Abstract
The P-selectin glycoprotein ligand-1 (PSGL-1) is a high-affinity ligand of P-selectin on myeloid cells and certain subsets of lymphoid cells. We generated the rat monoclonal antibody (MoAb) 2PH1 that recognizes an epitope within the first 19 amino acids at the N-terminus of the processed form of mouse PSGL-1. This antibody blocks attachment of mouse myeloid cells to P-selectin under both static and flow conditions. Intravenous administration of saturating amounts of 2PH1 reduced the number of rolling leukocytes in venules of the acutely exposed mouse cremaster muscle by 79% (±5.7%), whereas an anti–P-selectin MoAb reduced it completely. Examining the effect of the MoAb 2PH1 on the recruitment of neutrophils into chemically inflamed mouse peritoneum showed that blocking PSGL-1 inhibited neutrophil accumulation in the peritoneum by 82% (±7%) at 2 hours and by 59% (±7.9%) at 4 hours after stimulation. A similar effect was seen with the MoAb against P-selectin. Simultaneous administration of both antibodies at the 4-hour time point blocked neutrophil accumulation by 86% (±4.2%), arguing for an additional partner molecule for PSGL-1 besides P-selectin. This is the first demonstration of the importance of PSGL-1 in the recruitment of mouse neutrophils into inflamed tissue.
RECRUITMENT OF LEUKOCYTES into inflamed tissue is initiated by the selectins, a family of three carbohydrate-binding cell-adhesion molecules that mediate transient interactions between leukocytes and the endothelium.1,2 These interactions enable leukocytes to roll along the blood vessel wall, a process that is followed by firm adhesion and subsequent transendothelial migration.3 L-selectin is found on most circulating leukocytes, and P- and E-selectin are both expressed on activated endothelium.
All three selectins have been demonstrated to be important for the rolling of neutrophils along the blood vessel wall and the subsequent migration of neutrophils into inflamed tissues. Antibodies against each selectin can partially block rolling in vivo, as shown for L-selectin4,5 and P-selectin.6,7 With antibodies against E-selectin, the effect on rolling was either modest8 or needed simultaneous elimination of P-selectin.9,10 In fact, as shown recently, elimination of E-selectin by gene disruption or by blocking with an antibody only reduced the number of slowly rolling leukocytes.10 Antibodies against each of the three selectins and disruption of any of the three selectin genes lead to reduced neutrophil recruitment to inflamed peritoneum,11-14 although, again, the effect for E-selectin was only observed when P-selectin was simultaneously blocked.
While the physiological role of the selectins in leukocyte rolling and extravasation is well-established by numerous in vivo studies, little is known about the selectin ligands involved in this process. Several glycoprotein ligands of the selectins have been identified in recent years, which have in common that they need to be specifically glycosylated to function as selectin ligands.15-17 The functionally glycosylated forms of the L-selectin ligands GlyCAM-1,18 CD34,19 Sgp200,20 and MAdCAM-121 are locally restricted to high-endothelial venules in lymph node tissue, where they may serve as ligands in the lymphocyte homing process. L-selectin ligands involved in the process of inflammation have not yet been defined.
The E-selectin ligand ESL-1 was identified by affinity isolation as the major ligand on mouse neutrophils.22,23 Although it was found to be involved in the binding of mouse myeloid cells and neutrophils to E-selectin using in vitro adhesion assays24 and was demonstrated to be expressed on the surface of microvillous processes of mouse lymphoid cells,25 in vivo data about the physiologic function of ESL-1 are not yet available. Based on in vitro adhesion assays, human L-selectin was also reported to function as an E-selectin ligand on human neutrophils.26-28 In agreement with these reports, L-selectin could be affinity-isolated from human neutrophils as a major ligand by an E-selectin affinity probe.29 Surprisingly, L-selectin of mouse neutrophils did not bind to E-selectin.29 These biochemical results were confirmed in flow adhesion assays. Since human neutrophils but not mouse neutrophils seem to interact via L-selectin with E-selectin, no in vivo data for the physiologic relevance of the L-selectin/E-selectin interaction are yet available. Other E-selectin ligands defined by indirect evidence17 have not yet been studied in vivo.
The P-selectin glycoprotein ligand-1 (PSGL-1) was found by affinity isolation30 and by expression cloning.31 PSGL-1 requires for its binding activity sulfated tyrosine residues at its N-terminus32-34 plus certain carbohydrate modifications such as sialic acid, fucose,31 and branched carbohydrate side chains generated by the core-2 enzyme.35-37 The monoclonal antibody (MoAb) PL-1 against human PSGL-1 was demonstrated to block attachment of human neutrophils to P-selectin under flow conditions.38 This antibody was also shown to inhibit the rolling of human neutrophils injected into rat mesenteric venules.39 The first evidence of the physiologic importance of PSGL-1 for lymphocyte extravasation was reported recently for mouse Th1 lymphocytes. Recruitment of these cells into inflamed sites of the mouse skin in a delayed-type hypersensitivity reaction was found to be inhibited by affinity-purified rabbit antibodies against an N-terminal peptide of mouse PSGL-1.40 CD24 (also known as heat-stable antigen) was found as another ligand of P-selectin, since the purified protein could bind to P-selectin but not to E-selectin.41 Later, antibodies against CD24 were demonstrated to partially inhibit binding of myeloid cells to P-selectin using in vitro adhesion assays.42 43
Although PSGL-1 is a well-characterized ligand for P-selectin, direct evidence for its importance for neutrophil recruitment into sites of inflammation is still lacking. We have generated the MoAb 2PH1 against mouse PSGL-1, which blocks rolling of mouse neutrophils on P-selectin in flow adhesion assays. We could demonstrate that this antibody partially inhibits the migration of neutrophils into chemically inflamed peritoneum of the mouse. The in vivo effect of 2PH1 was compared with the effect of anti–L- and anti–P-selectin antibodies.
MATERIALS AND METHODS
Cells.The mouse myelomonocytic cell line WEHI-3B, the human monocytic cell line HL60, the mouse myeloma SP2/0, and all hybridomas were grown in Dulbecco's modified Eagle's medium (DMEM; GIBCO-BRL, Eggenstein, Germany) with 10% fetal calf serum (FCS). The mouse neutrophilic progenitor 32Dcl3 was cultured as previously described.22
Antibodies and selectin-IgG chimeras.The rat MoAb 2PH1 against mouse PSGL-1 was generated by immunizing Lewis rats with a peptide covering amino acids 42 to 60 of the sequence of murine PSGL-1, which was conjugated to ovalbumin via an additional N-terminal cysteine as previously described.44 Lymphocytes isolated from the spleen and lymph nodes of immunized rats were fused with the mouse myeloma SP2/0, and hybridoma supernatants were tested in enzyme-linked immunosorbent assays (ELISAs) for binding to the bovine serum albumin (BSA)-conjugated peptide. Cysteine-conjugated BSA was used as a negative control antigen. For purification of MoAbs for in vivo studies, hybridomas were grown in protein-free hybridoma medium (GIBCO-BRL) and antibodies were purified with protein G–Sepharose (Pharmacia, Uppsala, Sweden). The following hybridomas were used: MEL14 anti-mouse L-selectin,45 RB40.34 anti-mouse P-selectin,11 M1/70 anti-mouse Mac-1 (from the ATCC), 2PH1 anti-mouse PSGL-1 (this study), and 9DB3 anti-mouse VCAM-1.46 All antibody preparations were essentially free of endotoxin as tested in a standard Limulus assay (Sigma, München, Germany). The P-selectin-IgG and E-selectin-IgG chimeras were produced as previously described,47 as well as the VE-cadherin-IgG chimera.48 The rabbit antiserum 124 against mouse PSGL-1 was previously described.40 To construct an antibody-like fusion protein of mouse PSGL-1, the cDNA of mouse PSGL-1 was cloned by polymerase chain reaction from RNA of 32Dcl3 cells, and a DNA fragment covering basepairs 1 to 918 of mouse PSGL-1 was fused to a DNA fragment coding for the hinge region and domains C2 and C3 of human IgG1, similarly as previously described.47 This resulted in a fusion protein with mouse PSGL-1 ending with the membrane-proximal glutamine (amino acid 306) in front of the IgG1 hinge region.
Immunoprecipitation.WEHI-3B cells were surface-biotinylated with sulfo-N-hydroxysuccinimide-biotin (Pierce, Oud Beijerland, The Netherlands),40 and cell lysates were subjected to immunoprecipitations as previously described.23 Immunoprecipitated proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, blotted onto nitrocellulose, incubated with streptavidin conjugated to horseradish peroxidase (Dianova, Hamburg, Germany), and developed with the ECL-System (Amersham, Braunschweig, Germany).
Flow cytometry.Cells were incubated in hybridoma supernatant and stained with fluorescein isothiocyanate–labeled goat anti-rat IgG and IgM (Dianova) in phosphate-buffered saline (PBS) with 3% FCS and 0.04% azide. Analysis was performed on a Becton Dickinson FACSCalibur (San Jose, CA) with CellQuest software.
Treatment of 32Dcl3 cells with O-sialoglycoprotease.32Dc13 cells were washed twice in DMEM and resuspended in DMEM at a final concentration of 5 × 106 cells/mL. Then, 50 μL 2.5-mg/mL concentrated stock solution of O-sialoglycoprotease (Cedar Lane, Hornby, Canada) or 50 μL PBS was added to 1 mL cell suspension and incubated for 1 hour at 37°C. Subsequently, the cells were washed twice and subjected to FACS analysis.
Cell adhesion assay under static conditions.Microtiter plates (96 well; Falcon, San Jose, CA) were coated with 10 μg/mL selectin-IgG fusion proteins or human IgG for 1 hour at 37°C and subsequently blocked with 10% FCS in Hanks' balanced salt solution (HBSS) (Biochrom, Berlin, Germany) for another hour at 37°C. After washing the plates with HBSS, 3 × 105 cells in 200 μL HBSS were added per well and incubated for 20 minutes at 4°C under rotation (80 rpm). Subsequently, plates were washed three times with HBSS and fixed with 2% glutaraldehyde (Sigma) in HBSS for 30 minutes on ice. For antibody inhibition studies, 32Dcl3 cells were preincubated with fivefold-concentrated hybridoma supernatants for 30 minutes on ice and washed once before assay. Assays were evaluated by computer-aided image analysis using NIH image software.
Intravital microscopy.Six male C57BL/6 mice older than 8 weeks and weighing between 24 g and 48 g were used in these experiments. The mice were prepared for intravital microscopy as previously described.9 Briefly, they were anesthesized with an intraperitoneal (IP) injection of ketamine hydrochloride 100 mg/kg (Ketalar; Parke-Davis, Detroit, MI) after IP premedication with sodium pentobarbital 30 mg/kg (Nembutal; Abbott Laboratories, North Chicago, IL) and atropine 0.1 mg/kg (American Regent Laboratories, Shirley, NY). Mice received a tracheal cannula to facilitate normal respiration, a jugular cannula for injection of anesthetic and saline, and a carotid cannula for blood pressure monitoring, blood sampling, and antibody (MoAb RB40.34 or 2PH1) injection. During the experiment, the mice were thermocontrolled (model 50-7503 heating pad; Harvard Apparatus, South Natick, MA).
The cremaster muscle was prepared as previously described.9 The epididymis and testes were pinned to the side, leaving the vascular connection to the cremaster intact. During and subsequent to the surgical procedure, the muscle was superfused with thermocontrolled (37°C) bicarbonate-buffered saline (131.9 mmol/L NaCl, 18 mmol/L NaHCO3 , 4.7 mmol/L KCl, 2.0 mmol/L CaCl2⋅2H2O, and 1.2 mmol/L MgCl2 ). Microscopic observations were made using a Zeiss (Thornwood, NY) intravital microscope with a saline-immersion objective (SW 40, 0.75 numeric aperture). Venules with diameters between 17 and 35 μm were observed and recorded through a CCD camera system (model VE-1000 CD; Dage-MTI, Inc, Michigan City, IN) for approximately 60 seconds (Panasonic S-VHS recorder; Osaka, Japan). Centerline red blood cell velocity in the recorded microvessels was measured using a dual photodiode and a proprietary digital on-line cross-correlation program49 on a 486DX2-66 personal computer. Centerline velocities were converted to mean blood flow velocities by dividing the centerline velocity by an empirical factor of 1.6.50 Wall shear rates were estimated based on in vivo velocity profiles as previously described.10 Throughout the experiment, 10-μL blood samples were withdrawn at approximately 45-minute intervals from the carotid catheter and analyzed for leukocyte concentration and two-part differentials after Kimura staining.
Microvessel diameter was measured from videorecordings using a digital image-processing system,49 and the rolling leukocyte flux was determined by counting the number of leukocytes passing through a stationary plane perpendicular to the axis of each venule over a given period. Total leukocyte flux through a given venule was estimated by the product of the systemic leukocyte count and the volumetric flow rate of blood through the venule calculated using the microvessel diameter and the mean blood flow velocity assuming cylindrical geometry. The leukocyte rolling flux fraction is defined as the flux of rolling leukocytes as a percent of total leukocyte flux, and due to this definition, it is independent of variations in systemic leukocyte counts. Leukocyte rolling flux fractions were corrected for hemodynamic variation among individual vessels as previously described.9 51
Cell attachment assay under flow.Assays were essentially performed as previously described.29 Glass cover slips were coated for 2 hours at room temperature with 0.3 μg/mL selectin-IgG fusion proteins or human IgG and subsequently blocked with 5% BSA in HBSS. 32Dcl3 cells (1 × 107/mL) were preincubated at room temperature in 0.5 mL, fivefold-concentrated hybridoma supernatant for 10 minutes. To avoid unspecific activation of rolling cells via Fc-receptor binding to coated selectin-IgG fusion proteins, cells were preincubated with 50 μg/mL soluble human IgG (Sigma) and 0.04% azide. Cells were diluted to a final density of 1 × 106 cells/mL with DMEM containing 10% FCS and 0.04% azide and perfused through a rectangular transparent laminar-flow perfusion chamber containing the protein-coated cover slip.52 The flow rate was adjusted to yield a wall shear stress of 2.1 dyne/cm2. Evaluation was started 90 seconds after starting the pump. The number of rolling cells per field was counted for 10 adjacent areas of 0.25 mm2 each.
In vivo peritonitis model.Experiments were essentially performed as previously described.11 Briefly, 7- to 10-week-old female BALB/c mice were injected intravenously with 50 μg antibody (2 mg/kg body weight) in a total volume of 200 μL PBS, immediately followed by IP administration of 1 mL thioglycollate bouillon (Merck, Darmstadt, Germany). The MoAb 2PH1 was given a second time 2 hours after the first injection to ensure saturating conditions. Accumulated neutrophils in the peritoneum were removed, and their numbers were evaluated.11 Peripheral neutrophil counts were not affected by any of the used antibodies at 2 and 4 hours after injection of the antibodies, and were determined as described previously.11
RESULTS
Generation and characterization of an MoAb against mouse PSGL-1.To generate an MoAb against mouse PSGL-1, we immunized rats with an ovalbumin-conjugated peptide covering amino acids 42 to 60 of the sequence of murine PSGL-1. This sequence had been successfully used by Yang et al53 and Borges et al40 to generate adhesion-blocking polyclonal antibodies against mouse PSGL-1. The sequence starts after the cleavage site of the propeptide, and the analogous region of human PSGL-1 is known to be necessary for P-selectin binding54 and to harbor the binding epitope of the adhesion-blocking MoAb PL-1.55
Hybridoma supernatants were screened in an ELISA for binding to BSA-conjugated peptide. As a negative control, BSA was used carrying the cysteine-conjugated cross-linker alone. In this way, the antibody 2PH1 was found, which recognized the peptide and was classified as a rat IgG1. Binding of this antibody to mouse PSGL-1 was verified in subsequent ELISA using a mouse PSGL-IgG fusion protein as antigen (data not shown). VE-cadherin-IgG was used as a negative control antigen.
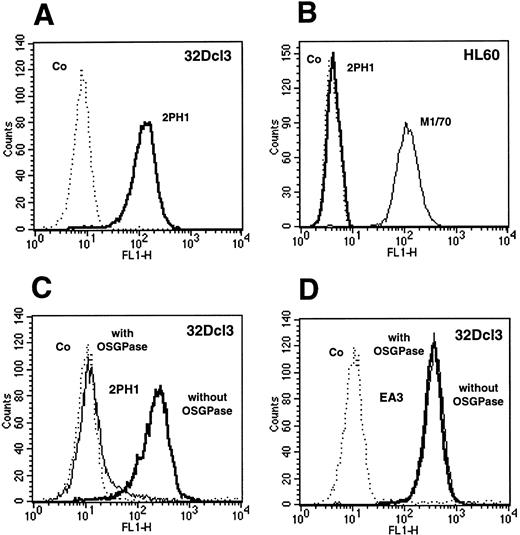
Binding of 2PH1 to the mouse neutrophil progenitor 32Dcl3 and the human monocytic cell line HL60 was analyzed by flow cytometry. Figure 1 shows that a clear signal was obtained with 32Dcl3 cells, while no binding was observed with HL60 cells. Since HL60 cells are known to express human PSGL-1, we conclude that 2PH1 most likely does not cross-react with human PSGL-1, which is in line with the low similarity of the N-termini of mouse and human PSGL-1. The sialomucin PSGL-1 is known to be sensitive to the O-sialoglycoprotease from Pasteurella hemolytica.56 Pretreatment of 32Dcl3 cells with this mucin-specific protease blocked subsequent binding of 2PH1 to these cells (Fig 1C and D).
FACS analysis of 32Dcl3 and HL60 cells with the anti–PSGL-1 MoAb 2PH1. 32Dcl3 cells and HL60 cells (as indicated) were analyzed by flow cytometry with MoAb 2PH1 and control antibodies. Binding of 2PH1 to 32Dcl3 cells (A) and to HL60 cells (B). Positive control staining of HL60 cells with the anti–Mac-1 antibody M1/70 is shown as a faint line. (C and D) 32Dcl3 cells were either pretreated with O-sialoglycoprotease (with OSGPase, faint line) or mock-treated (without OSGPase, bold line) and then analyzed for binding of 2PH1 (C) or binding of the anti–PECAM-1 antibody EA3 (D). All dotted lines (Co) show negative control staining with a nonbinding rat IgG.
FACS analysis of 32Dcl3 and HL60 cells with the anti–PSGL-1 MoAb 2PH1. 32Dcl3 cells and HL60 cells (as indicated) were analyzed by flow cytometry with MoAb 2PH1 and control antibodies. Binding of 2PH1 to 32Dcl3 cells (A) and to HL60 cells (B). Positive control staining of HL60 cells with the anti–Mac-1 antibody M1/70 is shown as a faint line. (C and D) 32Dcl3 cells were either pretreated with O-sialoglycoprotease (with OSGPase, faint line) or mock-treated (without OSGPase, bold line) and then analyzed for binding of 2PH1 (C) or binding of the anti–PECAM-1 antibody EA3 (D). All dotted lines (Co) show negative control staining with a nonbinding rat IgG.
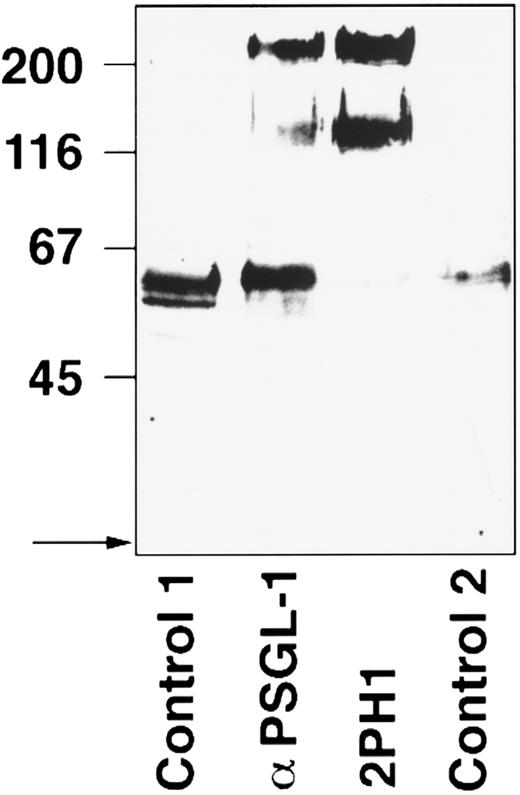
2PH1 was tested in immunoprecipitations with surface-biotinylated mouse WEHI-3B cells. For comparison, the cell lysate was immunoprecipitated with affinity-purified rabbit antibodies against mouse PSGL-1. The polyclonal antibodies and the MoAb 2PH1 recognized the same doublet of 230/130 kD (Fig 2). The 230-kD band represents the nonreduced form of the PSGL-1 dimer, which is difficult to reduce.37 No other specifically recognized protein was detected in these immunoprecipitations. Additional bands at about 50 to 60 kD were nonspecific and were also detected with control antibodies.
PSGL-1 can be immunoprecipitated from WEHI-3B cells with anti–PSGL-1 MoAb 2PH1. WEHI-3B cells were surface-biotinylated and subjected to immunoprecipitations with IgG from a rabbit nonimmune serum (control 1), affinity-purified antibodies from a rabbit antiserum against mouse PSGL-1 (α-PSGL-1), MoAb 2PH1 (2PH1), and an isotype-matched control rat antibody (control 2). Proteins were electrophoresed on an 8% polyacrylamide gel under reducing conditions. The front of the gel is marked by an arrow on the left, and molecular mass markers (in kD) are also indicated on the left.
PSGL-1 can be immunoprecipitated from WEHI-3B cells with anti–PSGL-1 MoAb 2PH1. WEHI-3B cells were surface-biotinylated and subjected to immunoprecipitations with IgG from a rabbit nonimmune serum (control 1), affinity-purified antibodies from a rabbit antiserum against mouse PSGL-1 (α-PSGL-1), MoAb 2PH1 (2PH1), and an isotype-matched control rat antibody (control 2). Proteins were electrophoresed on an 8% polyacrylamide gel under reducing conditions. The front of the gel is marked by an arrow on the left, and molecular mass markers (in kD) are also indicated on the left.
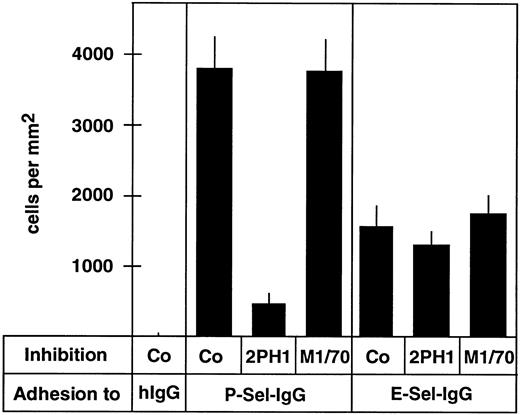
MoAb 2PH1 blocks adhesion of 32Dcl3 cells to P-selectin-IgG under static and flow conditions.To examine whether 2PH1 would block the cell adhesion function of PSGL-1, we analyzed the effect of this antibody on the binding of 32Dcl3 cells to plastic-coated P-selectin-IgG or E-selectin-IgG in 96-well microtiter plates. Figure 3 shows that 2PH1 inhibited the binding of 32Dcl3 cells to P-selectin-IgG by 88% (±4.5%), while no effect was seen with the anti-mouse Mac-1 antibody M1/70. In contrast, cell binding to E-selectin-IgG was not affected by 2PH1. These results are in line with the inhibitory effect of the MoAb PL1 in an analogous human system. PL-1 binds to an epitope between amino acids 49 and 62 of human PSGL-1,55 and was found to block the static binding of human neutrophils to P-selectin but not to E-selectin.57
Binding of 32Dcl3 cells to P-selectin-IgG but not to E-selectin-IgG can be blocked by anti–PSGL-1 MoAb 2PH1. Cell adhesion assays were performed with 32Dcl3 cells in 96-well microtiter plates coated with human IgG (hIgG), P-selectin-IgG (P-Sel-IgG), or E-selectin-IgG (E-Sel-IgG). Before the assay, cells were incubated with fivefold-concentrated hybridoma medium (Co), a fivefold-concentrated hybridoma supernatant of 2PH1 (2PH1), or a fivefold-concentrated hybridoma supernatant of the anti–Mac-1 antibody M1/70 (M1/70).
Binding of 32Dcl3 cells to P-selectin-IgG but not to E-selectin-IgG can be blocked by anti–PSGL-1 MoAb 2PH1. Cell adhesion assays were performed with 32Dcl3 cells in 96-well microtiter plates coated with human IgG (hIgG), P-selectin-IgG (P-Sel-IgG), or E-selectin-IgG (E-Sel-IgG). Before the assay, cells were incubated with fivefold-concentrated hybridoma medium (Co), a fivefold-concentrated hybridoma supernatant of 2PH1 (2PH1), or a fivefold-concentrated hybridoma supernatant of the anti–Mac-1 antibody M1/70 (M1/70).
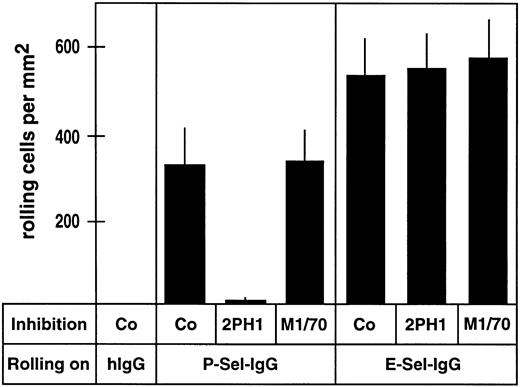
To examine the effect of 2PH1 on rolling of 32Dcl3 cells on P-selectin-IgG and E-selectin-IgG, adhesion assays were performed in parallel plate laminar-flow chambers. In these chambers, 32Dcl3 cells were allowed to flow over cover slips coated with the antibody like fusion proteins or with human IgG as a negative control. The number of rolling 32Dcl3 cells on P-selectin-IgG was reduced by greater than 97% (±2.6%) when cells were preincubated with the MoAb 2PH1, while no effect was seen with the anti-mouse Mac-1 MoAb M1/70 (Fig 4). Again, no significant effect was observed with 2PH1 on the rolling of 32Dcl3 cells on E-selectin-IgG (Fig 4). Thus, 2PH1 blocks the interaction of myeloid cells with P-selectin, but does not interfere with cell binding to E-selectin. Interestingly, in contrast to 2PH1, the anti-human PSGL-1 antibody PL1 was found to reduce the number of rolling human neutrophils on E-selectin–expressing CHO cells.57
Number of rolling 32Dcl3 cells on P-selectin-IgG but not on E-selectin-IgG is blocked by anti–PSGL-1 MoAb 2PH1. Flow adhesion assays were performed with 32Dcl3 cells in a planar laminar-flow chamber containing cover slips coated with human IgG (hIgG), P-selectin-IgG (P-Sel-IgG), or E-selectin-IgG (E-Sel-IgG). Before the assay, cells were incubated with fivefold-concentrated hybridoma medium (Co), a fivefold-concentrated hybridoma supernatant of 2PH1 (2PH1), or a fivefold-concentrated hybridoma supernatant of the anti–Mac-1 antibody M1/70 (M1/70).
Number of rolling 32Dcl3 cells on P-selectin-IgG but not on E-selectin-IgG is blocked by anti–PSGL-1 MoAb 2PH1. Flow adhesion assays were performed with 32Dcl3 cells in a planar laminar-flow chamber containing cover slips coated with human IgG (hIgG), P-selectin-IgG (P-Sel-IgG), or E-selectin-IgG (E-Sel-IgG). Before the assay, cells were incubated with fivefold-concentrated hybridoma medium (Co), a fivefold-concentrated hybridoma supernatant of 2PH1 (2PH1), or a fivefold-concentrated hybridoma supernatant of the anti–Mac-1 antibody M1/70 (M1/70).
Effect of MoAb 2PH1 on leukocyte rolling in venules of the exposed mouse cremaster muscle.Acute exposure of internal tissues, including the mouse cremaster muscle, leads to rapid induction of P-selectin on the surface of endothelial cells due to proinflammatory mediators released from mast cells during surgery.58-60 Within the first 60 minutes after exteriorization of the tissue, leukocyte rolling is almost exclusively mediated by P-selectin.6,7,13 51 We examined the effect of the MoAb 2PH1 on rolling leukocyte flux in venules of the acutely exposed mouse cremaster muscle and compared it with the effect of the anti–P-selectin MoAb RB40.34.
Intravenous administration of 2PH1 resulted in a dose-dependent reduction of leukocyte rolling flux (Table 1). The measured flow velocity, vessel diameter, and wall shear rate were not different between the groups, but systemic leukocyte counts decreased significantly and reproducibly on injection of 2PH1 (Table 1). This was taken into account by determining the leukocyte rolling flux fraction for each vessel. The lowest dose reduced the rolling leukocyte flux fraction by 17% (±13.6%), while MoAb 2PH1 at 2 mg/kg inhibited rolling by about 80% (±5.7%). This level of inhibition was not further increased by elevating the dose to 4 mg/kg, suggesting that a saturating concentration had been reached. In contrast to this partial inhibition, the MoAb RB40.34 completely inhibited leukocyte rolling (Table 1). These data establish that mouse PSGL-1 is of major importance for P-selectin–mediated leukocyte rolling in vivo. The fact that 2PH1 did not block leukocyte rolling completely may suggest that another, yet unidentified P-selectin ligand may exist on mouse leukocytes that may support a minor component of P-selectin–dependent rolling that becomes detectable in the absence of functional PSGL-1 molecules.
Effect of MoAb 2PH1 on Leukocyte Rolling in Venules of the Exposed Mouse Cremaster Muscle
| Treatment . | None . | |||
|---|---|---|---|---|
| . | 2PH1 . | RB40.34 . | ||
| . | 1 mg/kg . | 2 mg/kg . | 1 mg/kg . | |
| Wall shear rate (s−1) | 858 ± 81 | 823 ± 161 | 869 ± 202 | 638 ± 182 |
| Flow velocity (mm/s) | 2.0 ± 0.2 | 1.8 ± 0.3 | 2.5 ± 0.5 | 1.7 ± 0.5 |
| Diameter (μm) | 25.6 ± 1.0 | 24.0 ± 1.5 | 31.5 ± 1.8 | 27.4 ± 2.4 |
| WBC (cells/μL) | 5,990 ± 466 | 3,667 ± 256 | 3,086 ± 197 | 3,245 ± 202 |
| Rolling flux (cells/s) | 1.77 ± 0.20 | 0.79 ± 0.14 | 0.33 ± 0.05 | 0.01 ± 0.01 |
| Rolling flux (%) | 57.85 ± 7.02 | 48.35 ± 7.85 | 11.98 ± 3.31 | 0.41 ± 0.11 |
| Treatment . | None . | |||
|---|---|---|---|---|
| . | 2PH1 . | RB40.34 . | ||
| . | 1 mg/kg . | 2 mg/kg . | 1 mg/kg . | |
| Wall shear rate (s−1) | 858 ± 81 | 823 ± 161 | 869 ± 202 | 638 ± 182 |
| Flow velocity (mm/s) | 2.0 ± 0.2 | 1.8 ± 0.3 | 2.5 ± 0.5 | 1.7 ± 0.5 |
| Diameter (μm) | 25.6 ± 1.0 | 24.0 ± 1.5 | 31.5 ± 1.8 | 27.4 ± 2.4 |
| WBC (cells/μL) | 5,990 ± 466 | 3,667 ± 256 | 3,086 ± 197 | 3,245 ± 202 |
| Rolling flux (cells/s) | 1.77 ± 0.20 | 0.79 ± 0.14 | 0.33 ± 0.05 | 0.01 ± 0.01 |
| Rolling flux (%) | 57.85 ± 7.02 | 48.35 ± 7.85 | 11.98 ± 3.31 | 0.41 ± 0.11 |
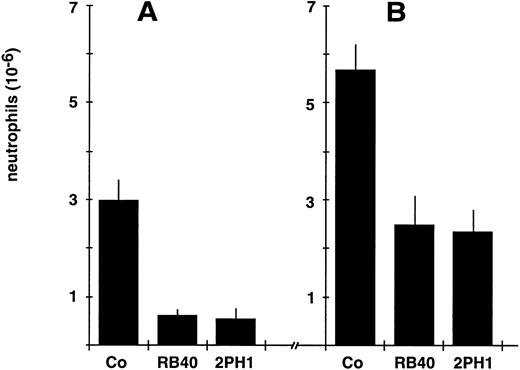
Recruitment of mouse neutrophils to inflamed peritoneum is inhibited by 2PH1.Chemically induced peritonitis has been widely used to study neutrophil recruitment into sites of acute inflammation. Each of the selectins has been shown to be involved in neutrophil recruitment, yet none of the known selectin ligands has been analyzed in such a model. We have examined whether intravenous injection of MoAb 2PH1 immediately before application of thioglycollate into the peritoneal cavity would affect neutrophil accumulation in the peritoneum. The number of accumulated neutrophils in the peritoneal exudate 2 hours after stimulation was reduced by 81.5% (±7.0%) in 2PH1-treated animals compared with control animals treated with the nonblocking antibody 9DB3 against mouse VCAM-1 (Fig 5A). A similar reduction of 79.5% (±3.5%) was determined for animals treated with the anti–P-selectin MoAb RB40.34 (Fig 5A). No difference was observed between 9DB3-treated and PBS-treated animals11,48 (and data not shown). At 4 hours after stimulation, 2PH1 reduced neutrophil accumulation by 58.5% (±7.9%), while RB40.34 reduced it by 56% (±10.6%) (Fig 5B). The decreased importance of P-selectin over time is probably due to the induction of E-selectin and potential cytokine-inducible endothelial ligands for L-selectin, and has been reported.13 Peripheral neutrophil counts at 2 and 4 hours after peritoneal stimulation were not affected by any of the two MoAbs. We conclude that blocking of PSGL-1 and blocking of P-selectin caused a similar reduction of neutrophil recruitment.
Inhibitory effect of the anti–PSGL-1 MoAb 2PH1 on leukocyte recruitment in vivo. Mice were intravenously injected with either 50 μg (in 200 μL PBS) of the nonblocking control antibody 9DB3 against VCAM-1 (Co), 50 μg of the anti–P-selectin MoAb RB40.34 (RB.40), or 50 μg MoAb 2PH1 (2PH1), immediately followed by IP administration of thioglycollate. Peritoneal leukocytes were removed at 2 hours (A) or 4 hours (B) after stimulation, and neutrophil counts were determined. For the 4-hour time point, 2PH1 was injected twice at time 0 and at 2 hours (50 μg each). The data represent the mean ± SEM of ≥ 4 mice in each group. The depicted experiment represents 1 of 3 (for other time points) independent experiments with similar results.
Inhibitory effect of the anti–PSGL-1 MoAb 2PH1 on leukocyte recruitment in vivo. Mice were intravenously injected with either 50 μg (in 200 μL PBS) of the nonblocking control antibody 9DB3 against VCAM-1 (Co), 50 μg of the anti–P-selectin MoAb RB40.34 (RB.40), or 50 μg MoAb 2PH1 (2PH1), immediately followed by IP administration of thioglycollate. Peritoneal leukocytes were removed at 2 hours (A) or 4 hours (B) after stimulation, and neutrophil counts were determined. For the 4-hour time point, 2PH1 was injected twice at time 0 and at 2 hours (50 μg each). The data represent the mean ± SEM of ≥ 4 mice in each group. The depicted experiment represents 1 of 3 (for other time points) independent experiments with similar results.
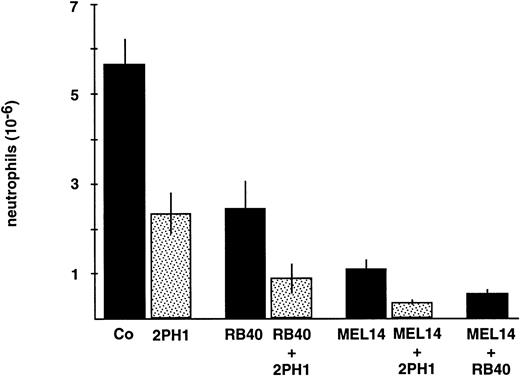
We next analyzed whether the simultaneous blocking of PSGL-1 and L-selectin or P-selectin, respectively, would have an additive effect on neutrophil recruitment. The inhibitory effect of RB40.34 was increased when RB40.34 and 2PH1 were given simultaneously (Fig 6). Similarly, the inhibitory effect of the anti–L-selectin antibody MEL14 was increased by simultaneously applying 2PH1. As previously demonstrated,11 the effects of anti–P- and anti–L-selectin antibodies were also additive (Fig 6). We conclude from the additive effect of the anti–P-selectin and anti–PSGL-1 antibodies that PSGL-1 is likely to interact with yet another partner molecule distinct from P-selectin, and that this interaction is relevant for neutrophil recruitment in vivo.
Effects of antibodies against PSGL-1, P-selectin, and L-selectin on neutrophil recruitment in vivo. Mice were intravenously injected with 9DB3 (Co), 2PH1 (2PH1), anti–P-selectin RB40.34 (RB.40), a mixture of RB40.34 and 2PH1 (RB.40 + 2PH1), anti–L-selectin MEL14 (MEL14), a mixture of MEL14 and 2PH1 (MEL14 + 2PH1), and a mixture of MEL14 and RB40.34 (MEL14 + RB.40). Peritoneal leukocytes were removed at 4 hours after stimulation, and neutrophil counts were determined. Fifty micrograms of each antibody was injected. 2PH1 was injected twice at time 0 and at 2 hours (50 μg each). Data represent the mean ± SEM of ≥ 4 mice in each group. The depicted experiment represents 1 of 3 (for other time points) independent experiments with similar results.
Effects of antibodies against PSGL-1, P-selectin, and L-selectin on neutrophil recruitment in vivo. Mice were intravenously injected with 9DB3 (Co), 2PH1 (2PH1), anti–P-selectin RB40.34 (RB.40), a mixture of RB40.34 and 2PH1 (RB.40 + 2PH1), anti–L-selectin MEL14 (MEL14), a mixture of MEL14 and 2PH1 (MEL14 + 2PH1), and a mixture of MEL14 and RB40.34 (MEL14 + RB.40). Peritoneal leukocytes were removed at 4 hours after stimulation, and neutrophil counts were determined. Fifty micrograms of each antibody was injected. 2PH1 was injected twice at time 0 and at 2 hours (50 μg each). Data represent the mean ± SEM of ≥ 4 mice in each group. The depicted experiment represents 1 of 3 (for other time points) independent experiments with similar results.
DISCUSSION
In this study, we examined whether mouse PSGL-1 is important for the recruitment of neutrophils into inflamed tissue. We generated a MoAb against the N-terminus of mouse PSGL-1 that blocks adhesion of mouse myeloid cells to P-selectin under static and flow conditions. With the help of this antibody, we were able to show that PSGL-1 is responsible for most of the P-selectin–mediated rolling in vivo. Furthermore, this antibody was able to inhibit neutrophil recruitment into the chemically inflamed peritoneum of the mouse to a similar extent as an antibody against mouse P-selectin. This is the first demonstration of the importance of PSGL-1 for neutrophil recruitment in vivo.
Our examination of leukocyte rolling in vivo was made under conditions where P-selectin is the major rolling receptor.6,7,13,51 Consequently, leukocyte rolling was completely abolished by the anti–P-selectin MoAb RB40.34. The MoAb 2PH1 reduced the number of rolling leukocytes in the venules of the exteriorized mouse cremaster muscle by 80%. This strong inhibitory effect shows that PSGL-1 is the major rolling-mediating ligand for P-selectin on mouse leukocytes. This is in line with the study by Norman et al,39 who inhibited the rolling of human neutrophils in rat mesenteric venules with the anti-human PSGL-1 antibody PL1 by about 80%. The fact that MoAb 2PH1 did not completely inhibit leukocyte rolling in vivo, although given in saturating amounts, argues for the possibility that further P-selectin ligand(s) could be involved in leukocyte rolling. A candidate for such a ligand would be the heat-stable antigen CD24, which was reported to mediate neutrophil binding to P-selectin.41-43
In addition to the correct glycosylation of PSGL-1, it is well documented that the N-terminus of mature PSGL-1 protein is important for the adhesion function of human and mouse PSGL-1. Sulfation of tyrosine residues in this peptide sequence is essential for binding to P-selectin,32-34 cleavage of the N-terminal amino acids of human PSGL-1 with a specific protease blocks neutrophil binding to P-selectin54; the adhesion-blocking MoAb PL1 binds to this region55; and polyclonal antibodies against this region of mouse PSGL-1 block binding of myeloid cells to P-selectin.40,53 In light of these data, it is interesting that the anti-human PSGL-1 MoAb PL1 and the anti-mouse PSGL-1 MoAb 2PH1 differ in the ability to interfere with the binding of PSGL-1 to E-selectin. Neither antibody interferes with myeloid cell binding to E-selectin under static conditions; however, PL1 partially blocks rolling of human neutrophils on E-selectin,57 while 2PH1 has no effect on the rolling of 32Dcl3 cells on E-selectin. It has been shown that sulfation of N-terminal tyrosine residues in human PSGL-1 is necessary for P-selectin binding, but not for binding to E-selectin.32,33 36 Thus, it is possible that the N-terminal part of murine PSGL-1 might not even be involved in the binding to murine E-selectin. While this is speculative, our results demonstrate that 2PH1 is a valuable tool for selective inhibition of P-selectin interactions with PSGL-1 without interfering with E-selectin–mediated interactions. Further antibodies against PSGL-1 need to be generated to analyze the physiologic relevance of interactions between E-selectin and PSGL-1.
Although anti–P-selectin and anti–PSGL-1 MoAbs each substantially reduced neutrophil recruitment into the mouse peritoneum to a similar extent, the effects of both antibodies were additive when given simultaneously. Since the anti–P-selectin antibody RB40.34 was used at saturating conditions,11 the additive effects of both antibodies argue for the existence of a second partner molecule for PSGL-1 besides P-selectin. Binding of PSGL-1 to E-selectin23,31,36 and to L-selectin61,62 has been reported. Since 2PH1 does not seem to block interactions with E-selectin, L-selectin is a better candidate to explain the additive effects of 2PH1 and RB40.34. It has been shown recently that neutrophils roll on neutrophils via L-selectin, and this phenomenon has been described as secondary tethering.63-65 Walcheck et al66 have shown that this process is mediated by L-selectin and PSGL-1 and can be blocked in vitro by the MoAb PL1, although blocking by PL1 was not found by others.65 It is intriguing to speculate that 2PH1 might interfere in vivo with the secondary tethering of neutrophils, and that this might be the reason for the additive effects of 2PH1 and RB40.34. Our data do not allow verification of this speculation, but they do suggest that yet another partner molecule of PSGL-1, distinct from P-selectin, is relevant for the process of neutrophil recruitment in vivo.
ACKNOWLEDGMENT
We thank Uta Staufer and Norbert Joswig for excellent technical support in the peritonitis model.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB293) to D.V. and by USPHS Grant No. HL-54136 to K.L.
Address reprint requests to Dietmar Vestweber, PhD, Institute of Cell Biology, ZMBE, Technologiehof, Mendelstr. 11, D-48149 Münster, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal