Abstract
We have previously reported that the serine protease plasmin triggers chemotaxis in human peripheral monocytes, but not in polymorphonuclear leukocyte. We now show that the structurally related lipoprotein(a) (Lp[a]) as well as recombinant apolipoprotein(a) (apo[a]) trigger chemotactic responses in human monocytes equipotent to that observed with the standard chemoattractant FMLP. The chemotactic effects of Lp(a) and FMLP were additive. Low density lipoprotein (LDL) did not elicit any significant chemotactic response nor did it interfere with that triggered by Lp(a). As assessed by checkerboard analysis, Lp(a)-mediated monocyte locomotion was a true chemotaxis. Both plasminogen as well as catalytically inactivated plasmin inhibited monocyte migration elicited by Lp(a), suggesting binding of Lp(a) to plasminogen binding sites. Lp(a)-mediated signaling proceeds through a pertussis toxin-sensitive guanosine triphosphate (GTP)-binding protein and activation of protein kinase C as implicated by the effects of 1-O-hexadecyl-2-O-methyl-rac-glycerol and chelerythrine. Lp(a) induced generation of guanosine 3′,5′-cyclic monophosphate (cGMP), apparently crucial for the Lp(a)-mediated chemotaxis, because an inhibitor of soluble guanylyl cyclase, LY83583, reduced both the Lp(a)-induced cGMP formation as well as the monocyte migration. The latter effect of LY83583 was antagonized by the stable cGMP analog 8-pCPT-cGMP. The data indicate that Lp(a) triggers chemotaxis in human monocytes by way of a cGMP-dependent mechanism. Our findings may have important implications for the atherogenesis associated with elevated levels of Lp(a).
LIPOPROTEIN(A) (LP[A]) consists of a low-density lipoprotein (LDL)–like particle containing a protein moiety composed of apolipoprotein B-100 and the unique, highly glycosylated glycoprotein apolipoprotein(a) (apo[a]), which exhibits a striking size polymorphism. The two proteins are linked by disulfide bridges. Apo(a) is remarkably homologous to plasminogen and contains multiple repeats of kringle 4–like domains.1,2 Lp(a) has gained increasing attention because of its putative role as a novel major risk factor for atherothrombosis. Indeed, in many human studies high plasma levels of Lp(a) (> approximately 30 mg/dL in terms of particle concentration) have been found to be associated with a marked increase in risk of premature cardiovascular disease.2-4 However, the molecular and cellular mechanisms by which Lp(a) contributes to the acceleration of atherosclerosis remain obscure. It has been proposed that the seemingly deleterious effects of elevated levels of Lp(a) might arise in part from the 80% homology of the apo(a) moiety with the plasminogen molecule.1-3 Thus, it has been shown that Lp(a) exerts antifibrinolytic effects by competitively inhibiting the binding of plasminogen to cells and to fibrin.5-11 To date, in vitro data support this hypothesis,5-11 but the in vivo significance of such a mechanism remains undetermined.12
In previous studies we have shown that plasmin is a potent and selective proinflammatory stimulus for human peripheral monocytes.13-15 Indeed, low concentrations of plasmin were able to trigger release of potent lipid mediators, including chemotactic leukotriene B4 .13,14 Moreover, plasmin was found to be equipotent to the standard compound FMLP as a chemoattractant for human peripheral monocytes.15 Because Lp(a) interacts with plasminogen binding sites on monocytoid U937 cells,8,11 we investigated whether Lp(a) might elicit a chemotactic response in human monocytes resembling that of plasmin. Such an effect could contribute significantly to Lp(a)-mediated atherogenesis, as Lp(a) accumulates in the vessel wall16-18 and would thereby enhance the intimal recruitment of monocyte-derived macrophages.
MATERIALS AND METHODS
Materials.FMLP, pertussis toxin, human serum albumin, and essentially fatty acid free bovine serum albumin (BSA) were purchased from Sigma Chemical Co (St Louis, MO). 8-(para-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate (8-pCPT-cGMP) was from Biolog Life Science Institute (Bremen, Germany). Human plasmin (13.83 Committee on Thrombolytic Agents [CTA] U/mg, lot # 320 018) was obtained from Fluka (Buchs, Switzerland), and plasmin standard solution was from Boehringer (Mannheim, Germany). D-valyl-L-phenylalanyl-L-lysine chloromethyl ketone, 1-O-hexadecyl-2-O-methyl-rac-glycerol (HMG), LY83583 (6-anilino-5,8-quinolinodione), and Aquacid II were from Calbiochem (San Diego, CA). The plasmin substrate S-2251 (H-D-valyl-leucyl-L-lysine-para-nitroanilide dihydrochloride) was supplied by Chromogenix (Mölndal, Sweden). Percoll was obtained from Pharmacia (Uppsala, Sweden) and lysine-Sepharose from Pharmacia Biotech (St Quentin Yveslines, France). Chelerythrine chloride was from Biomol (Plymouth Meeting, PA) and the radioimmunoassay kit for guanosine 3′,5′-cyclic monophosphate from Amersham (Braunschweig, Germany). Fluorescein isothiocyanate (FITC)-conjugated mouse antihuman monoclonal antibodies against CD14 were from Immunotech (Hamburg, Germany). Recombinant human monocyte chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1α (MIP-1α) and respective neutralizing mouse monoclonal antibodies were purchased from Pepro Tech Inc (Rocky Hill, NJ). Recombinant human anaphylatoxin C5a19 and neutralizing mouse monoclonal antibodies20 were kindly provided by Dr J. Köhl (Medical School Hannover, Germany). Recombinant apo(a) of the 18 kringle type21 was generously donated from Dr E. Anglés-Cano (INSERM U143, Bicêtre, France). Pyrogen-free water was from standard clinical supplies. Other chemicals of analytical grade were received from Merck (Darmstadt, Germany).
Cells.Human peripheral monocytes were isolated by Percoll (Pharmacia) gradient centrifugation essentially as described elsewhere.13,15,22 Briefly, the mononuclear cell fraction was washed twice with 150 mmol/L NaCl after the first gradient centrifugation. Preparations with purity greater than 92% were used. Contamination with lymphocytes and platelets represented 2% to 8% and less than 2 platelets per 100 monocytes, respectively. Human neutrophils were isolated as described.13 Neutrophils were found to be greater than 98% pure with less than 2% lymphocytes. Cell purity was verified microscopically after staining and by phase contrast microscopy. The identity of monocytes was further confirmed by staining for α-naphtyl-acetate esterase (>92% positive staining). In addition, scanning and transmission electron microscopy as well as flow cytometric analysis of CD14+ cells were used for the characterization of monocytes.
Chemotaxis.Cell migration was evaluated in triplicate using sterile 24-well Transwell plates (Costar, Cambridge, MA) with tissue culture-treated polycarbonate membranes (pore size: 5 μm for monocytes and 3 μm for neutrophils).23 Monocytes and neutrophils were suspended in Hanks' balanced salt solution containing essentially fatty acid–free BSA at a concentration of 0.4% (HBSS) at a density of 2 × 106 cells/mL; 100 μL were added to the upper compartment of each well. Chemoattractants or solvents were added to the lower compartments, and cells were permitted to migrate at 37°C (90 minutes for monocytes and 60 minutes for neutrophils).23 Lipoproteins were added in phosphate-buffered saline supplemented with 1 mmol/L CaCl2 at pH 7.4. At the end of incubation, cells adherent to the membranes were fixed, stained with hematoxylin (Accustain, Sigma), and seven high-power oil-immersion fields (100 ×) of the lower side of the membrane were counted. The checkerboard assay was used to distinguish between true chemotaxis and chemokinesis.23
In experiments with plasminogen and inactivated plasmin, the compounds were added to both the upper and lower compartments of the chemotaxis chamber.
The protein kinase C (PKC) inhibitors, HMG24,25 and chelerythrine chloride,26 were added to both the upper and lower compartments of the chambers. Because HMG binds to albumin,24 the PKC inhibitor experiments were performed with HBSS containing 0.1% (wt/vol) human serum albumin.
In experiments with LY83583 (6-anilino-5,8-quinolinodione),27 the compound was added to both upper and lower compartments of the chemotaxis chambers and was preincubated for 15 minutes at 37°C before addition of Lp(a) as the chemoattractant.
When the stable cGMP analog, 8-pCPT-cGMP28 was used, this compound was added together with the chemoattractant Lp(a) to the lower compartment.
Monocytes were pretreated with 1 μg/mL pertussis toxin (PTX) for 90 minutes at 37°C.29 30 At the end of incubation cells were washed twice in HBSS. Toxin treatment did not significantly affect the viability of the cells as assessed by Trypan blue dye exclusion. Controls always received the appropriate solvents.
Analysis of cGMP.For the determination of the cellular content of cGMP, monocytes were incubated in HBSS at a density of 107cells/mL. After adaptation for 6 minutes at 37°C, Lp(a) was added and incubation was continued. The incubation of 100 μL of cell suspension was stopped by addition of 25 μL of perchloric acid (35%, wt/vol) at 4°C for 15 minutes. After centrifugation, to remove the precipitate the supernatants were collected and neutralized with 5.82 mol/L KOH. After 60 minutes at 4°C, the tubes were centrifuged again, and the supernatants were acetylated and used for radioimmunological determination of cGMP according to the instructions of the Amersham assay kit.
Plasmin.Human plasmin was measured as activity against the substrate S-2251 (H-D-Val-Leu-L-Lys-para-nitroanilide dihydrochloride).31 Plasminogen was determined in the same amidolytic assay after activation with streptokinase (10,000 U/mL) at 37°C for 10 minutes.31 In those experiments, the different reaction kinetics of streptokinase-activated plasminogen as compared with plasmin were taken into account (Chromogenix Laboratory Instruction).
Inactivated plasmin was obtained by incubation of 4 μmol/L plasmin in 100 mmol/L phosphate buffer, pH 7.4, with 4 mmol/L D-Val-L-Phe-L-Lys CH2Cl at 37°C for 15 minutes.32 33 After exhaustive dialysis against phosphate-buffered saline, inactivated plasmin had no residual activity using S-2251 as substrate. Considering the sensitivity of the assay, more than 99% of plasmin activity had been inactivated.
Isolation of lipoproteins.Lp(a) and LDL protected against oxidation by butylated hydroxytoluene (BHT) were isolated from plasma by sequential ultracentrifugation as previously described.34 The lipoproteins isolated in the 1.050 to 1.100-g/mL interval were dialyzed against 20 mmol/L Na2HPO4 , pH 7.3, and applied to a lysine-Sepharose column. After washing the column we eluted Lp(a) with the lysine analog, ε-aminocaproic acid 200 mmol/L in a buffer containing 100 mmol/L Na2HPO4 , 150 mmol/L NaCl, ethylenediaminetetraacetic acid (EDTA) 0.01% (wt/vol), and NaN3 0.01% (wt/vol) at pH 7.3. Lp(a) and LDL passed through the lysine-Sepharose column were concentrated against Aquacid II and extensively dialyzed against phosphate-buffered saline, pH 7.4. The concentration of total protein was determined by the method of Lowry et al.35 Lp(a) has been prepared within 2 days, and under those conditions no oxidation was noticed. Various isoforms of Lp(a) from several donors have been used for the experiments.
Statistics.Values are expressed as mean ± SEM. Statistical analysis was performed using the Student's t-test for unpaired data or the Newman-Keuls test in cases of multigroup comparisons. A threshold P value less than .05 was considered significant.
RESULTS
Effect of Lp(a) on monocyte migration.Incubation of human monocytes in HBSS in the absence of chemoattractant led to a minor basal migration of monocytes across polycarbonate membranes (Fig 1). However, when the standard chemoattractant FMLP was added to the lower compartment of the chemotaxis chamber at an optimal concentration of 10 nmol/L,23 monocyte locomotion was significantly enhanced (n = 6, P < .01 v basal controls). Addition of Lp(a) at concentrations from 25 to 400 μg/mL (in this report lipoprotein concentrations are generally expressed in terms of protein concentration except when stated otherwise) to the lower compartments led to a concentration-dependent stimulation of monocyte migration with a maximum chemotactic response at 300 μg/mL (Fig 1). At this concentration the chemotactic activity of Lp(a) was found to be equipotent to that of 10 nmol/L FMLP (Fig 1).
Concentration-dependent effect of Lp(a) on human monocyte migration. Monocytes incubated at 37°C in Hanks' balanced salt solution containing BSA 0.4% (HBSS) were permitted to migrate across polycarbonate membranes (pore size 5 μm) for 90 minutes. After fixation and staining, migrated cells attached to the lower side of the membrane were counted under the microscope using oil-immersion at a 1,000-fold magnification. **P < .01 versus basal value without chemoattractant (HBSS). Cell numbers represent the mean ± SEM of migrated monocytes per high-power field of six independent experiments. Seven fields were counted and experiments were performed in triplicate.
Concentration-dependent effect of Lp(a) on human monocyte migration. Monocytes incubated at 37°C in Hanks' balanced salt solution containing BSA 0.4% (HBSS) were permitted to migrate across polycarbonate membranes (pore size 5 μm) for 90 minutes. After fixation and staining, migrated cells attached to the lower side of the membrane were counted under the microscope using oil-immersion at a 1,000-fold magnification. **P < .01 versus basal value without chemoattractant (HBSS). Cell numbers represent the mean ± SEM of migrated monocytes per high-power field of six independent experiments. Seven fields were counted and experiments were performed in triplicate.
Lp(a) and FMLP apparently synergize in chemotaxis, because Lp(a) 300 μg/mL and FMLP 10 nmol/L triggered monocyte migration of 21.7 ± 1.8 and 22.0 ± 0.7 cells/high-power field (n = 4 each), respectively, whereas addition of the combination of both chemoattractants to the lower compartment yielded 41.0 ± 1.3 cells/high-power field (n = 4, P < .01 v Lp(a) or FMLP alone). In controls without any chemoattractant, 5.2 ± 0.8 cells/high-power field were observed (n = 4).
We also tested whether neutrophils respond to Lp(a) with a chemotactic reaction. When 10 nmol/L FMLP was used as a chemoattractant, neutrophils showed significant cell migration across polycarbonate membranes, leading to 26.2 ± 1.15 cells/high-power field versus 6.0 ± 1.3 cells/high-power field under basal conditions (n = 3, P < .01). However, no significant increase in cell migration (6.1 ± 0.8 cells/high-power field, n = 3, P value not significant) could be detected in the presence of Lp(a) (300 μg/mL).
Effects of neutralizing antibodies directed against monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and anaphylatoxin C5a on Lp(a)-induced monocyte migration.As judged by polyacrylamide gel electrophoresis, our preparations of Lp(a) appeared to be free of contaminating proteins (data not shown). To exclude trace contaminations of Lp(a) with other potent monocyte chemoattractants, such as the CC chemokines MCP-1 or MIP-1α36,37 or anaphylatoxin C5a,37 38 we performed additional experiments with neutralizing antibodies. When used at optimal chemotactic concentrations, MCP-1 50 ng/mL, MIP-1α 50 ng/mL, and C5a 1.0 nmol/L elicited monocyte migration comparable to that induced by Lp(a) 300 μg/mL (Table 1). Addition of corresponding neutralizing antibodies abolished the chemotactic effects of MCP-1, MIP-1α, and C5a to the level of basal HBSS controls (n = 4, P < .01 v corresponding chemoattractant). However, none of the neutralizing antibodies had any significant effect on the Lp(a)-induced chemotactic response (n = 4, P value was not significant v Lp[a]; Table 1). Moreover, by immunostaining of Western blots we could not detect any contamination of Lp(a) with either MCP-1, MIP-1α, or C5a (data not shown).
Effects of Neutralizing Antibodies on Monocyte Migration Elicited by MCP-1, MIP-1α, C5a, and Lp(a)
| . | No. of Migrated Cells . | n‡ . |
|---|---|---|
| Control | 4.8 ± 0.5 | 5 |
| MCP-1 (50 ng/mL) | 21.9 ± 1.8* | 4 |
| + anti–MCP-1 (10 μg/mL) | 6.1 ± 0.2† | 4 |
| MIP-1α (50 ng/mL) | 15.3 ± 1.2* | 4 |
| + anti–MIP-1α (10 μg/mL) | 5.7 ± 0.3† | 4 |
| C5a (1.0 nmol/L) | 22.0 ± 0.7* | 4 |
| + anti-C5a (80 μg/mL) | 5.2 ± 0.7† | 4 |
| Lp(a) (300 μg/mL) | 19.7 ± 0.8* | 5 |
| + anti–MCP-1 (10 μg/mL) | 19.4 ± 0.3 | 4 |
| + anti–MIP-1α (10 μg/mL) | 20.9 ± 1.3 | 4 |
| + anti-C5a (80 μg/mL) | 20.8 ± 0.9 | 4 |
| . | No. of Migrated Cells . | n‡ . |
|---|---|---|
| Control | 4.8 ± 0.5 | 5 |
| MCP-1 (50 ng/mL) | 21.9 ± 1.8* | 4 |
| + anti–MCP-1 (10 μg/mL) | 6.1 ± 0.2† | 4 |
| MIP-1α (50 ng/mL) | 15.3 ± 1.2* | 4 |
| + anti–MIP-1α (10 μg/mL) | 5.7 ± 0.3† | 4 |
| C5a (1.0 nmol/L) | 22.0 ± 0.7* | 4 |
| + anti-C5a (80 μg/mL) | 5.2 ± 0.7† | 4 |
| Lp(a) (300 μg/mL) | 19.7 ± 0.8* | 5 |
| + anti–MCP-1 (10 μg/mL) | 19.4 ± 0.3 | 4 |
| + anti–MIP-1α (10 μg/mL) | 20.9 ± 1.3 | 4 |
| + anti-C5a (80 μg/mL) | 20.8 ± 0.9 | 4 |
Monocytes were permitted to migrate across polycarbonate membranes with a pore size of 5 μm for 90 minutes. Results represent the mean ± SEM of migrated cells in seven oil-immersion fields.
P < .01 versus HBSS control.
P < .01 versus positive control without neutralizing antibodies.
The number of independent experiments is given as n.
Checkerboard analysis.To clarify whether the migration of monocytes through the membranes depends on the presence of a concentration gradient of Lp(a) between the lower and upper compartments of the chambers, checkerboard experiments were performed. Maximal induction of migration occurred in the presence of a positive concentration gradient between the two compartments, ie, with the higher concentration below the polycarbonate membrane (Table 2). With equal concentrations of Lp(a) below and above the membranes, only a slightly higher migration of monocytes was observed, indicating a weak chemokinetic effect of Lp(a) on monocytes.
Checkerboard Analysis of Lp(a)-Induced Monocyte Migration Across Polycarbonate Membranes
| Below Membrane . | Above Membrane . | |||
|---|---|---|---|---|
| . | . | . | . | . |
| . | . | |||
| . | . | . | . | . |
| . | HBSS . | Lp(a) 33 . | Lp(a) 100 (μg/mL) . | Lp(a) 300 . |
| HBSS | 5.0 ± 0.6 | 8.3 ± 0.6 | 7.6 ± 0.2 | 7.6 ± 0.9 |
| Lp(a) (μg/mL) | ||||
| 33 | 10.4 ± 1.1 | 8.3 ± 0.6 | 8.3 ± 0.5 | 7.7 ± 0.3 |
| 100 | 15.0 ± 0.8* | 12.8 ± 0.8† | 9.6 ± 0.5 | 7.2 ± 0.5 |
| 300 | 23.9 ± 1.1* | 20.8 ± 0.9* | 16.0 ± 1.1* | 9.6 ± 2.2 |
| Below Membrane . | Above Membrane . | |||
|---|---|---|---|---|
| . | . | . | . | . |
| . | . | |||
| . | . | . | . | . |
| . | HBSS . | Lp(a) 33 . | Lp(a) 100 (μg/mL) . | Lp(a) 300 . |
| HBSS | 5.0 ± 0.6 | 8.3 ± 0.6 | 7.6 ± 0.2 | 7.6 ± 0.9 |
| Lp(a) (μg/mL) | ||||
| 33 | 10.4 ± 1.1 | 8.3 ± 0.6 | 8.3 ± 0.5 | 7.7 ± 0.3 |
| 100 | 15.0 ± 0.8* | 12.8 ± 0.8† | 9.6 ± 0.5 | 7.2 ± 0.5 |
| 300 | 23.9 ± 1.1* | 20.8 ± 0.9* | 16.0 ± 1.1* | 9.6 ± 2.2 |
Monocytes were permitted to migrate for 90 minutes across polycarbonate membranes with a pore size of 5 μm. Different concentrations of Lp(a) were placed in the upper and/or lower compartment of the Transwell chambers. Cell numbers represent the mean ± SEM of migrated monocytes in seven oil immersion fields counted in triplicates from four experiments.
P < .01 and † P < .05 by Newman-Keuls test versus corresponding values with HBSS in the lower compartment of the chemotaxis chamber.
Effect of recombinant apo(a) on monocyte migration.When recombinant apo(a) was added to the lower compartment of the chemotaxis chamber, a concentration-dependent stimulation of monocyte locomotion was observed from 25 to 150 μg/mL apo(a) (n = 4, P < .01 v basal controls; Table 3). At a concentration of 150 μg/mL the chemotactic activity of recombinant apo(a) was equipotent to that of 10 nmol/L FMLP (Table 3).
Effect of Recombinant Apolipoprotein(a) on Monocyte Migration Across Polycarbonate Membranes
| . | No. of Migrated Cells . |
|---|---|
| Control | 7.1 ± 0.3 |
| FMLP (10 nmol/L) | 22.9 ± 1.53-150 |
| Apo(a) (μg/mL) | |
| 150 | 22.1 ± 1.03-150 |
| 100 | 16.7 ± 1.13-150 |
| 50 | 14.7 ± 0.43-150 |
| 25 | 11.8 ± 0.73-150 |
| . | No. of Migrated Cells . |
|---|---|
| Control | 7.1 ± 0.3 |
| FMLP (10 nmol/L) | 22.9 ± 1.53-150 |
| Apo(a) (μg/mL) | |
| 150 | 22.1 ± 1.03-150 |
| 100 | 16.7 ± 1.13-150 |
| 50 | 14.7 ± 0.43-150 |
| 25 | 11.8 ± 0.73-150 |
Monocytes were allowed to migrate for 90 minutes across polycarbonate membranes with a pore size of 5 μm. Different dilutions of recombinant apo(a) were added to the lower compartments of the Transwell chambers. Results represent the mean ± SEM for migrated cells in 7 oil-immersion fields counted in triplicate from 4 experiments.
P < .01 versus HBSS-BSA 0.4% controls by Neuman-Keuls test.
Effect of LDL on monocyte migration.When LDL instead of Lp(a) was added to the lower compartment of the chemotaxis chambers, a minor but significant increase (n = 8, P < .01 v basal HBSS control) in monocyte locomotion was observed at a concentration of 300 μg/mL (Table 4). Such enhanced migration was not significantly different from the chemokinetic effects observed with Lp(a) as shown in Table 1. However, the chemotactic response elicited with Lp(a) at 300 μg/mL (n = 6; Fig 1) was significantly higher (P < .01) as compared with the cell migration induced by LDL (300 μg/mL; n = 8).
Effect of LDL on Monocyte Migration
| . | No. of Migrated Cells . | n4-151 . |
|---|---|---|
| Control | 5.6 ± 0.5 | 8 |
| FMLP (10 nmol/L) | 19.6 ± 1.04-150 | 8 |
| LDL (μg/mL) | ||
| 300 | 9.4 ± 0.94-150 | 8 |
| 200 | 7.6 ± 0.9 | 6 |
| 100 | 6.1 ± 0.6 | 7 |
| . | No. of Migrated Cells . | n4-151 . |
|---|---|---|
| Control | 5.6 ± 0.5 | 8 |
| FMLP (10 nmol/L) | 19.6 ± 1.04-150 | 8 |
| LDL (μg/mL) | ||
| 300 | 9.4 ± 0.94-150 | 8 |
| 200 | 7.6 ± 0.9 | 6 |
| 100 | 6.1 ± 0.6 | 7 |
Different dilutions of LDL were added to the lower compartment of the Transwell chambers. Monocytes were permitted to migrate across polycarbonate membranes with a pore size of 5 μm for 90 minutes. Results represent the mean ± SEM of migrated cells in seven oil immersion fields.
P < .01 by Newman-Keuls test versus HBSS control.
The number of independent experiments is given as n.
LDL does not interfere with the Lp(a)-induced monocyte migration as shown in additional experiments. In those experiments monocyte locomotion was 22.5 ± 1.5 and 8.2 ± 0.7 cells/high-power field with Lp(a) 300 μg/mL and LDL 200 μg/mL, respectively (n = 5 each) When Lp(a) 300 μg/mL and LDL 200 μg/mL were added together to the lower compartment, monocyte migration was 22.8 ± 0.9 cells/high-power field (n = 4, P value was not significant v Lp(a) 300 μg/mL). In basal controls migration was 5.7 ± 0.5 cells/high-power field (n = 5).
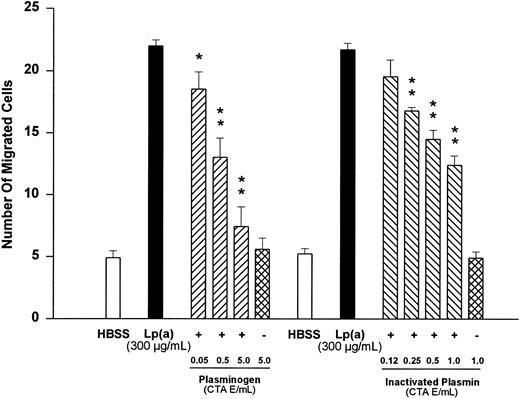
Effects of plasminogen and active site-blocked plasmin on Lp(a)-induced monocyte chemotaxis.Because Lp(a) has been shown to compete with plasminogen for the same binding site in several systems,5-11 we tested the effects of plasminogen and active site-blocked plasmin on Lp(a)-induced monocyte migration. Plasminogen at concentrations from 0.05 to 5.0 CTA unit equivalents/mL led to a significant (n = 4, P < .05 v Lp[a] control) and concentration-dependent inhibition of monocyte migration induced by Lp(a) (300 μg/mL; Fig 2). At 5.0 CTA unit equivalents/mL, plasminogen almost completely prevented monocyte locomotion triggered by 300 μg/mL of Lp(a) (n = 4, P < .01 v Lp[a] control). Plasminogen at 5.0 CTA unit equivalents/mL and added in the absence of Lp(a) did not significantly affect monocyte locomotion as compared with the basal HBSS value (n = 4, P value was not significant; Fig 2).
Effects of various concentrations of plasminogen or inactivated plasmin on human monocyte migration triggered by Lp(a) (300 μg/mL). HBSS values show random cell migration in the absence of the chemoattractant Lp(a). *P < .05 and **P < .01 versus appropriate Lp(a) control. Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments in each group.
Effects of various concentrations of plasminogen or inactivated plasmin on human monocyte migration triggered by Lp(a) (300 μg/mL). HBSS values show random cell migration in the absence of the chemoattractant Lp(a). *P < .05 and **P < .01 versus appropriate Lp(a) control. Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments in each group.
Similarly, when Lp(a) (300 μg/mL) was added together with increasing concentrations of catalytically-inactivated plasmin to the lower compartment, a concentration-dependent reduction of the chemotactic response was observed. Inactivated plasmin, at concentrations from 0.25 to 1.0 CTA unit equivalents/mL, significantly reduced Lp(a)-induced chemotaxis (n = 4, P < .01 v Lp(a) control). Inactivated plasmin (1.0 CTA unit equivalent/mL), added in the absence of Lp(a), did not significantly affect monocyte locomotion as compared with the basal HBSS value (n = 4, P value was not significant; Fig 2).
Effects of pertussis toxin and PKC inhibitors on Lp(a)-induced monocyte chemotaxis.Pretreatment of monocytes with 1 μg/mL PTX for 90 minutes, which is known to block G protein-mediated monocyte responses,29 30 led to complete blockade (n = 4, P < .01 v appropriate Lp[a] control) of the chemotactic activity of monocytes towards Lp(a) (300 μg/mL; Fig 3). This finding indicates that a PTX-sensitive G protein is involved in the signal transduction pathway activated by Lp(a).
Effect of pretreatment with PTX (1 μg/mL) for 90 minutes and effects of the protein kinase C inhibitors HMG and chelerythrine on human monocyte migration elicited by Lp(a) (300 μg/mL). HBSS shows random cell migration in the absence of the chemoattractant Lp(a). **P < .01 versus appropriate Lp(a) control. Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments.
Effect of pretreatment with PTX (1 μg/mL) for 90 minutes and effects of the protein kinase C inhibitors HMG and chelerythrine on human monocyte migration elicited by Lp(a) (300 μg/mL). HBSS shows random cell migration in the absence of the chemoattractant Lp(a). **P < .01 versus appropriate Lp(a) control. Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments.
Moreover, the PKC inhibitor HMG,24,25 at concentrations of 30 and 60 μmol/L, significantly reduced the chemotactic response of monocytes elicited by Lp(a) (300 μg/mL) by 66.2 ± 6.0% (n = 4, P < .01 v appropriate Lp[a] control) and 92.7 ± 8.4% (n = 4, P < .01 v appropriate Lp[a] control), respectively (Fig 3). Another structurally unrelated PKC inhibitor, chelerythrine,26 at concentrations of 1 and 10 μmol/L, similarly inhibited Lp(a)-induced locomotion by 50.0 ± 10.7% (n = 4, P < .01 v appropriate Lp[a] control) and 85.9 ± 11.4% (n = 4, P < .01 v appropriate Lp[a] control), respectively (Fig 3).
Effect of Lp(a) on cGMP formation.Cyclic GMP may play an important role in the signal transduction induced by chemoattractants, and specifically in monocytes.39 40 Therefore, we investigated the effect of Lp(a) on cGMP formation by monocytes. Stimulation of monocytes with Lp(a) (100 and 300 μg/mL) triggered a time- and concentration-dependent generation of cGMP (Fig 4). Within 1 minute of exposure to Lp(a), significant increases in migration (n = 4, P < .01 v basal control) were observed, with maximum values at Lp(a) concentrations of 100 and 300 μg/mL (203 ± 19 fmol/106 cells and 798 ± 64 fmol/106 cells, respectively). At both Lp(a) concentrations used, cGMP levels remained significantly elevated for up to 30 minutes (n = 4, P < .05 v basal control).
Time-dependent effects of Lp(a) at 100 μg/mL (▪) and 300 μg/mL (•) on cGMP formation by human peripheral monocytes incubated at 37°C in HBSS at a density of 107 cells/mL. The incubations were stopped by addition of perchloric acid and rapid cooling. After neutralization, cGMP contents were determined radioimmunologically. *P < .05 and **P < .01 versus basal values at time point 0 minutes. The data show mean ± SEM for four independent experiments.
Time-dependent effects of Lp(a) at 100 μg/mL (▪) and 300 μg/mL (•) on cGMP formation by human peripheral monocytes incubated at 37°C in HBSS at a density of 107 cells/mL. The incubations were stopped by addition of perchloric acid and rapid cooling. After neutralization, cGMP contents were determined radioimmunologically. *P < .05 and **P < .01 versus basal values at time point 0 minutes. The data show mean ± SEM for four independent experiments.
Significance of cGMP-dependent mechanisms for the Lp(a)-induced monocyte chemotaxis.The potential significance of cGMP in Lp(a)-mediated chemotaxis was further investigated using LY83583, an inhibitor of the soluble guanylyl cyclase.27 As expected, LY83583 inhibited Lp(a)-induced cGMP formation in monocytes in a concentration-dependent manner when the cells were stimulated with Lp(a) (300 μg/mL) for 2 minutes after 15 minutes preincubation with various concentrations of LY83583 (n = 4; Fig 5). The IC50 value for cGMP generation triggered by Lp(a) was calculated as 7.8 μmol/L (confidence limits: 1.4 to 42.6 μmol/L). At a concentration of 100 μmol/L, LY83583 almost completely inhibited cGMP formation.
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on cGMP formation by human peripheral monocytes incubated in the presence of Lp(a) (300 μg/mL) for 2 minutes. Monocytes were pretreated with LY83583 for 15 minutes before addition of Lp(a). The incubations were stopped with ice-cold perchloric acid and by rapid cooling. After neutralization, cGMP contents were analyzed radioimmunologically. **P < .01 versus samples without LY83583. The data show mean ± SEM of four independent experiments.
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on cGMP formation by human peripheral monocytes incubated in the presence of Lp(a) (300 μg/mL) for 2 minutes. Monocytes were pretreated with LY83583 for 15 minutes before addition of Lp(a). The incubations were stopped with ice-cold perchloric acid and by rapid cooling. After neutralization, cGMP contents were analyzed radioimmunologically. **P < .01 versus samples without LY83583. The data show mean ± SEM of four independent experiments.
As Fig 6 shows, LY83583 induced a concentration-dependent inhibition of the chemotactic reaction of monocytes stimulated with Lp(a) (300 μg/mL). Furthermore, LY83583 (100 μmol/L) led to a nearly complete blockade (n = 4, P < .01 v Lp[a] control) of the chemotactic response of monocytes towards Lp(a) (300 μg/mL). Lp(a)-induced chemotaxis was inhibited with an IC50 of 10.4 μmol/L (confidence limits: 3.9 to 28.1 μmol/L). The inhibitory effect of 10 μmol/L LY83538 on monocyte locomotion was mediated by inhibition of guanylyl cyclase, because it could be completely antagonized with the stable cGMP analog 8-pCPT-cGMP (10 μmol/L; Fig 6). When added in the absence of LY83583, 10 μmol/L 8-pCPT-cGMP did not significantly affect the Lp(a)-induced chemotactic response.
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on human monocyte migration elicited by Lp(a) (300 μg/mL). Monocytes were preincubated with LY83583 for 15 minutes before addition of the chemoattractant Lp(a) (300 μg/mL). When the stable cGMP analog 8-pCPT-cGMP was used, the compound was added together with Lp(a) to the lower compartment of the chemotaxis chamber. *P < .05 and **P < .01 versus Lp(a) (300 μg/mL). Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments.
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on human monocyte migration elicited by Lp(a) (300 μg/mL). Monocytes were preincubated with LY83583 for 15 minutes before addition of the chemoattractant Lp(a) (300 μg/mL). When the stable cGMP analog 8-pCPT-cGMP was used, the compound was added together with Lp(a) to the lower compartment of the chemotaxis chamber. *P < .05 and **P < .01 versus Lp(a) (300 μg/mL). Cell numbers are expressed as mean ± SEM per high-power field of four independent experiments.
DISCUSSION
For the first time we showed that Lp(a) is a potent and specific chemoattractant for human peripheral monocytes, but not for human neutrophils. At levels as low as 300 μg/mL, Lp(a) was equipotent to a 10 nmol/L concentration of the standard chemoattractant FMLP, the optimal chemotactic concentration for this tripeptide.23 Preliminary experiments using the air pouch model in C57/BL6J mice41 42 indicate that Lp(a) acts as chemoattractant in vivo as well (Philippou et al, unpublished results). Our data therefore suggest that accumulation of Lp(a) in the arterial wall potentiates recruitment of monocytes to the vessel wall during atherogenesis.
Two key events in the initiation of atherogenesis involve the influx and accumulation of LDL at arterial sites displaying a predilection for formation of atherosclerotic lesions and for the recruitment of peripheral monocytes to these lesion-prone sites; monocytes mature into macrophages, accumulate lipid, are transformed into foam cells, and, together with the accompanying lymphocytes, contribute directly to formation of fatty streaks.43 Hence, chemotaxis constitutes a critical event in the development of atherosclerotic lesions. Several growth factors including colony-stimulating factors in addition to MCP-1 are chemoattractants for human peripheral monocytes.36,37,43 Moreover, oxidized LDL, but not native LDL, has been reported to induce monocyte chemotaxis.44 The chemotactic activity of oxidized LDL resides in the lipid fraction44 and is apparently caused by the presence of lysophosphatidylcholine generated by phospholipase A2 activity during LDL oxidation.45 During the isolation of Lp(a) and LDL for our experiments, the lipoproteins were protected against oxidation by BHT and no oxidation could be noticed. The minor increase in monocyte locomotion that we observed in our control experiments with LDL is not in fact at variance with the results reported earlier by these previous authors; indeed, at a concentration of native LDL of 100 μg/mL, they observed a 1.4 ± 0.2-fold increase in monocyte locomotion,44 whereas at an LDL concentration of 300 μg/mL we detected a 1.7 ± 0.2-fold increase in monocyte migration. In contrast, monocyte locomotion induced by Lp(a) (300 μg/mL) led to a chemotactic index which was typically greater than 4. As shown in our checkerboard experiments, monocyte migration represented a true chemotactic response requiring a concentration gradient between the lower and upper compartment of the chemotaxis chamber.
The second key event in the initiation of atherogenesis involves the subendothelial accumulation of LDL. In this context, it is relevant that Lp(a) binds avidly to components of the connective tissue matrix, such as glycosaminoglycans46 and fibronectin.47 In murine and human vessels, Lp(a) accumulation is primarily located extracellularly in the intima and subintima.17,18,48 Indeed, the relative amounts of apo(a) and apo B present in the arterial wall at sites of atherosclerotic plaque formation indicate that Lp(a) is preferentially retained in comparison to non-apo(a) containing LDL.18 49
Despite extensive studies,8,11,50-53 the precise nature of the binding sites for plasminogen and Lp(a) on membranes of monocytes and related cell lines remains undetermined. This situation arises from the heterogeneous nature of the binding sites for these ligands,8,11,50-53 which encompass even such diverse molecules as α-enolase54 as well as annexin II.55 Indeed, monocytes and monocyte-like U937 cells have been shown to carry binding sites that bind both plasminogen as well as plasmin.50,53 Furthermore, Lp(a) competes with plasminogen for the same binding sites on U937 cells.8,11,53 The binding of Lp(a) consists of two separate components, one that depends upon the lipoprotein character of Lp(a) and that is inhibitable by LDL, and one that is dependent upon the lysine binding function of apo(a), the latter sites being shared by plasminogen.11 These binding sites, which have been proposed to play a role in the Lp(a)-associated atherogenesis,11 apparently mediate Lp(a)-induced monocyte chemotaxis, because plasminogen as well as active site-blocked plasmin antagonized the chemotactic effect of Lp(a) in our experiments. This hypothesis has been further substantiated by our experiments with purified recombinant apo(a).21 Apo(a) clearly possesses chemotactic activity. Indeed, considering the differences in molecular weight between apo(a) and Lp(a), one has to conclude that on a molar basis both agents yield identical concentration-response curves.
With this as background, low affinity binding sites for LDL, which may also mediate binding of Lp(a) on U937 cells,11 monocyte-derived macrophages,52 or a murine macrophage cell line,51 are unlikely to propagate the monocyte activation observed. In keeping with this, LDL exhibited only a weak effect on monocyte locomotion and did not interfere with the Lp(a)-induced monocyte chemotaxis. Moreover, in contrast to the effects observed in our study, binding of Lp(a) to scavenger receptors on murine macrophages could not be antagonized by plasminogen.51 Thus, the common binding site for plasminogen and Lp(a) described by Miles et al8,11 53 should transmit the chemotactic signal elicited by Lp(a).
This point gains further support from our recent finding that plasmin, similar to Lp(a), is a potent chemoattractant for human peripheral monocytes.15 For such activity, it is apparently necessary that the plasmin molecule carries an intact catalytic domain.15 Apo(a) similarly harbors a “pseudo” protease segment nearly identical to that of plasmin.1,2,12 Although some authors described an enzymatic activity associated with Lp(a) or apo(a),47,56,57 careful studies with recombinant and wild-type apo(a) could not confirm those previous findings.58 Thus, for receptor activation the conformational integrity of the serine protease and pseudo serine protease region in the plasmin and Lp(a) molecule, respectively, appear to be important, but perhaps less so a proteolytic activity.
Studies with the specific bacterial toxin PTX, as well as molecular cloning and sequencing experiments, indicate that the receptors for chemoattractants belong to the family of G protein–coupled receptors.59 Indeed, PTX has been shown to inhibit FMLP, C5a, and MCP-1–induced signal transduction and chemotaxis in monocytes.29 30 In our experiments, a concentration of PTX, which blocked monocyte chemotaxis induced by FMLP, C5a, and MCP-1, was equally effective in blocking Lp(a)-induced chemotaxis. These findings indicate that a pertussis toxin-sensitive G protein plays a key role in Lp(a)-induced monocyte activation.
An enhanced activity of protein kinase C is also thought to play a key role in the numerous responses of leukocytes to chemoattractants.59 HMG, an ether lipid that interacts with the diacylglycerol binding site of PKC, has been shown to inhibit both the FMLP- and the phorbol ester–stimulated respiratory burst in human neutrophils.24 This compound, which supposedly is noncytotoxic and does not inhibit cAMP-dependent or Ca2+/calmodulin-dependent kinases,24,25 inhibited the Lp(a)-induced monocyte locomotion in a concentration-dependent manner. In addition, chelerythrine, another inhibitor of PKC that is known to interact with the catalytic domain of PKC without affecting phorbol ester binding to the enzyme,26 similarly led to a concentration-dependent inhibition of Lp(a)-induced monocyte chemotaxis. Thus, two structurally but mechanistically unrelated inhibitors of PKC exhibited profound inhibitory effects, thereby providing clear evidence for a crucial role of PKC in Lp(a)-induced monocyte migration.
Cyclic nucleotides might also play a major role as second messengers in chemoattractant signal transduction. More specifically, cGMP has been implicated in monocyte chemotaxis, as agents that elevate cGMP levels in human monocytes equally enhance the chemotactic response. In contrast, agents that increase cAMP levels led to reduction in monocyte migration.39,40 Our data clearly show that Lp(a) induces a prolonged elevation in monocyte cGMP content. Such formation of cGMP appears to be critical for Lp(a)-induced chemotactic activation, as both parameters were inhibited in a concentration-dependent fashion by LY83583, an inhibitor of the soluble guanylyl cyclase.27 The IC50 values for cGMP formation and Lp(a)-induced chemotaxis were very similar (7.8 and 10.4 μmol/L, respectively) and compare favorably with the reported Ki of 10 μmol/L for isolated soluble guanylyl cyclase.27 Additionally, it was possible to antagonize the inhibitory effect of 10 μmol/L LY83583 on Lp(a)-induced monocyte chemotaxis with the stable cGMP analog, 8-pCPT-cGMP at a concentration of 10 μmol/L,28 thereby indicating that the effect of LY83583 is caused by endogenous cGMP formation. These data clearly support the view that the soluble guanylyl cyclase plays a pivotal role in the Lp(a)-induced chemotactic signal transduction.
In conclusion, our data reveal that Lp(a) is a potent chemoattractant for human peripheral monocytes, which acts through a cGMP-dependent pathway. When the selective accumulation of Lp(a) in the intima and subintima of the arterial wall16, 17,48,49 is taken into account, then this mechanism may, by attracting monocyte-derived macrophages into the vessel wall, contribute significantly to atherogenesis associated with elevated levels of Lp(a) as a result of the attraction of monocyte-derived macrophages into the vessel wall. Because Lp(a) has been detected by immunohistochemical techniques in the microvasculature associated with inflammatory lesions, including Crohn's disease and pericarditis,60 61 it cannot be excluded that Lp(a) may play an even more general role in inflammatory reactions.
ACKNOWLEDGMENT
We thank Dr S. Philippou (Department of Pathology, Ruhr University, Bochum) for scanning and transmission electron microscopy and the histopathological evaluation of the Lp(a)-induced chemotaxis in vivo; and M. Rieks (Department of Neurology, Ruhr University, Bochum) for flow cytometry. The technical assistance of B. Tippler is gratefully acknowledged. Recombinant apolipoprotein(a) and recombinant human anaphylatoxin C5a as well as corresponding monoclonal neutralizing antibodies were generous gifts from Dr E. Anglés-Cano (INSERM U143, Bicêtre, France) and Dr J. Köhl (Department of Microbiology, Medical School Hannover, Germany), respectively. Glu-plasminogen and streptokinase were gifts from Dr J. Römisch (Behring, Marburg).
Supported in part by the Deutsche Forschungsgemeinschaft and by INSERM.
Address reprint requests to Thomas Simmet, MD, Department of Pharmacology and Toxicology, Ruhr University, Universitätsstrasse 150, D-44780 Bochum, Germany.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal