Abstract
Many B-cell malignancies express the CD22 antigen on their cell surface. To kill cells expressing this antigen, the RFB4 monoclonal antibody (MoAb) has been linked chemically with either deglycosylated ricin A chain or truncated versions of Pseudomonas exotoxin. These immunotoxins exhibited selective cytotoxic activity for CD22+ cells and antitumor activity in nude mouse models bearing human B-cell lymphomas. To construct a recombinant immunotoxin targeted to CD22, we first cloned the variable portions of the heavy and light chains of RFB4. The cloned Fv fragments were joined by a newly created disulfide bond to form a disulfide stabilized (ds) construct. The RFB4 construct was combined by gene fusion with PE38, a truncated version of PE. The recombinant immunotoxin was then expressed in Escherichia coli, purified by column chromatography and tested for cytotoxicity activity. RFB4(dsFv)PE38 retained its binding activity for CD22, was very stable at 37°C and exhibited selective cytotoxic activity for CD22+-cultured cell lines. Because of its favorable binding characteristics and potency for CD22-positive cell lines, RFB4(dsFv)PE38 was tested for antitumor activity in a nude mouse model of human lymphoma. CA46 cells were injected subcutaneously and then treated with the RFB4(dsFv)PE38 immunotoxin. Antitumor activity was dose responsive and was not evident when an irrelevant immunotoxin was administered on the same schedule.
LEUKEMIAS AND LYMPHOMAS are attractive targets for treatment with immunotoxins. The response of patients with B-cell malignancies has been extensively investigated in phase I/II clinical trials of immunotoxin activity.1-4 To date, some antitumor responses have been noted but immunotoxin-mediated toxicity to normal tissue often prevented dose escalations to therapeutic levels. Several B-cell–specific antigens such as CD19, CD22, and CD40 have been targeted by immunotoxins made with plant toxins such as ricin A-chain and bacterial toxins, such as Pseudomonas exotoxin A (PE).5-7
CD22, a lineage-restricted B-cell antigen that belongs to the Ig superfamily, is expressed on the surface of many types of malignant B cells, including chronic B-lymphocytic cells (B-CLL), B lymphoma cells such as Burkitt's lymphomas, and hairy cell leukemias, as well as on normal mature B lymphocytes. CD22 is not present on the cell surface in the early stages of B-cell development and is not expressed on stem cells.8 Additionally, no shed antigen can be detected in normal human serum or serum from patients with CLL.9
RFB4 IgG is an anti-CD22 monoclonal antibody that has been chemically conjugated to both ricin and Pseudomonas exotoxin A (PE) and has shown excellent activity against B cells both in vitro and in vivo.6,10,11 RFB4 is highly specific for cells of the B lineage and has no detectable cross-reactivity with any other normal cell type.9 RFB4 IgG has previously been covalently coupled to both ricin A-chain and a truncated form of PE called PE35.6,11 These conjugate molecules were effective against experimental lymphoma xenograft models, and in the clinical setting, the ricin-based immunotoxin also has shown some efficacy against human disease.1 2
While chemical conjugates are frequently very stable and potent, they are relatively large and often heterogeneous at their linkage sites, which may result in suboptimal activity.12,13 The requirements for making large quantities of IgG and chemical conjugation also put some limitations on the ability to manufacture the drug. Because the ability to penetrate tumors is inversely related to the size of the penetrating molecule, the large size of whole antibody-toxin conjugates may impair their ability to penetrate tumor masses such as those found in lymphomas.13
A number of antibody Fv fragments have recently been cloned and genetically fused to PE derivatives such as PE40, PE38, and PE35.14 The recombinant molecules produced from these constructs are one third the size of IgG-toxin chemical conjugates and are homogeneous in composition. A reduction in size is predicted to improve drug penetration in solid tumors, while elimination of the constant portion of the IgG molecule results in faster clearance from the circulation of experimental animals and patients. Together, these properties may lessen side effects by reducing the time in which the immunotoxin can interact with nontarget tissues and tissues that express very low levels of antigen. Finally homogeneous preparations of recombinant immunotoxins can easily be produced in large quantities.
To take advantage of the benefits of recombinant immunotoxin technology, we have cloned the variable light and heavy chains of RFB4 IgG and expressed them as a disulfide-linked (ds) Fv fusion with PE38. In disulfide-linked immunotoxins, cysteine replaces a single amino acid in each chain, whose location is chosen using predictions from structural models. Here we describe the cloning, mutagenesis, and expression of RFB4(dsFv)PE38, and characterize its antigen binding, cytotoxicity and antitumor activity properties.
MATERIALS AND METHODS
Cell lines.HUT102 cells were a gift from T. Waldmann (NCI) and CA46 and JD38 lines were from I. McGrath (NIH). Daudi, Raji, and Namalwa cells were purchased from ATCC, Rockville, MD.
Cloning.The RFB4 IgG-producing hybridoma was from the Royal Free Hospital School of Medicine, University of London. Purified RFB4 IgG was provided by E. Vitteta, UTSMC, Dallas, TX. RFB4 IgG was reduced with 10 mmol/L dithiothreitol (DTT) and light and heavy chains were separated on 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Novex, San Diego, CA) and blotted onto a PVDF membrane. Light and heavy chain bands were cut from the membrane and subjected to N-terminal sequence analysis. DNA fragments encoding the light and heavy chains were prepared by polymerase chain reaction (PCR) amplification as described15 except using total RNA and RFB4-specific 5′ primers RFB4 VH5 and RFB4 VL5, which were designed from N-terminal protein sequence data. The sequences of all primers used in cloning are listed in Table 1. PCR products were cloned into the PCR cloning vector (Invitrogen, San Diego, CA) and sequenced using Sequenase (US Biochemical Corp, Cleveland, OH) reagents and protocols. To make RFB4(dsFv)PE38, VL was amplified using a 5′ primer, RFB4 VL5 dsFv, introducing an Nde I site, and a 3′ primer, RFB4 VL3 dsFv, introducing an EcoRI site and mutating glycine residue 100 to cysteine. VH was amplified using RFB4 VH5 and RFB4 VH3 dsFv, which introduces a HindIII site and a lysine residue at the C-terminus of VH . PCR products were digested with Nde I and either HindIII (VH ) or EcoRI (VL ) and were used to replace VH -linker-VL of pUL17 (VH ) or to replace the entire VH -linker-VL -PE38 (VL ). VH -PE38 was mutagenized to change Arg44 to Cys using the Muta-Gene site-directed mutagenesis kit and protocol (Bio-Rad, Hercules, CA) and a phosphorylated primer, VHdsFv(cys). Clones that had incorporated the Cys44 mutation were identified by DNA sequencing.
PCR Primers
| Heavy chain primers | |
| RFB4 VH5 | GGACCTCATATGGAAGTGCAGCTGGTGGAGTCT |
| γCH1 | AGCAGATCCAGGGGCCAGTGGATA |
| RFB4 VH3 | AGATCCGCCACCACCGGATCCGCCTCCGCCTGCAGAGACAGTGACCAGAGTCCC |
| RFB4 VH3 dsFv | CCGGAAGCTTTTGCAGAGACAGTGACC |
| RFB4 VH dsFv(cys) | GACCCACTCCAGGCACTTCTCCGGAGTC |
| Light chain primers | |
| RFB4 VL5 | GGTGGCGGATCTGGAGGTGGCGGAAGCGATATCCAGATGACACAGACT |
| C-κ | TGGTGGGAAGATGGATACAGTTGG |
| RFB4 VL3 | CCGGAAGCTTTGATTTCCAGCTTGG |
| RFB4 VL5 dsFv | GGACCTCATATGGATATCCAGATGACCC |
| RFB4 VL3 dsFv | CCGGAATTCA TTATTTGATTTCCAGCTTGGTGCCGCAACCGAACGTCC |
| Heavy chain primers | |
| RFB4 VH5 | GGACCTCATATGGAAGTGCAGCTGGTGGAGTCT |
| γCH1 | AGCAGATCCAGGGGCCAGTGGATA |
| RFB4 VH3 | AGATCCGCCACCACCGGATCCGCCTCCGCCTGCAGAGACAGTGACCAGAGTCCC |
| RFB4 VH3 dsFv | CCGGAAGCTTTTGCAGAGACAGTGACC |
| RFB4 VH dsFv(cys) | GACCCACTCCAGGCACTTCTCCGGAGTC |
| Light chain primers | |
| RFB4 VL5 | GGTGGCGGATCTGGAGGTGGCGGAAGCGATATCCAGATGACACAGACT |
| C-κ | TGGTGGGAAGATGGATACAGTTGG |
| RFB4 VL3 | CCGGAAGCTTTGATTTCCAGCTTGG |
| RFB4 VL5 dsFv | GGACCTCATATGGATATCCAGATGACCC |
| RFB4 VL3 dsFv | CCGGAATTCA TTATTTGATTTCCAGCTTGGTGCCGCAACCGAACGTCC |
Primers are given 5′ to 3′. Primers γCH1 and C-κ were designed as described.15 RFB4 VH5 and RFB4 VL5 were designed according to the N-terminal protein sequences determined by Edman degradation. RFB4 VH5 encodes an Nde I site (bold) and an initiator Met (bold italicized). RFB4 VL3 encodes a HindIII site (italicized). Primers RFB4 VH3 and RFB4 VL3 were designed according to the nucleotide sequence determined from cDNA clones. Primers RFB4 VH3 and RFB4 VL5 include partial Gly4Ser linker sequences which partially overlap (underlined italicized). Primer RFB4 VL3 dsFv mutates VL Gly100 residue to Cys (underlined), includes a terminator codon (bold) and an EcoRI site (italicized). Primer RFB4 VH dsFv(cys) mutates VH Arg44 to Cys (underlined). RFB4 VH3 dsFv includes an additional Lys codon and a HindIII site (italicized). RFB4 VL5 dsFv includes an Nde I (bold) and an initiator Met (bold italicized).
Expression and purification of recombinant clones.Expression plasmids encoding RFB4 VH -PE38 and RFB4 VL were separately expressed in Escherichia coli BL21(λDE3).16 Disulfide-linked immunotoxins were produced by refolding of purified inclusion body protein generally as described.17 Briefly, inclusion bodies were prepared from cell paste by lysis and washing in nonionic detergent, then solubilized in 6 mol/L guanidine-HCl, 0.1 mol/L Tris, pH 8, 2 mmol/L EDTA. Solubilized protein at 10 mg/mL was reduced with 10 mg/mL dithioerythritol (DTT) (65 mmol/L), then rapidly diluted into 100 vol 0.1 mol/L Tris, 0.5 mol/L L-arginine, 0.9 mmol/L oxidized glutathione, 2 mmol/L EDTA at 10°C. Equal weight amounts of VL and VH -PE38 were used to refold RFB4(dsFv)PE38. Disulfide-linked immunotoxin was refolded in buffer adjusted to pH 9.5 (room temperature). Immunotoxin was allowed to refold for 48 hours, then was dialyzed against 100 mmol/L urea, 20 mmol/L Tris, pH 8 to a conductivity of less than 3.5 mMho. Properly refolded proteins were purified by sequential anion-exchange FPLC on Q-Sepharose and MonoQ (Pharmacia, Arlington Heights, IL) followed by gel filtration on 30 mL TSK G3000SW (TosoHaas, Montgomeryville, PA).
Binding.Relative binding affinities of recombinant immunotoxins was measured by competition against 125I-labeled RFB4 IgG for binding to CA46 target cells at 4°C. CA46 cells grown to >106/mL were washed twice in ice cold binding buffer (RPMI, 50 mmol/L BES, pH 6.8, 1% bovine serum albumin [BSA]), and plated at 106 cells/150 μL binding buffer/well in 96-well plates on ice. To the cells was added 0.35 ng 125I-RFB4 (2.5 × 109 cpm/nmol) in binding buffer and varying concentrations of RFB4(dsFv)PE38. Cells were incubated for 3 hours on ice, washed twice in cold binding buffer, and solubilized in 200 μL 0.5% SDS/TE. Bound 125I-RFB4 was quantitated on a Wallac 1470 Wizard gamma counter (Turku, Finland). The means of duplicate samples were used for calculations.
Cytotoxicity assays.Cells were maintained in RPMI 1640 containing 20% (Daudi) or 10% fetal bovine serum (FBS) (all other lines), 50 U/mL penicillin, 50 μg/mL streptomycin, 1 mmol/L sodium pyruvate, and an additional 2 mmol/L L-glutamine. For cytotoxicity assays, 4 × 104 cells/well in 200 μL culture medium were plated in 96-well plates. Immunotoxins were serially diluted in phosphate-buffered saline (PBS)/0.2% human serum albumin (HSA) and 10 μL added to cells. Plates were incubated for the indicated times at 37°C, then pulsed with 1 μCi/well 3H-leucine in 10 μL PBS for 4 to 5 hours at 37°C. Radiolabeled material was captured on filtermats and counted in a Betaplate scintillation counter (Pharmacia, Gaithersburg, MD). Triplicate sample values were averaged and inhibition of protein synthesis determined by calculating percent incorporation compared with control wells without added toxin.
Toxicity in mice.Six- to eight-week-old Balb/C female mice were obtained from the National Cancer Institute, Frederick, MD. Multiple-dose intravenous (IV) LD50 values were determined for a treatment schedule of dose × 3 qod. Various amounts of immunotoxins were diluted to 200 μL with PBS/0.2% HSA and injected into the tail veins every other day for three doses. Two mice were injected with each dose, and mice were monitored for weight loss and death for 14 days after last injection.
Inhibition of CA46 tumor establishment.Six- to eight-week-old female athymic nude mice were obtained from the National Cancer Institute, Frederick, MD. Mice were treated with 300 rad of gamma irradiation 3 or 4 days before injection of malignant cells. CA46 cells were seeded at 1.8 × 105/mL 2 days before injection. On day 0, CA46 cells were washed in RPMI without serum and adjusted to either 108 cells/mL or 5 × 107 cells/mL in RPMI. Each mouse was given 100 μL of the cell suspension by subcutaneous injection. Mice were treated by tail vein injection every day for 4 consecutive days with various amounts of immunotoxins or with control materials in 200 μL volume. The appearance of tumors was monitored daily or every other day for 21 days following first treatment.
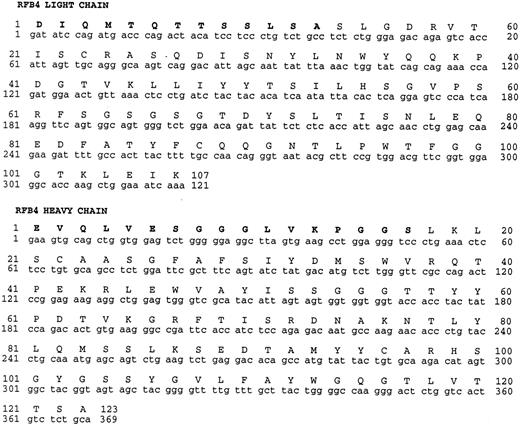
Deduced amino acid sequence of the variable region of RFB4 light and heavy chains. Amino acids shown in bold were determined by N-terminal protein sequence analysis.
Deduced amino acid sequence of the variable region of RFB4 light and heavy chains. Amino acids shown in bold were determined by N-terminal protein sequence analysis.
Cytotoxicity of RFB4(dsFv)PE38 after timed exposure to cells.RFB4(dsFv)PE38 dilutions were incubated with CA46 and JD38 cells for 2, 24, and 48 hours in standard cytotoxicity assays, except that for the 2-hour time point, immunotoxin was removed from the medium by washing with RPMI + 10% FCS, and replaced with standard medium for the remaining 22 hours of the assay. For 48-hour assays, the cells were incubated continuously for 48 instead of 24 hours.
RESULTS
Cloning of cDNAs encoding the heavy and light chain variable regions of RFB4 monoclonal antibody (MoAb).N-terminal amino acid analysis yielded sequence data for both the light and heavy chains of the RFB4 MoAb, which is provided in Fig 1 (bold type). To obtain cDNAs encoding the heavy and light chain variable regions of RFB4, total RNA was prepared from RFB4 hybridoma cells and reverse transcribed to yield first strand cDNA. Subsequently PCR was performed to amplify the heavy and the light chains. Heavy and light chain specific primers were synthesized based on the N-terminal amino acid data, and amplification was performed using these primers together with primers from the constant regions CH1 (heavy) and C-κ (light). This resulted in the amplification of the variable portion of the heavy chain plus part of CH1 and amplification of the variable region of the light chain plus part of C-κ. These products were cloned and sequenced as described in Materials and Methods. There was complete agreement between the amino acid sequence data from the Edman degradations and the deduced sequence from our antibody clones. The nucleotide and deduced amino acid sequences of VH and VL are shown in Fig 1.
Construction of RFB4(dsFv)PE38.The binding portion of disulfide-linked immunotoxins consists of VH and VL chains that are covalently linked through a single key residue in each chain that has been mutated to cysteine. Based on predictions using molecular models and empirical evidence with recombinant PE-containing immunotoxins, several different amino acids in the heavy and light chains can be mutated to cysteine to create a stable disulfide linkage between the chains.18-21 Using this data, we chose one amino acid in each chain (Gly100 of the light chain and Arg44 of the heavy chain) to mutate to cysteine to create a new disulfide linkage between heavy and light chains that would retain the binding affinity of the whole antibody. To construct the disulfide-linked immunotoxin (dsFv), RFB4 VH and VL clones were reamplified using primers VH5 and VH3-dsFv, and VL5-dsFv and VL3-dsFv, respectively. RFB4 VL3-dsFv, in addition to introducing a termination codon and an EcoRI site, mutates the naturally occurring Gly100 of VL to Cys. The amplified products were used to replace Fv and Fv-PE38 sequences of the plasmid pULI7. The cloned product pEM16 (encoding RFB4 VL -cys100 ) was sequenced and shown to have incorporated the Gly to Cys mutation. RFB4 VH -PE38 was mutated using site-directed mutagenesis on single-stranded DNA with primer RFB4 VH dsFv(cys), which replaces VH Arg44 with a cysteine. The resulting mutated construct, pEM15, was sequenced and shown to have incorporated the Arg to Cys mutation.
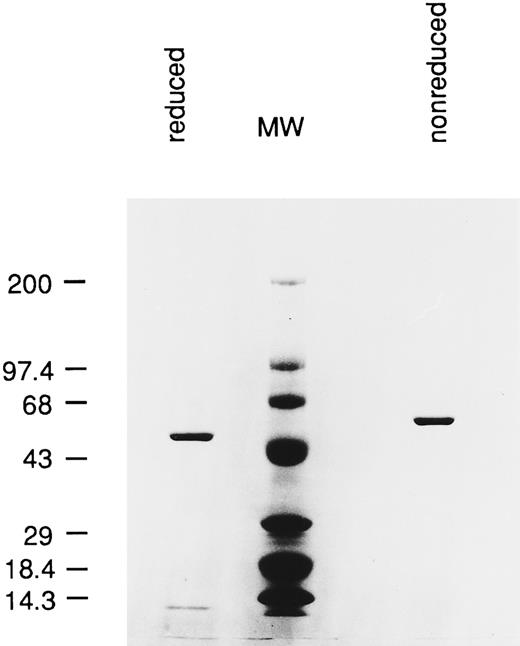
SDS-PAGE analysis of purified RFB4(dsFv)PE38. A total of 2 μg of immunotoxin were loaded in each lane. Reduced: RFB4(dsFv)PE38 was reduced with 10 mmol/L DTT. The variable portion of the heavy chain fused with PE38 migrated with an apparent molecular mass of 48 kD. The light chain migrated faster than the 14.3 kD standard. MW, molecular mass standards. Nonreduced: nonreduced RFB4(dsFv)PE38 migrated with an apparent molecular mass of 58 kD.
SDS-PAGE analysis of purified RFB4(dsFv)PE38. A total of 2 μg of immunotoxin were loaded in each lane. Reduced: RFB4(dsFv)PE38 was reduced with 10 mmol/L DTT. The variable portion of the heavy chain fused with PE38 migrated with an apparent molecular mass of 48 kD. The light chain migrated faster than the 14.3 kD standard. MW, molecular mass standards. Nonreduced: nonreduced RFB4(dsFv)PE38 migrated with an apparent molecular mass of 58 kD.
Expression and purification of RFB4(dsFv)PE38.Plasmids pEM15 and pEM16 were used to transform BL21(λDE3). Cultures of transformed bacteria were induced with IPTG (isopropyl-β-d-Thiogalacto-Pyranoside) for high level expression, with the protein products accumulating in inclusion bodies. Inclusion bodies were isolated and the proteins solubilized, reduced, and refolded as described in Materials and Methods. Properly refolded immunotoxins were purified to near-homogeneity by ion exchange and gel filtration column chromatography. SDS-PAGE analysis of the purified immunotoxin, run under reduced and nonreduced conditions, is shown in Fig 2. Purified immunotoxins were stored at −80°C.
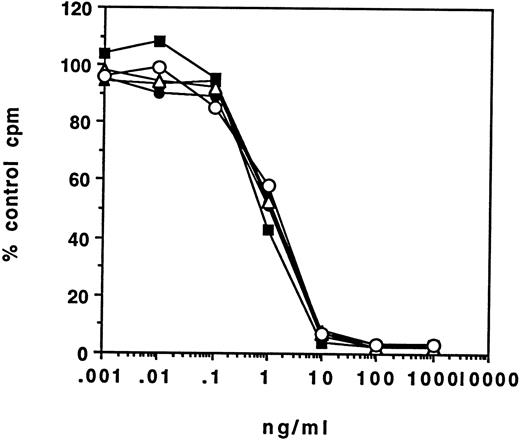
Cytotoxicity of RFB4(dsFv)PE38.RFB4(dsFv)PE38 (Fig 3) was tested on five Burkitt's lymphoma cell lines (CA46, Daudi, JD38, Namalwa, and Raji) and HUT 102, a T-cell line that is CD22-negative. Of the B-cell lines tested, there were variations in sensitivity to RFB4(dsFv)PE38, which had IC50 values ranging from 0.25 to 0.6 ng/mL on CA46, JD38 and Raji, 1.5 ng/mL on Namalwa and 20 ng/mL on Daudi (Table 2). Subsequent studies have shown that Daudi cells cannot efficiently proteolytically process PE38 (Biochemical Soc Transactions, 1997, in press).
Cytotoxicity of RFB4(dsFv)PE38 for various cell lines after a 24-hour incubation. [3H]leucine incorporation is expressed as a percentage of cpm incorporated by control cells incubated without immunotoxin. (○), CA46; (▵), JD-38; (+), Raji; (▪), Namalwa; (•), Daudi; (▴), HUT102. Data are representative of at least three trials for each cell line.
Cytotoxicity of RFB4(dsFv)PE38 for various cell lines after a 24-hour incubation. [3H]leucine incorporation is expressed as a percentage of cpm incorporated by control cells incubated without immunotoxin. (○), CA46; (▵), JD-38; (+), Raji; (▪), Namalwa; (•), Daudi; (▴), HUT102. Data are representative of at least three trials for each cell line.
Cytotoxicity of RFB4(dsFv)PE38 Toward Various Cell Lines
| Cell line* . | Source . | IC50 (ng/mL)† of RFB4(dsFv)PE38 . |
|---|---|---|
| CA46 | Burkitt's lymphoma | 0.6 |
| Daudi | 20 | |
| JD-38 | 0.3 | |
| Namalwa | 1.5 | |
| Raji | 0.4 | |
| HUT102 | T-cell leukemia | >1,000 |
| Cell line* . | Source . | IC50 (ng/mL)† of RFB4(dsFv)PE38 . |
|---|---|---|
| CA46 | Burkitt's lymphoma | 0.6 |
| Daudi | 20 | |
| JD-38 | 0.3 | |
| Namalwa | 1.5 | |
| Raji | 0.4 | |
| HUT102 | T-cell leukemia | >1,000 |
All the cell lines are of human origin.
Cytotoxicity data are given as IC50s , which are the concentrations of immunotoxin that cause a 50% reduction in protein synthesis compared with controls after incubation with cells for 24 hours.
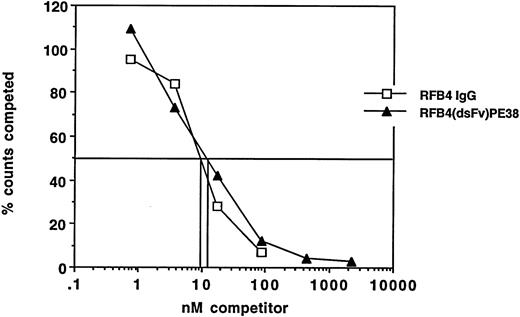
Antigen binding of RFB4(dsFv)PE38.The ability of the recombinant RFB4 immunotoxin to bind to antigen-positive cells was analyzed using competition assays in which increasing concentrations of immunotoxin were used to compete the binding of a fixed concentration of 125I-labeled RFB4 IgG at 4°C. As shown in Fig 4, binding to CA46 cells was reduced by 50% at 10 nmol/L RFB4(dsFv)PE38. Native RFB4 IgG reduced the binding of labelled RFB4 IgG to CA46 cells by 50% at 8.5 nmol/L (Fig 4).
The relative binding activity of RFB4(dsFv)PE38 compared with native antibody on CA46 cells. Whole antibody and recombinant immunotoxin were used to compete for binding of trace amounts of 125I-labeled RFB4 IgG. Counts competed are expressed as a percentage of counts from cells that were incubated without any competitor. (□), RFB4 IgG; (▴), RFB4(dsFv)PE38.
The relative binding activity of RFB4(dsFv)PE38 compared with native antibody on CA46 cells. Whole antibody and recombinant immunotoxin were used to compete for binding of trace amounts of 125I-labeled RFB4 IgG. Counts competed are expressed as a percentage of counts from cells that were incubated without any competitor. (□), RFB4 IgG; (▴), RFB4(dsFv)PE38.
Stability of recombinant RFB4 immunotoxin at 37°C.The stability of RFB4(dsFv)PE38 was investigated by incubation of the immunotoxin for extended periods at 37°C. Stability of PE-based recombinant immunotoxins has been correlated with their activity in vitro.15 Accordingly, RFB4(dsFv)PE38 was incubated in PBS at 37°C for 1 to 7 days and its cytotoxic activity after incubation was compared with samples which were kept at −80°C. In keeping with the previous finding of high stability of dsFv immunotoxins at 37°C, RFB4(dsFv)PE38 was also very stable over the entire 7 days, as judged by maintenance of full cytotoxic activity toward CA46 cells in a 24-hour assay (Fig 5).
Stability of RFB4(dsFv)PE38. RBF4(dsFv)PE38 was incubated at 37°C for the number of days indicated and cytotoxicity on CA46 cells was compared with a sample that was stored at −80°C. (○), 7 days; (▵), 5 days; (•), 3 days; (▴), 1 day; (▪), 0 days.
Stability of RFB4(dsFv)PE38. RBF4(dsFv)PE38 was incubated at 37°C for the number of days indicated and cytotoxicity on CA46 cells was compared with a sample that was stored at −80°C. (○), 7 days; (▵), 5 days; (•), 3 days; (▴), 1 day; (▪), 0 days.
Temporal measurement of cytotoxic activity of RFB4 (dsFv)PE38.The time required for cells to internalize and process immunotoxin is of therapeutic interest because blood levels of immunotoxin in patients must remain above a cytotoxic threshold for long enough to intoxicate the malignant cells. Therefore, we evaluated the consequences of varying exposure time of cells to immunotoxin. RFB4(dsFv)PE38 was added to cells and incubated at 37°C for 2, 24, or 48 hours. The 2-hour exposure was followed by 22 additional hours of incubation in immunotoxin-free medium to allow time for the intracellular trafficking that is required for intoxication. For both CA46 and JD38 cells, increasing the exposure time to immunotoxin from 2 to 24 hours decreased the IC50 by fivefold to 10-fold. Incubation of cells for 48 hours continuously with RFB4(dsFv)PE38 resulted in little, if any, additional effect on cytotoxicity over that observed after 24 hours of incubation. Therefore, we conclude that the cell lines used require greater than 2 hours of exposure to bind and internalize maximal amounts of immunotoxin and are intoxicated to nearly the fullest extent possible by 24 hours. Increasing exposure to times greater than 24 hours does not provide any advantage in vitro.
Toxicity of RFB4(dsFv)PE38.An evaluation of RFB4 (dsFv)PE38 toxicity was performed to select appropriate doses of immunotoxin for antitumor experiments. A maximum tolerated dose (MTD) value was determined by multiple-dose IV injection of RFB4(dsFv)PE38 in varying amounts (Table 3). Two mice were injected for each dose level. At a dose of 3 × 10 μg or 3 × 8 μg, no mice survived. At 3 × 7 μg or less, all mice survived.
LD50 of RFB4(dsFv)PE38
Doses were given IV every other day for three doses.
Mice were observed for 7 days following last dose.
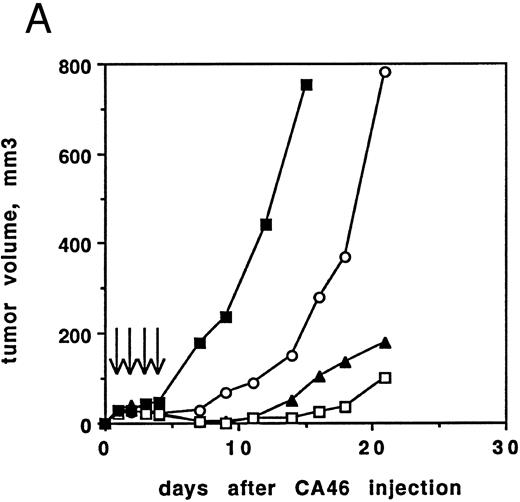
Antitumor activity of RFB4(dsFv)PE38 against CA46 cells in nude mice.The excellent cytotoxicity and stability of RFB4(dsFv)PE38 in vitro predicted that this molecule would have antitumor activity in an animal model of lymphoma. We evaluated the ability of RFB4(dsFv)PE38 to inhibit tumor development in a subcutaneous solid tumor model using CA46 Burkitt's lymphoma cells injected into nude mice. Two protocols were used. In the first, female athymic nude mice were irradiated on day −3 and then injected subcutaneously with 107 CA46 cells on day 0, while in the second, mice were irradiated on day −4 and injected with 5 × 106 CA46 cells. Mice were divided into groups of five and treated beginning 24 hours after injection of CA46 cells for 4 consecutive days (days 1 to 4) with various amounts of immunotoxin, in addition to diluent control (Fig 6). Tumor volumes were recorded for 21 days. Mice inoculated using the first protocol and treated with either 5 or 2 μg of immunotoxin developed small tumor cell nodules that regressed on treatment and then slowly regrew (Fig 6A). Treatment with 1 μg of immunotoxin delayed tumor development compared with controls, but tumors grew rapidly thereafter.
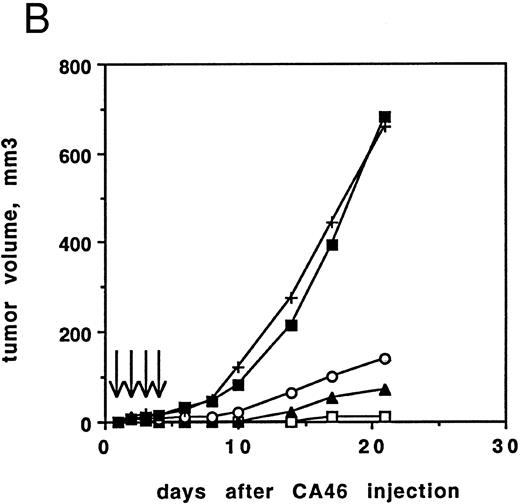
(A) Antitumor activity of RFB4(dsFv)PE38. Athymic nude mice irradiated on day −3 were inoculated with 107 CA46 cells on day 0. Beginning on day 1, injections of 5, 2, or 1 μg RFB4(dsFv)PE38 or PBS/0.2% BSA were given every day for four doses. Tumor growth was monitored by measuring tumor volume and is expressed as the average tumor volume of each group. Control mice were killed before the end of the 21-day observation period because their tumor volumes would have exceeded humane limits. (□), 5 μg; (▴), 2 μg; (○), 1 μg; (▪), PBS/0.2% BSA diluent control. Data are from one of two experiments that gave similar results. (B) Antitumor activity of RFB4(dsFv)PE38. Athymic nude mice irradiated on day −4 were inoculated with 5 × 106 CA46 cells on day 0. Beginning on day 1, injections of 5, 3, or 1 μg RFB4(dsFv)PE38, 5 μg nonrelevant control dsFv-PE38 immunotoxin, or PBS/0.2% BSA diluent were given every day for four doses. Tumor growth was monitored by measuring tumor volume and is expressed as the average tumor volume of each group. (□), 5 μg; (▴), 3 μg; (○), 1 μg; (+), 5 μg nonrelevant control dsFv-PE38 immunotoxin; (▪), PBS/0.2% BSA diluent control. Data are from one of two experiments that gave similar results.
(A) Antitumor activity of RFB4(dsFv)PE38. Athymic nude mice irradiated on day −3 were inoculated with 107 CA46 cells on day 0. Beginning on day 1, injections of 5, 2, or 1 μg RFB4(dsFv)PE38 or PBS/0.2% BSA were given every day for four doses. Tumor growth was monitored by measuring tumor volume and is expressed as the average tumor volume of each group. Control mice were killed before the end of the 21-day observation period because their tumor volumes would have exceeded humane limits. (□), 5 μg; (▴), 2 μg; (○), 1 μg; (▪), PBS/0.2% BSA diluent control. Data are from one of two experiments that gave similar results. (B) Antitumor activity of RFB4(dsFv)PE38. Athymic nude mice irradiated on day −4 were inoculated with 5 × 106 CA46 cells on day 0. Beginning on day 1, injections of 5, 3, or 1 μg RFB4(dsFv)PE38, 5 μg nonrelevant control dsFv-PE38 immunotoxin, or PBS/0.2% BSA diluent were given every day for four doses. Tumor growth was monitored by measuring tumor volume and is expressed as the average tumor volume of each group. (□), 5 μg; (▴), 3 μg; (○), 1 μg; (+), 5 μg nonrelevant control dsFv-PE38 immunotoxin; (▪), PBS/0.2% BSA diluent control. Data are from one of two experiments that gave similar results.
Mice inoculated using the second protocol and treated with 5 or 3 μg of RFB4(dsFv)PE38 developed very small tumor nodules that completely regressed on treatment in 9 of 10 mice at the 5 μg dose level and in 7 of 10 mice at the 3 μg dose level (Fig 6B). No mice achieving complete regression at the 5 μg × 4 dose regrew tumors after extended observation (up to 90 days), while 4 of 7 mice receiving 3 μg × 4, and showing a complete regression, regrew tumors (within 90 days). Treatment with 1 μg of immunotoxin delayed tumor development significantly throughout the observation period, but produced complete regressions in only 2 of 10 mice. Mice that were treated with 5 μg of a nonrelevant dsFv-PE38 immunotoxin that is nonbinding for CA46 cells developed tumors that grew at the same rate as those in the diluent-treated control group, demonstrating specificity of the RFB4(dsFv)PE38 immunotoxin (Fig 6B).
DISCUSSION
The variable portions of the heavy and light chains of the MoAb RFB4 were cloned and sequenced. This antibody recognizes the CD22 antigen, which is present exclusively on B cells and is found on many B-cell malignancies. RFB4 has already been extensively tested for its ability to direct deglycosylated ricin A chain to antigen-positive cells. Recently, it has also been used to make chemical conjugates with PE35, a truncated form of PE. As a way to prepare a wholly recombinant immunotoxin, a disulfide-linked Fv was constructed and then was fused with PE38.
Analysis of the recombinant immunotoxin showed that RFB4(dsFv)PE38 is stable at 37°C for extended incubation times. Cytotoxicity profiles on several antigen-positive and antigen-negative cell lines showed that the recombinant immunotoxin was highly and selectively toxic towards CD22-bearing cells and was nontoxic toward CD22− lines. The duration of incubation required for maximum intoxication of cells was determined to be greater than 2 hours, but little additional benefit was noted at incubation times greater than 24 hours. The stability of RFB4(dsFv)PE38 is therefore compatible with the time required for efficient intoxication.
Binding of RFB4(dsFv)PE38 to CD22 antigen on CA46 cells was nearly identical to that of whole RFB4 IgG, although the recombinant molecule is monovalent. Previously, it was shown that a chemically conjugated immunotoxin, RFB4-PE35, bound with identical affinity to RFB4 IgG on Daudi cells.11 It is, therefore, clear that RFB4(dsFv)PE38 and a chemically conjugated RFB4-PE35 are also very similar in ability to bind antigen. RFB4(dsFv)PE38 has slightly improved cytotoxicity towards CA46 cells compared with RFB4-PE35 (0.6 ng/mL v 1 ng/mL) and is equally stable over 24 hours at 37°C, demonstrating that the recombinant RFB4(dsFv)PE38 has all of the advantages of the chemical conjugate in vitro, with the additional benefit that it can be easily prepared in large homogeneous batches.
Because the disulfide-linked immunotoxin was quite stable, had binding properties similar to the whole antibody, and superior cytotoxic effects, it was chosen for further evaluation in animal models. We evaluated its antitumor activity by assessing its ability to prevent formation of tumors after injection of CA46 cells into irradiated nude mice. We chose this model, which has been used previously in testing anti-CD22 immunotoxins, for several reasons. First, the subcutaneous tumors formed by CA46 cells are structurally very similar to human soft tissue lymphomas (unpublished observation, August 1994). This model then may more accurately represent this type of disease than do dissiminated models, which are more similar to leukemic phase lymphomas usually associated with advanced disease and a high tumor burden. Secondly, we would predict that RFB4(dsFv)PE38 would not be effective in the widely-used Daudi/SCID model because Daudi cells are unique in their relative inability to process PE to an active form (Biochemical Soc Transactions, 1997, in press). Finally, this model produces quantitative, rapid, and reproducible results in terms of tumor reduction rather than relying on development of terminal disease for antitumor activity measurement. Using the CA46/nude mouse model, we showed that RFB4 (dsFv)PE38 could eradicate tumor cells when immunotoxin administration began 24 hours after tumor implantation, in contrast to mice that were treated with diluent or a nonrelevant dsFv-PE38 immunotoxin.
Based on the data presented here, it would appear that RFB4(dsFv)PE38 merits further preclinical development as a possible treatment for B-cell lymphoma.
Address reprint requests to David J. FitzGerald, PhD, Laboratory of Molecular Biology, DBS, National Cancer Institute, National Institutes of Health, 37/4E16, 37 Convent Dr, MSC 4255, Bethesda, MD 20892.

![Fig. 3. Cytotoxicity of RFB4(dsFv)PE38 for various cell lines after a 24-hour incubation. [3H]leucine incorporation is expressed as a percentage of cpm incorporated by control cells incubated without immunotoxin. (○), CA46; (▵), JD-38; (+), Raji; (▪), Namalwa; (•), Daudi; (▴), HUT102. Data are representative of at least three trials for each cell line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.2020/4/m_bl_0012f3.jpeg?Expires=1767776507&Signature=ARYXFUUAyRlVIygndRcKTY~b~hKKNnfbKv0uVkRdBRemiArFCkJt3s22C47BNL08Y4psBR2rkbKopLzm8txTzVwB7XR9qFPhEw7qUUon3ZvL1zTQjbvb6Eb9m1gBbzB2oij8w31oqe~VY9IGBl0Qu8Cjy~LYGG4aNUDseXILo9ZSB0xhuqPXRdqo91iTqzWlmNJ4J2AYYJeNKYXQl8JRvhlp6pkUQBcZpl7PaXrypxUXqwQZB5XdmcAc159pMt2tlh4WkIjL-EWnZ5Z9SyjS0Z~V7m8~ggH1ipEL9hN7q9GE~J3KQr-JfFPK8RidIM0ujZeSPlgQXwz7Db9Zx1tHhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal