in vivo.1-3,12 31 Although 2 × 1011 platelets are produced every day, the sites and modalities of platelet production in vivo are still incompletely understood. In fact, the process of platelet production, similar to that of megakaryocyte apoptosis, is very rarely observed in biopsy sections owing to the speed of the action.
Because apoptotic cell death is a highly regulated genetic program,40 it remains to be determined how the induction of apoptosis relates to the normal megakaryocyte maturation and release of functional platelets. In a recent study performed on E2F-1 transgenic mice, it was demonstrated that E2F-1 transgenic megakaryocytes show an abnormally increased proliferation accompanied by a block in terminal differentiation followed by apoptosis.41 This study suggests that, at least under certain circumstances, apoptosis may follow an impaired megakaryocytic differentiation.
Although we were unable to investigate in depth the relationship existing between megakaryocyte maturation and apoptosis, we found that apoptosis occurred predominantly in mature megakaryocytes rather than in immature megakaryoblasts. Moreover, the kinetics of platelet release in the culture supernatants by CD34-derived megakaryocytes were delayed with respect to the peak of megakaryocyte maturation and rather corresponded to the onset of apoptosis in these cells. This temporal correlation suggests, although it does not prove, that maximal platelet production and megakaryocyte apoptosis are closely related events.
Another interesting point that should be underlined is that apoptosis in primary megakaryocytes was only partially modulated by TPO and was not significantly affected by either Epo or IL-3. In fact, TPO showed some protection of mature BM megakaryocytes from apoptosis only for a short time period. The behavior of TPO is similar to that of other hematopoietic growth factors, which show pleiotropic activities on cell survival, growth, and maturation, depending on the stage of differentiation of their target cells.42 For instance, granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor promote the survival and growth of and allow the differentiation of hematopoietic progenitor cells committed towards the granulocytic lineage and delay the onset of apoptosis of mature circulating neutrophils, which occurs spontaneously during short-term in vitro culturing.43 44
Supported by “Blood and AIDS projects” of the Italian Ministry of Health and by AIRC.
Address reprint requests to Giorgio Zauli, MD, PhD, Institute of Human Anatomy, Via Fossato di Mortara 66, 44100 Ferrara, Italy.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal