Abstract
Intrathymic expression of endogenous mouse mammary tumor virus (MMTV)–encoded superantigens (SAg) induces the clonal deletion of T cells bearing SAg-reactive T-cell receptor (TCR) Vβ elements. However, the identity of the thymic antigen-presenting cells (APC) involved in the induction of SAg tolerance remains to be defined. We have analyzed the potential of dendritic cells (DC) to mediate the clonal deletion of Mtv-7-reactive TCR αβ P14 transgenic thymocytes in an in vitro assay. Our results show that both thymic and splenic DC induced the deletion of TCR transgenic double positive (DP) thymocytes. DC appear to be more efficient than splenic B cells as negatively selecting APC in this experimental system. Interestingly, thymic and splenic DC display a differential ability to induce CD4+SP thymocyte proliferation. These observations suggest that thymic DC may have an important role in the induction of SAg tolerance in vivo.
VIRAL SUPERANTIGENS (SAg) are proteins encoded by the 3′ long terminal repeat (LTR) of exogenous or endogenous mouse mammary tumor viruses (MMTV).1,2 MMTV-SAg expression at the cell surface, in association with major histocompatibility complex class II (MHC II) molecules, induces a strong proliferation/deletion response of the SAg-reactive peripheral T cells expressing a particular T-cell receptor (TCR) Vβ chain.3 Within the thymus presentation of MMTV-SAg by specialized thymic antigen-producing cells (APC) results in the generation of tolerance by the clonal deletion of T cells bearing SAg-reactive TCR Vβ elements.2,3 However, although the essential role of B cells in the presentation of MMTV-SAg to SAg-reactive T cells in the periphery has been firmly shown,4 the thymic APC involved in the elimination of SAg-specific clones are still largely unknown. In this regard, for many years B cells have been considered the only APC with capacity for MMTV-SAg expression and presentation, despite several functional studies showing the potential of dendritic cells (DC) as SAg-presenting cells in mixed leukocyte reaction (MLR) as well as in vivo assays.5-7 However, we have recently shown that both thymic and splenic DC express MMTV-SAg at the mRNA level,8 supporting the hypothesis that DC, whose role in the negative selection against conventional antigens has been firmly established,9 could be responsible for the induction of SAg-tolerance. In the present report we have used an in vitro system to analyze the potential of thymic DC in inducing the negative selection of Mtv-7-reactive TCR αβ transgenic thymocytes. Our results show that thymic DC are more effective than B cells in this assay system, suggesting their participation in this process in vivo.
MATERIALS AND METHODS
Animals.BALB/c mice were purchased from IFFA Credo (L'Arbresle, France). Mtv-7+ (Mls-1a) congenic BALB/c (BALB.D2) (H-2d, Mtv-7+) mice were bred from breeding pairs originally obtained from Dr H. Festenstein (London Hospital Medical College, London, UK). P14 αβ TCR transgenic mice (Vα2-Jα -TA31/Vβ8.1-D-Jβ2.4) expressing a TCR with double specificities for lymphocytic choriomeningitis virus (LCMV) and the Mtv SAg10 were obtained from Dr H.P. Pircher (Department of Experimental Pathology, University Hospital, Zürich, Switzerland) and crossed on a BALB/c background. In all experiments, 4- to 6-week-old female mice were used.
Antibodies.The following antibodies were used in this study: KT3-1.1, anti-CD3; GK1.5, 172.4 and CT-CD4, anti-CD4; CT-CD8a, 53-6.7 and 31M, anti-CD8; AT83, anti-Thy-1.2; PC61.5, anti-CD25 (IL-2Rα), H1-2F3, anti-CD69; RA3-6B2, anti-B220; M5-114, anti-MHC II; C1.A3-1, anti-macrophage antigen F4/80; and N418, anti-CD11c.
Isolation of splenic B cells.Splenic B cells were purified by depletion of T cells by complement-mediated cytotoxicity. Splenocytes were incubated with anti–Thy-1 (clone AT83), anti-CD4 (clone 172.4), and CD8 (clone 31M) monoclonal antibodies (MoAbs) for 60 minutes at 4°C, followed by 30 minutes at 37°C after the addition of rabbit complement (Saxon Europe, Suffolk, UK) and DNAse I (1 mg/mL; Boehringer-Mannheim, Mannheim, Germany) to prevent excessive clumping of dead cells and debris. Dead cells were removed by centrifugation on Ficoll-Paque (1.077 g/mL; Pharmacia, Uppsala, Sweden). Analysis of B220, CD4, and CD8 expression confirmed that the B-cell preparation obtained had a purity greater than 95% (data not shown). Contaminating DC and macrophages represent less than 1% and 2%, respectively, as assessed by the expression of CD11c and F4/80 (data not shown).
Isolation of DC.Thymic and splenic DC were purified following an isolation protocol modified from our previously described method.11 Thymuses and spleens were cut into small fragments and then digested with collagenase (0.5 mg/mL) and DNase I (1 mg/mL), (Boehringer-Mannheim) in RPMI 1640 medium (adjusted to mouse osmolarity and supplemented with HEPES buffer, pH 7.2) for 10 minutes at 37°C with continuous agitation. Digested fragments were filtered through a stainless steel screen, and the cell suspension washed in the same medium at 37°C and recovered by centrifugation at 500g for 7 minutes. The cells were then resuspended in cold isosmotic Metrizamide solution (Nyegaard Diagnostics, Oslo, Norway), pH 7.2, density 1.075 g/L, containing 5 mmol/L ethylenediaminetetraacetic acid (EDTA) to dissociate DC-thymocyte complexes, and a low-density cell fraction, accounting for 8% to 10% of the starting cell population, obtained by centrifugation at 1,700g for 10 minutes. Low-density cells were washed in RPMI 1640 medium, resuspended in the same medium supplemented with 5% fetal calf serum (FCS) at 5 × 107 cells/mL and cultured in 35-mm Petri dishes (Nunc, Roskilde, Denmark) for 60 minutes at 37°C. The nonadherent cells were removed by gentle washing with warm RPMI 1640 medium, and the remaining cells, mostly adherent DC and macrophages, were cultured in RPMI 1640 medium with 5% FCS for 12 to 16 hours at 37°C. After this culture period DC were released from the culture surface whereas macrophages remained adherent. The DC-enriched DC preparation collected from the culture supernatant was then depleted of T-lineage cells, B cells, and macrophages by treating the recovered cells for 30 minutes at 4°C with a MoAb cocktail including anti-CD3 (clone KT3-1.1), anti-CD4 (clone GK1.5), anti-IL-2Rα (clone PC61.5), and anti-macrophage antigen F4/80 (clone C1.A3-1) then removing magnetically the unwanted cells after incubation for 30 minutes at 4°C using a 1:1 mixture of anti-rat immunoglobulin (Ig)–and anti-mouse Ig–coated magnetic beads (Dynabeads; Dynal, Oslo, Norway) using an 8:1 bead-to-cell ratio. Analysis of the expression of MHC II, B220, and CD11c (recognized by the MoAb N418) confirmed that the DC preparations obtained had a purity greater than 90% (Fig 1). Contaminating cells were MHC II−/low, B220−, CD11c−, CD3−, CD4−, CD8− cells, ie, double-negative (DN) thymocytes and MHC II−/low macrophages in the thymic DC preparations; granulocytes, NK cells, and MHC II−/low macrophages in the splenic DC preparations (data not shown).
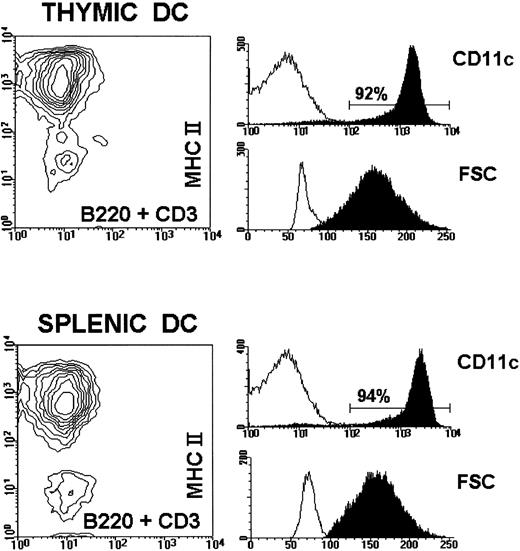
Phenotypic analysis of DC preparations used in this study. Purified thymic and splenic DC from either BALB.D2 or BALB/c mice were tripled stained with PE-conjugated anti-MHC II, FITC-conjugated anti-B220 and anti-CD3, and biotin-conjugated anti-CD11c followed by streptavidin-tricolor. The figure shows the MHC II versus B220 + CD3 contour profile of BALB.D2 thymic and splenic DC, as well as the corresponding histograms for CD11c and FSC (black profiles). White profiles in the CD11c histograms represent streptavidin-tricolor background staining of the DC preparation. White profiles in the FSC histograms of the thymic and splenic DC preparations represent the FSC of total thymocytes and total splenocytes, respectively. The results shown demonstrate that the DC preparations had a purity greater than 90%. Similar results were obtained for DC isolated from BALB/c mice.
Phenotypic analysis of DC preparations used in this study. Purified thymic and splenic DC from either BALB.D2 or BALB/c mice were tripled stained with PE-conjugated anti-MHC II, FITC-conjugated anti-B220 and anti-CD3, and biotin-conjugated anti-CD11c followed by streptavidin-tricolor. The figure shows the MHC II versus B220 + CD3 contour profile of BALB.D2 thymic and splenic DC, as well as the corresponding histograms for CD11c and FSC (black profiles). White profiles in the CD11c histograms represent streptavidin-tricolor background staining of the DC preparation. White profiles in the FSC histograms of the thymic and splenic DC preparations represent the FSC of total thymocytes and total splenocytes, respectively. The results shown demonstrate that the DC preparations had a purity greater than 90%. Similar results were obtained for DC isolated from BALB/c mice.
In vitro deletion assay.Single cell suspensions of thymocytes were prepared by squeezing the whole thymuses through a wire screen. Thymocytes (2 × 105/well) from Mtv-7-reactive P14 αβ TCR transgenic mice were cultured with purified thymic DC or splenic B cells (105/well) from either BALB/c (H-2d, Mtv-7−) or BALB.D2 (H-2d, Mtv-7+), in RPMI 1640 medium supplemented with 10% FCS, in 96-well plates for the indicated times. Cell surface marker analysis of double-positive (DP) thymocytes was performed after removing magnetically the B cells used as APC using anti-mouse Ig–coated magnetic beads (Dynal) to avoid B cell background in the FL3 channel.
Flow cytometry.Purity of the B cell preparation was assessed after double staining with FITC-conjugated anti-B220 (clone RA3-6B2; Caltag, San Francisco, CA) and phycoerythrin (PE)-conjugated anti-CD4 and anti-CD8 (clones CT-CD4 and CT-CD8a, Caltag). Purity of the DC preparation was assessed after triple staining with PE-conjugated anti-MHC II (clone M5-114, Boehringer-Mannheim), FITC-conjugated anti-B220 (clone RA3-6B2, Caltag) and anti-CD3 (clone KT3-1.1), and biotin-conjugated anti-CD11c (clone N418) followed by streptavidin-tricolor (Caltag). Deletion of DP thymocytes was determined after double staining with PE-conjugated anti-CD4 (clone CT-CD4; Caltag) and FITC-conjugated anti-CD8 (clone 53-6.7). Expression of the activation marker CD69 by thymocytes was analyzed after triple staining with tricolor-conjugated anti-CD4 (clone CT-CD4; Caltag), FITC-conjugated anti-CD8 (clone 53-6.7), and biotin-conjugated anti-CD69 (clone H1-2F3) followed by streptavidin-PE (Caltag). Blast formation was estimated by mean of the light forward scatter (FSC) profile; blast cells were defined as cells with FSC greater than 100 (see Fig 5). Propidium iodide was included at 10 μg/mL in the final wash to selectively stain dead cells (Fig 2). All the staining steps were performed at 0 to 4°C in PBS containing 5 mmol/L EDTA and 2% FCS. Analysis was performed on a FACScan (Becton Dickinson, Mountain View, CA) cell analyzer using Lysys II and PC-Lysys softwares (Becton Dickinson) for data evaluation.
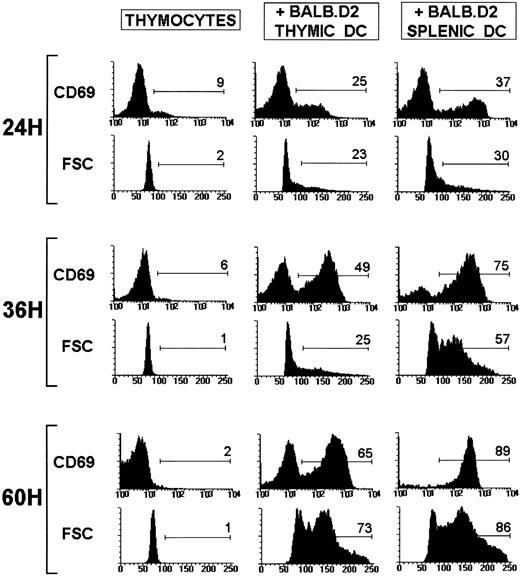
CD69 expression and FSC profiles of viable CD4+ SP thymocytes after culture of TCR transgenic thymocytes alone or with BALB.D2 thymic or splenic DC for 24, 36, and 60 hours. The cells were triple stained with tricolor-conjugated anti-CD4, FITC-conjugated anti-CD8, and biotin-conjugated anti-CD69 followed by streptavidin-PE. The percentage of CD69+ CD4+ SP cells and the percentage of CD4+ SP blasts (cells with FSC < 100) are shown. The experiment shown is representative of a series of three experiments with similar results.
CD69 expression and FSC profiles of viable CD4+ SP thymocytes after culture of TCR transgenic thymocytes alone or with BALB.D2 thymic or splenic DC for 24, 36, and 60 hours. The cells were triple stained with tricolor-conjugated anti-CD4, FITC-conjugated anti-CD8, and biotin-conjugated anti-CD69 followed by streptavidin-PE. The percentage of CD69+ CD4+ SP cells and the percentage of CD4+ SP blasts (cells with FSC < 100) are shown. The experiment shown is representative of a series of three experiments with similar results.
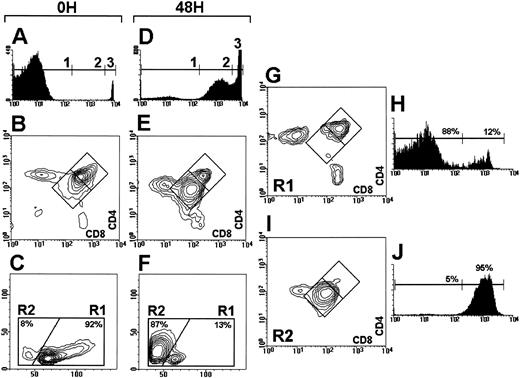
A through E: PI staining (A,D), CD4 versus CD8 expression (B,E), and FSC versus SSC profile (C,F ) of αβ TCR transgenic thymocytes uncultured (A through C), and cultured for 48 hours (D through F ). PIlow, PIint, and PIhigh cells (regions 1, 2, and 3) represented 97%, 0.5%, and 2.5%, respectively in (A), and 10%, 44%, and 46% in (D). (G through J): correlation between CD4 versus CD8 expression and PI staining levels in the live cell gate (R1), and in the apoptotic cell gate (R2) of 48-hour cultured thymocytes.
A through E: PI staining (A,D), CD4 versus CD8 expression (B,E), and FSC versus SSC profile (C,F ) of αβ TCR transgenic thymocytes uncultured (A through C), and cultured for 48 hours (D through F ). PIlow, PIint, and PIhigh cells (regions 1, 2, and 3) represented 97%, 0.5%, and 2.5%, respectively in (A), and 10%, 44%, and 46% in (D). (G through J): correlation between CD4 versus CD8 expression and PI staining levels in the live cell gate (R1), and in the apoptotic cell gate (R2) of 48-hour cultured thymocytes.
Quantitation method.The absolute number of DP and single-positive (SP) thymocytes has been obtained by multiplying the total number of cells per well by the percentage of viable cells, and by the percentage of each thymocyte subset calculated within the total viable cell population.
To allow the direct comparison of the results obtained in different experimental conditions, the percentages shown in Figs 3 and 4 correspond to corrected values, which have been calculated considering DP plus CD4+SP plus CD8+SP equals 100%, ie, excluding DC and DN thymocytes.
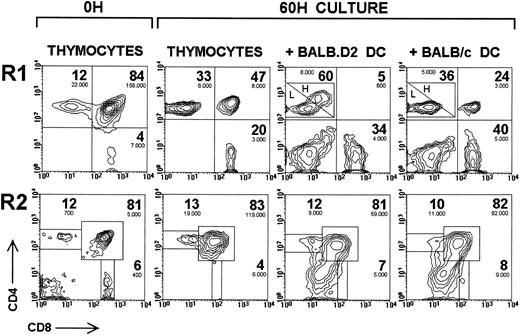
Deletion of αβ TCR transgenic DP thymocytes by thymic DC. CD4 versus CD8 expression in R1 and R2 of αβ TCR transgenic thymocytes uncultured or cultured for 60 hours alone or with thymic DC isolated from BALB.D2 mice or nontransgenic BALB/c littermate mice. The percentage (large figures) and the absolute number per well (small figures) are indicated for each thymocyte subset. The absolute number of DP and SP thymocytes has been obtained by multiplying the total number of cells per well by the percentage of viable cells, and by the percentage of each thymocyte subset calculated within the total viable cell population. The percentages shown correspond to corrected values, which have been calculated considering DP plus CD4+ SP plus CD8+ SP equals 100%, ie, excluding DC and DN thymocytes, to allow the direct comparison of the results obtained in different experimental conditions. The percentage of CD4high SP cells was 50% with BALB.D2 thymic DC and 6% with BALB/c thymic DC. H is CD4high SP cells; L is CD4low SP cells. The experiment shown is representative of a series of four experiments with similar results.
Deletion of αβ TCR transgenic DP thymocytes by thymic DC. CD4 versus CD8 expression in R1 and R2 of αβ TCR transgenic thymocytes uncultured or cultured for 60 hours alone or with thymic DC isolated from BALB.D2 mice or nontransgenic BALB/c littermate mice. The percentage (large figures) and the absolute number per well (small figures) are indicated for each thymocyte subset. The absolute number of DP and SP thymocytes has been obtained by multiplying the total number of cells per well by the percentage of viable cells, and by the percentage of each thymocyte subset calculated within the total viable cell population. The percentages shown correspond to corrected values, which have been calculated considering DP plus CD4+ SP plus CD8+ SP equals 100%, ie, excluding DC and DN thymocytes, to allow the direct comparison of the results obtained in different experimental conditions. The percentage of CD4high SP cells was 50% with BALB.D2 thymic DC and 6% with BALB/c thymic DC. H is CD4high SP cells; L is CD4low SP cells. The experiment shown is representative of a series of four experiments with similar results.
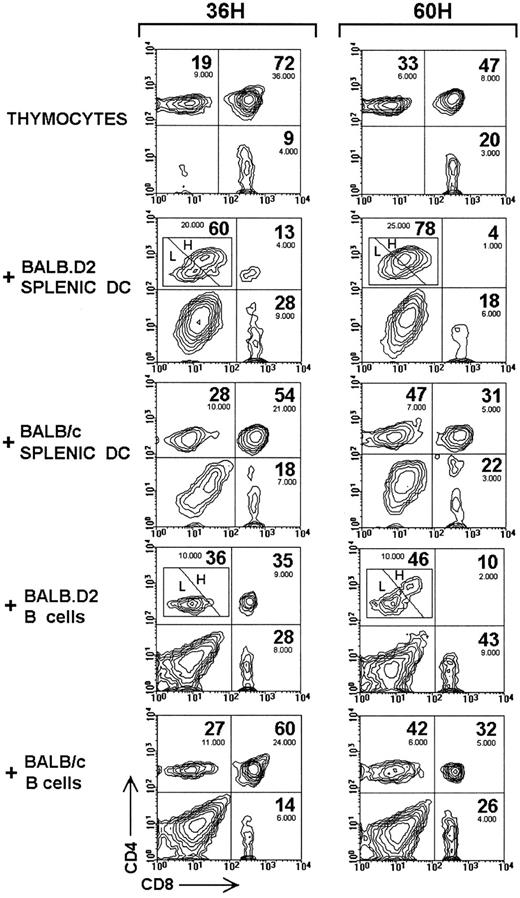
Comparison of the deletion of αβ TCR transgenic DP thymocytes induced by different APC from BALB.D2 or BALB/c mice. CD4 versus CD8 expression in R1 of control viable αβ TCR transgenic thymocytes cultured for 36 hours or 60 hours alone or with splenic DC and B cells from BALB.D2 or BALB/c mice. The percentage of CD4high SP thymocytes (H) at 36 hours and 60 hours was 63% and 75%, respectively with BALB.D2 splenic DC, and 8% and 35% with BALB.D2 splenic B cells. The absolute number of DP and SP thymocytes has been obtained by multiplying the total number of cells per well by the percentage of viable cells, and by the percentage of each thymocyte subset calculated within the total viable cell population. The percentages shown correspond to corrected values, which have been calculated considering DP plus CD4+SP plus CD8+SP equals 100%, ie, excluding DC or B cells and DN thymocytes. The experiment shown is representative of a series of four experiments with similar results.
Comparison of the deletion of αβ TCR transgenic DP thymocytes induced by different APC from BALB.D2 or BALB/c mice. CD4 versus CD8 expression in R1 of control viable αβ TCR transgenic thymocytes cultured for 36 hours or 60 hours alone or with splenic DC and B cells from BALB.D2 or BALB/c mice. The percentage of CD4high SP thymocytes (H) at 36 hours and 60 hours was 63% and 75%, respectively with BALB.D2 splenic DC, and 8% and 35% with BALB.D2 splenic B cells. The absolute number of DP and SP thymocytes has been obtained by multiplying the total number of cells per well by the percentage of viable cells, and by the percentage of each thymocyte subset calculated within the total viable cell population. The percentages shown correspond to corrected values, which have been calculated considering DP plus CD4+SP plus CD8+SP equals 100%, ie, excluding DC or B cells and DN thymocytes. The experiment shown is representative of a series of four experiments with similar results.
RESULTS
FSC versus light side scatter (SSC) profile and CD4 versus CD8 expression of thymocytes in culture.Figure 2 shows the FSC versus SSC profile as well as the CD4 versus CD8 expression and propidium iodide (PI) staining of P14 αβ TCR transgenic thymocytes before and after 48 hours in culture. After PI staining 97% of uncultured thymocytes (Fig 2A) had low levels of PI (PIlow) and consequently displayed a normal CD4 versus CD8 profile (Fig 2B); 92% of them had a live cell FSC versus SSC profile (region R1, Fig 2C). As shown in Fig 2D, after 48 hours in culture 46% of the cells had high levels of PI (PIhigh) and 44% had intermediate levels for PI staining (PIint). A downregulation of CD4 and CD8 expression was observed in 86% of DP thymocytes (Fig 2E), which was paralleled by an accumulation of cells with an apoptotic cell FSC versus SSC profile, ie, reduced FSC and increased SSC (region R2, Fig 2F ). Analysis of the correlation between FSC versus SSC and CD4 versus CD8 profiles after gating off the PIhigh cells indicated that the cells located within the R2 region had intermediate PI levels and decreased CD4 and CD8 expression (Fig 2I and J), whereas viable cells (within the R1 region) displayed normal CD4 and CD8 levels and had low PI levels (PIlow).
Deletion of transgenic DP thymocytes by thymic dendritic cells in vitro.As shown in Fig 3, culture of αβ TCR transgenic thymocytes with thymic DC isolated from BALB.D2 (Mtv-7+) mice induced a strong deletion of DP thymocytes after 60 hours, as assessed by the decrease in their percentage as well as in their absolute number within the R1 gate. In addition, the absolute number of DP thymocytes was also notably reduced in R2 after 60 hours when thymocytes were cultured with BALB.D2 thymic DC compared with thymocytes cultured alone. Interestingly, although the absolute number of SP cells did not undergo significant changes when thymocytes were cultured alone or with BALB.D2 thymic DC, 50% of CD4+ SP cells underwent an upregulation of CD4 expression paralleled by the acquisition of low levels of CD8. Analysis of FSC and CD69 expression of these CD4high SP cells revealed that they correspond to activated CD4+ SP blast cells (data not shown). Activation of peripheral lymph node CD4+ T cells by Mtv-7+ APC also induced the appearance of CD4high activated cells (I. Ferrero, unpublished observations, October 1996), indicating that the CD4high SP thymocytes did not originate from CD8 downregulation of DP when cultured with BALB.D2 thymic DC.
When thymocytes were cultured with syngeneic thymic DC from nontransgenic BALB/c littermates, CD4high SP cells were not observed (Fig 3); however a partial but detectable elimination of DP thymocytes occurred, as evidenced by their percentage and absolute number in the viable and apoptotic cell gates (R1 and R2, respectively). Comparison of the absolute number of viable DP thymocytes cultured alone or with BALB.D2 or BALB/c thymic DC, after 36 and 60 hours, is shown in Table 1. Although a reduction in the DP cell number occurred when thymocytes were cultured with BALB/c thymic DC compared with thymocytes cultured alone, a stronger reduction occurred with BALB.D2 thymic DC, because of the SAg-mediated deletion of DP thymocytes. As very few viable DP thymocytes remained after 60 hours, even when cultured alone, the SAg-specific DP deletion effect, estimated by comparing results with BALB.D2 and BALB/c, was more easily noticeable at 36 hours.
Absolute Number of Viable DP and CD4+ SP Thymocytes per Well at 24, 36, and 60 Hours After Culture of αβ TCR Transgenic Thymocytes Alone or With Different APC From BALB.D2 or BALB/c Mice
| . | 24 h . | 36 h . | 60 h . | |||
|---|---|---|---|---|---|---|
| . | DP . | CD4 . | DP . | CD4 . | DP . | CD4 . |
| Thymocytes cultured alone | 75.400 ± 4.100 | 7.300 ± 400 | 41.000 ± 3.000 | 7.700 ± 550 | 10.300 ± 1.000 | 7.500 ± 600 |
| + Thymic DC | ||||||
| BALB.D2 | 43.900 ± 5.200 | 7.700 ± 450 | 4.700 ± 250 | 8.300 ± 450 | 900 ± 300 | 8.200 ± 600 |
| BALB/c | ND | ND | 23.100 ± 1.200 | 7.900 ± 500 | 4.300 ± 550 | 7.200 ± 700 |
| + Splenic DC | ||||||
| BALB.D2 | 41.000 ± 6.100 | 14.600 ± 700 | 5.500 ± 750 | 24.500 ± 3.100 | 1.100 ± 200 | 28.900 ± 2.700 |
| BALB/c | ND | ND | 24.500 ± 1.900 | 8.100 ± 600 | 5.200 ± 400 | 7.600 ± 800 |
| + Splenic B cells | ||||||
| BALB.D2 | ND | ND | 10.100 ± 950 | 11.100 ± 1.400 | 2.100 ± 200 | 13.000 ± 800 |
| BALB/c | ND | ND | 28.000 ± 1.900 | 7.300 ± 750 | 6.100 ± 650 | 7.700 ± 500 |
| . | 24 h . | 36 h . | 60 h . | |||
|---|---|---|---|---|---|---|
| . | DP . | CD4 . | DP . | CD4 . | DP . | CD4 . |
| Thymocytes cultured alone | 75.400 ± 4.100 | 7.300 ± 400 | 41.000 ± 3.000 | 7.700 ± 550 | 10.300 ± 1.000 | 7.500 ± 600 |
| + Thymic DC | ||||||
| BALB.D2 | 43.900 ± 5.200 | 7.700 ± 450 | 4.700 ± 250 | 8.300 ± 450 | 900 ± 300 | 8.200 ± 600 |
| BALB/c | ND | ND | 23.100 ± 1.200 | 7.900 ± 500 | 4.300 ± 550 | 7.200 ± 700 |
| + Splenic DC | ||||||
| BALB.D2 | 41.000 ± 6.100 | 14.600 ± 700 | 5.500 ± 750 | 24.500 ± 3.100 | 1.100 ± 200 | 28.900 ± 2.700 |
| BALB/c | ND | ND | 24.500 ± 1.900 | 8.100 ± 600 | 5.200 ± 400 | 7.600 ± 800 |
| + Splenic B cells | ||||||
| BALB.D2 | ND | ND | 10.100 ± 950 | 11.100 ± 1.400 | 2.100 ± 200 | 13.000 ± 800 |
| BALB/c | ND | ND | 28.000 ± 1.900 | 7.300 ± 750 | 6.100 ± 650 | 7.700 ± 500 |
Data are indicated as the mean ± SD of three separate experiments. The differences between the absolute numbers of DP thymocytes obtained after coculture with BALB.D2 thymic (or splenic) DC and BALB.D2 splenic B cells were statistically significant as assessed by the Student's t-test (P < .0005 for data at 36 hours; P < .0025 for data at 60 hours).
Abbreviations: DP, double positive thymocytes; CD4, CD4+SP thymocytes; ND, not determined.
Comparative effect of different APC from BALB.D2 or BALB/c mice on the deletion and proliferation of αβ TCR transgenic DP and CD4+ SP thymocytes.Figure 4 shows the CD4 versus CD8 profiles of viable αβ TCR transgenic thymocytes (within the R1 gate) cultured for 36 hours and 60 hours alone or with different splenic APC. These data, together with those shown in Fig 4, show that BALB.D2 splenic DC are as effective as their thymic counterparts in inducing DP thymocyte deletion in vitro. BALB.D2 splenic B cells induced a weaker and slower DP deletion than BALB.D2 thymic or splenic DC. In addition, after 60 hours BALB.D2 splenic B cells induced CD4 upregulation in 35% of CD4+ SP thymocytes, and an increase in the absolute number of this thymocyte subset. Results obtained at 24, 36, and 60 hours with different thymic or splenic APC from BALB.D2 or BALB/c mice are summarized in Table 1.
Concerning the elimination of DP thymocytes occurring in syngeneic culture conditions, reduction in the absolute number of viable DP thymocytes was smaller with syngeneic BALB/c B cells than with syngeneic BALB/c thymic or splenic DC (Table 1).
BALB.D2 splenic DC induced a faster and stronger CD4 up-regulation in the CD4+ SP thymocytes than thymic DC, and therefore than splenic B cells. After 36 hours in culture with BALB.D2 splenic DC, 63% of CD4+ SP were CD4high, and after 60 hours CD4high cells represented more than 75% of CD4+ SP (versus 50% of CD4high cells obtained with BALB.D2 thymic DC). Furthermore, from 24 to 60 hours, the absolute CD4+ SP cell number underwent a strong and progressive increase when thymocytes were cultured with BALB.D2 splenic DC. However, when BALB.D2 thymic DC were used as APC the appearance of CD4high SP cells was not paralleled by an increase in the CD4+ SP cell number (Table 1). BALB/c APC induced neither CD4 upregulation nor increase of CD4+ SP cell number as shown in Figures 3 and 4 and Table 1. Therefore, thymic and splenic DC display a differential capacity to induce the proliferation of CD4+ SP. However, both thymic and splenic DC from BALB.D2 mice activated CD4+ SP thymocytes, as assessed by the appearance of CD4high SP cells (Figs 3 and 4) and by the expression of CD69 and the percentage of blast cells within this thymocyte subset (Fig 5). Interestingly, splenic DC induced a stronger CD4+ SP activation than thymic DC.
As illustrated in Table 2, showing the absolute number of viable DP and CD4+ SP thymocytes at 36 hours after culture with different thymocyte to APC ratios, proliferation of CD4+ SP thymocytes induced by BALB.D2 splenic DC is dependent on the thymocyte to APC ratio. This effect was also observed when splenic B cells were used, although these APC induced a weaker CD4+ SP thymocyte proliferation. In contrast, no significative proliferative response of CD4+ SP thymocytes was observed with different thymocyte to BALB.D2 thymic DC ratios.
Absolute Number of Viable DP and CD4+ SP Thymocytes per Well at 36 Hours After Coculture of αβ TCR Transgenic Thymocytes With Thymic DC, Splenic DC, or Splenic B Cells From BALB.D2 Mice at 2:1, 3:1, 5:1, or 10:1, Thymocyte to APC Ratios
| . | + Thymic DC . | + Splenic DC . | + Splenic B cells . | |||
|---|---|---|---|---|---|---|
| . | DP . | CD4 . | DP . | CD4 . | DP . | CD4 . |
| 2:1 | 4.800 | 8.200 | 5.900 | 24.700 | 9.000 | 10.500 |
| 3:1 | 5.800 | 8.600 | 6.600 | 18.000 | 12.900 | 9.200 |
| 5:1 | 5.400 | 7.800 | 7.500 | 11.300 | 15.900 | 7.800 |
| 10:1 | 6.700 | 7.500 | 7.300 | 10.800 | 16.300 | 7.200 |
| . | + Thymic DC . | + Splenic DC . | + Splenic B cells . | |||
|---|---|---|---|---|---|---|
| . | DP . | CD4 . | DP . | CD4 . | DP . | CD4 . |
| 2:1 | 4.800 | 8.200 | 5.900 | 24.700 | 9.000 | 10.500 |
| 3:1 | 5.800 | 8.600 | 6.600 | 18.000 | 12.900 | 9.200 |
| 5:1 | 5.400 | 7.800 | 7.500 | 11.300 | 15.900 | 7.800 |
| 10:1 | 6.700 | 7.500 | 7.300 | 10.800 | 16.300 | 7.200 |
Cultures were set with 2 × 105 thymocytes and varying numbers of APC. Data are representative of three independent experiments with similar results.
In addition, these data indicate that differences in the thymocyte to DC ratio for both BALB.D2 thymic and splenic DC, ranging from 2:1 to 10:1, determined minor variations at 36 hours in the DP thymocyte number, showing that, even at a 10:1 ratio, DC induced the deletion of DP thymocytes. However, a correlation between thymocyte to APC ratio and the depth of DP deletion was noticed when BALB.D2 splenic B cells were used, indicating the lower efficiency of B cells compared with DC, in inducing the in vitro deletion of DP thymocytes, in agreement with the results shown in Fig 4 and Table 1.
DISCUSSION
The identity of the cells controlling the induction of intrathymic tolerance against viral SAg is a controversial issue of T-cell development. Although thymic epithelial cells have been shown to be involved in the elimination of certain T-cell clones,12,13 different experimental approaches have indicated that thymic DC have an essential role in negative selection of both MHC class I and II restricted T cells with specificities for MHC molecules, minor histocompatibility antigens, viral antigens, and circulating proteins.12,14-18 The negative selection potential of thymic DC for conventional antigens supports the notion that they could also be involved in viral SAg tolerance. In this context, although expression and efficient presentation of viral SAg to peripheral T cells has been considered to be restricted to B cells,4 we have recently shown by reverse transcriptase–polymerase chain reaction (PCR) using MMTV-SAg mRNA specific primers, that both thymic and splenic DC express MMTV-SAg.8 Extending these previous data, in the present report we have described the clonal deletion of Mtv-7–specific P14 αβ TCR transgenic thymocytes by thymic and splenic DC in an in vitro assay.
Thymocytes cultured alone or cocultured with different APC underwent a marked downregulation of CD4 and CD8 accompanied by a decreased FSC. The FSC profile of cultured thymocytes allowed the definition of the regions R1 and R2, corresponding to the live and apoptotic cells, respectively. The correlation between CD4/CD8 downregulation, transition to the R2 region, and induction of apoptosis has been clearly shown by propidium iodide and Hoechst 33342 staining, DNA gel electrophoresis, and electron microscopy of thymocytes treated by etoposide and dexamethasone.19 In addition, reduction of CD4/CD8 expression and decreased FSC profile have been reported in the in vitro deletion of P14 and F5 TCR transgenic thymocytes by antigen,16,17 and in the clonal deletion of thymocytes reacting to the staphyloccocal enterotoxin A or B SAg in vitro.20
On the basis of the percentage and absolute cell number of the different thymocyte subsets in R1 and R2, our results showed that BALB.D2 thymic DC induced the clonal deletion of DP TCR transgenic thymocytes in vitro, and consequently that DC present MMTV-SAg in a functional form. Furthermore, comparison of the negative selection potential of different APC in the same experimental conditions revealed that splenic DC are as efficient as thymic DC, and that DC induced a faster and stronger DP thymocyte deletion than splenic B cells. These data confirm previous reports stating that DC are the most efficient APC in presenting both endogenous and exogenous antigens.21 22 Moreover, DC did not cause SP thymocyte deletion but induced a strong activation of CD4+ SP thymocytes, as shown by their CD69 expression and the percentage of blast CD4+ SP, providing further support for the SAg-presenting potential of DC.
With respect to the elimination of DP thymocytes induced by syngeneic DC, and to a lower extent by syngeneic B cells from BALB/c mice, similar results were obtained in the in vitro deletion of H-Y TCR transgenic thymocytes when APC not expressing the male antigen or the correct MHC molecules were used, although this point was not discussed in these reports.15,23 A partial stimulation of DP thymocytes by syngeneic APC could explain our data, as a syngeneic stimulation of peripheral T cells by DC has been reported in MLR experiments dealing with both Mtv-SAg5 and conventional antigens.24
Activation of CD4 SP thymocytes was accompanied by an increase in their CD4 levels and by the acquisition of low CD8 levels, which allowed the distinction of a CD4high subpopulation of activated cells. Interestingly, BALB.D2 splenic DC induced a stronger CD4+ SP activation than thymic DC, as estimated by the kinetics and size of the CD4high subset, and by the CD69 expression and the percentage of CD4+ SP blast cells. More importantly, whereas no proliferation of CD4+ SP was detected when thymic DC were used, a strong increase in the CD4+ SP cell number was induced by splenic DC. As shown in Table 2, whereas the proliferation of CD4+ SP thymocytes induced by BABL.D2 splenic DC is dependent on the thymocyte to APC ratio, minor variations in the deletion of DP thymocytes were observed at different thymocytes to splenic DC ratios. This is in agreement with previous results showing that negative selection occurs at lower dose of antigen than activation.25
The differential capacity of thymic versus splenic DC to induce the proliferation of CD4+ SP thymocytes is supported by a recent report by Süss and Shortman26 showing in an MLR system that a strong peripheral CD4+ SP cell proliferative response was obtained with CD8− splenic DC, whereas CD8+ DC induced a lesser response associated with marked T cell apoptosis. In this context, although splenic DC can be subdivided in a CD8+ and a CD8− subpopulations, all thymic DC express CD8.27 Therefore, it can be speculated that splenic DC induced activation and proliferation of CD4+ SP thymocytes, whereas CD8+ thymic DC induced activation but no net proliferative response, perhaps because proliferation was accompanied by cell death. The differential activation-proliferation potential of thymic versus splenic DC is currently under investigation in our laboratory.
Our results indicate that in vitro deletion of SAg-negative TCR transgenic thymocytes occurred at the DP stage. In this regard other data concerning the timing of SAg-tolerance in vivo are controversial. Pircher et al16 reported that Mtv-7 reactive (Vβ8.1) TCR transgenic thymocytes were deleted at the SP stage. In support of these results, Surh and Sprent28 have shown by in situ detection of apoptotic cells that deletion of Mtv-8,9-reactive Vβ5 cells occurred in the medulla and cortico-medullary junction, where most SP thymocytes are located. On the other hand, deletion of Mtv-7-reactive Vβ6 thymocytes has been described at the postpositive selection TCRint CD4+8low and CD4low8+ stages.29 Interestingly, Crompton et al30 reported that deletion of Mtv-7-reactive (Vβ8.1) TCR transgenic thymocytes occurred at the CD3high stage irrespective of their DP/SP phenotype, and was therefore dependent on TCR density. In support of this hypothesis, it has been shown that deletion of SAg-reactive Vβ3 DP thymocytes occurred in Vα11-Vβ3 transgenic mice, but not in Vβ3 transgenic mice, where TCR density is lower.31 Collectively, these data suggest that the stage of T cell differentiation at which deletion of SAg-reactive cells takes place depends on both the density of the transgenic or nontransgenic SAg-specific TCR and on the level of expression of the corresponding MMTV SAg. In support of this concept it has been shown that deletion of Vβ3 thymocytes specific for both Mtv-1 and Mtv-6 SAg occurs at different differentiation stages depending on the SAg to B10.BR-Mtv-6 mice delete Vβ3 DP thymocytes, whereas B10.BR-Mtv-1 mice delete Vβ3 SP thymocytes.32 In addition, these authors reported that during ontogeny B10.BR-Mtv-6 mice deleted Vβ3+ T cells by day 2, whereas deletion only occurred at day 15 in B10.BR-Mtv-1 mice. Concerning Mtv-7 reactive P14 TCR transgenic thymocytes, the results obtained in vivo suggest that negative selection occurs at a postpositive selection TCRhigh stage, most likely upon Mtv-7 presentation by DC at the cortico-medullary junction and in the medulla.33 TCR up-regulation occurring on P14 TCR transgenic thymocytes in culture (C. Ardavı́n et al, unpublished observations, October 1996), as well as overexpression of Mtv-7 caused by the high proportion of DC used, may be responsible for deletion occurring at the DP stage in our in vitro assay.
In summary our in vitro data show the SAg tolerance induction potential of thymic DC, thus suggesting their involvement in this process in vivo. In support of this hypothesis, thymic B cells do not seem to be required for SAg tolerance induction, as clonal elimination of TCR Vβ clones occurred in B-cell–deficient mice expressing the corresponding endogenous MMTV-SAg.34 Furthermore, the results obtained by Speiser et al.35 in irradiation bone marrow chimeras showed that clonal deletion of SAg-reactive T cells is controlled by lymphohemopoietic cells and not by thymic epithelial cells. Although this conclusion is challenged by the report of Burkly et al36 that showed that medullary epithelial cells induce partial deletion of Vβ5+ T cells, their data can be explained by MHC II/SAg transfer from epithelial to DC, as previously shown in bone marrow chimeras.37 38 Therefore, our results suggest that thymic DC might have an essential role in the induction of SAg tolerance in vivo.
Supported in part by grants to C.A. from the DGICYT, Ministerio de Educación y Ciencia, Spain and to H.R.M. from the Human Frontiers of Science Program.
Address reprint requests to Carlos Ardavı́n, PhD, Department of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal