Abstract
Leukemia cells may express tumor specific antigens in association with Class I and II major histocompatability complex (MHC) molecules. However, lack of expression of conventional costimulator molecules means that these cells tend to induce specific T-cell anergy rather than activation. CD40 ligand (CD40L) is a costimulator molecule that directly activates T cells and may promote antigen presentation by CD40-expressing cells, which include professional antigen presenting cells and B-acute lymphoblastic leukemia (ALL) cells from many patients. We determined whether transgenic expression of CD40L could enhance an antileukemia immune response using a CD40+ murine lymphoblastic (A20) leukemia and a CD40− myeloblastic (WEHI-3) leukemia in a tumor treatment model. Injection of otherwise nonimmunogenic A20 cells in the presence of CD40L induced an immune response active against preexisting A20 tumor at a distant site. Moreover, concomitant local secretion of transgenic interleukin-2 (IL-2) further amplified the antileukemic response induced and increased protection against preexisting tumor. In ex vivo studies, CD40 activation of A20 cells enhances the antigen presenting potential of A20 cells by upregulating expression of B7.1 (CD80), Class I and II MHC molecules, and increases expression of fas antigens. The importance of CD40 activation to the resulting antitumor response is further emphasized by the failure of transgenic CD40L to protect against the CD40− WEHI myeloblastic leukemia. Depletion studies showed the protective effects against A20 cells to be mediated by a combination of CD4+ and CD8+ T lymphocytes and by natural killer (NK) cells. These results suggest a means by which CD40+ leukemia cells may be rendered immunogenic in vivo.
IT IS EVIDENT that human tumors may express tumor-associated or tumor-specific antigens and that these antigens may then be processed and presented on the cell surface in association with major histocompatability complex (MHC) molecules (reviewed in Henderson and Finn1 ). This observation implies that it should be possible to generate tumor-specific T cells and thereby induce an antitumor immune response. On first consideration, acute lymphoblastic leukemia (ALL) cells should be excellent candidates for such an approach; a high proportion of these leukemic blasts express identifiable malignancy specific fusion proteins or mutant oncogenes, and they also express both class I and class II MHC molecules, enabling presentation of these unique products to both CD8 and CD4 T cells. However, these cells generally lack expression of costimulatory surface molecules such as B7.1 or B7.2, which are necessary for induction of T-cell response. Consequently, they induce specific T-cell anergy instead of specific T-cell activation.2 3
Notwithstanding their lack of conventional costimulatory molecules, 40% or more of ALL express the CD40 antigen, the receptor for the CD40 ligand (CD40L). This ligand is a potent costimulator molecule in its own right. Although CD40-CD40L interactions were first described as playing a key role in B-cell activation and differentiation, it has become increasingly clear that engagement of CD40L also augments the antigen presenting function of B cells and of professional antigen-presenting cells (APC),4-6 and in addition directly stimulates CD4 and CD8 T cells that have become activated by engagement of antigen on APC.5,7-11 We have therefore studied whether transgenic expression of the CD40L molecule in the vicinity of CD40 expressing leukemic blast cells is able to produce a milieu favorable to presentation of leukemia antigens and to generate an antileukemic immune response. We have also investigated whether concurrent local release of interleukin-2 (IL-2) serves to amplify the response further by expanding the leukemia specific T-cell population.12-14 To discover whether or not CD40 expression by leukemia cells is critical for any antitumor activity resulting from transgenic CD40L expression, we repeated these experiments using a CD40− myeloid leukemia cell line. Our results suggest a strategy for combining CD40L and IL-2 for the immunotherapy of CD40+ leukemias.
MATERIALS AND METHODS
Cell lines.The pre-B lymphoblastoid cell line A20, the myeloid leukemia cell line WEHI-3, and the fibroblast cell line CL7.115,16 are derived from BALB/C mice and were obtained from American Type Tissue Collection (ATCC; Rockville, MD).
Vetor construction.To generate a retroviral vector containing the murine CD40L gene, the CD40L cDNA was first cloned by nested polymerase chain reaction (PCR)17 from an activated BALB/C T cell CDNA library made in λecc kindly provided by Dr C. Coleclough (St Jude Children's Research Hospital).18 The primers 5′-GCGGTACCTTTCAGTCAGCATGATAGAAAC and 5′-CTTATTCCAGCTCT-ATGTGCCT were used for the first round of amplification and the same forward primer and the primer 5′-GGATCGATATACGGAAGACTGCCAGC for the second round. The PCR product was subcloned into pGEM7Z (Pharmacia, Piscataway, NJ). DNA sequence analysis confirmed the identity of the cloned CD40L CDNA with the published murine CDNA sequence.19 Subsequently, the plasmid pG1a.mCD40L was created by subcloning the CD40L CDNA into the retroviral vector pG1a (Genetic Therapy Inc [GTI], Gaithersburg, MD) with the CD40L gene driven by the long terminal repeat (LTR) promoter.
The producer cell line BOSC, kindly provided by Dr M. Scott (St Jude Children's Research Hospital), was transfected with the plasmid pG1amCD40L by calcium precipitation as previously described.20 Supernatants were collected from the BOSC cells at confluence and then used to transfect the CL7.1 fibroblast cell line. After transduction in the presence of 6 μg/mL polybrene, fibroblasts were stained with the CD40L antibody (Ab) MR1 (Pharmingen, San Diego, CA) and then sorted on a Facs-Star Plus (Becton Dickinson, Mansfield, MA).
Supernatants containing the retroviral vector G1Na21,22 or G1NaCvI2,23 both generously provided by GTI, were used to transfer the neomycin phosphotransferase gene (neo ) and the IL-2 gene, respectively, into the CL7.1 fibroblast cell line. Transduction was performed at 37°C, 5% C02 in the presence of 6 μg/mL polybrene. The transduced CL7.1 cells were selected in G418 sulfate (GIBCO, Gaithersburg, MD) at an active concentration of 1 μg/mL.
Characteristics of transduced fibroblasts.IL-2 production was assessed by enzyme-linked immunosorbent assay (ELISA) (R & D, Minneapolis, MN) and was 80 to 100 IU IL-2/106 cells over 24 hours. CL7.1 fibroblasts transduced with G1Na were used as controls. A total of 50% to 60% of fibroblasts transduced with PG1mCD40L stably expressed CD40L for a minimum of 4 weeks after selection by fluorescence-activated cell sorting (FACS) and subsequent cell culture; these cells were therefore used within 3 weeks of selection.
Tumor growth in vivo.Experiments to determine the effects of IL-2 and CD40L on tumor growth were performed in female BALB/CBYJ mice aged 12 to 18 weeks (The Jackson Laboratory, Bar Harbor, ME). This system was syngeneic, as both the A20 and CL7.1 cell lines were derived from BALB/C mice. Cells were washed twice in phosphate-buffered saline (PBS) before injection. Trypan blue-negative cells were adjusted to the desired concentrations in a total volume of 200 μL.
For assessment of inhibitory effects on preexisting leukemia, mice were inoculated subcutaneously (SQ) with 1 × 105 A20 or 1 × 105 WEHI-3 cells on day 1. This was double the cell dose shown in preliminary experiments to produce tumors in greater than 90% of control mice. To mimic treatment of established, but nonbulky disease, inoculation was followed on days 4 and 14 by two SQ injections at a distant site consisting of a mixture of 2 × 105 fibroblasts, each expressing the IL-2, CD40L or the neo gene, and 1 × 105 A20/WEHI-3 tumor cells. Immediately before injection, the cell mixture was irradiated to 1,000 cGy.
Tumor volumes were determined by measuring the transverse and perpendicular diameters. Tumors with a measurement greater than 10 mm3 were considered positive. Animals were killed when tumor growth resulted in an increase of more than 30% body weight, in ulceration or in distress. Tumor volumes are reported as mean mm3 ± standard error (SE). The Wilcoxon test was used to compare the tumor inhibitory effects produced by IL-2 in the presence and absence of CD40L.
T-cell depletion.Mice were depleted of T lymphocytes by intraperitoneal (IP) injection of the Gk1.5 (anti-CD4) and 2.43 (anti-CD8) monoclonal antibodies (MoAbs),24 25 kindly provided by Dr Peter Doherty (St Jude Children's Research Hospital). The antibodies were injected IP 1 day before tumor challenge and then every other day for a week, followed by four weekly injections. For depletion of NK cells, mice were injected intravenously (IV) with antiasialo GM1 antibody. After euthanasia of tumor-bearing animals, the depletion status was greater than 98% of the relevant subset, as demonstrated by FACS analysis of splenocytes and peripheral blood.
Coculture experiments.A total of 106 lymphoblastoid A20 cells were cocultured with 2 × 105 fibroblasts expressing either the neo gene as a control or CD40L. After 3 days of culture, A20 cells were stained with B7.1, Fas or MHC Class I or II MoAb, and analyzed by FACS. MoAbs were obtained from Pharmingen (San Diego, CA). To assess the extent of fas-mediated cell death, a cell-killing assay was performed.26 After 3 days of coculture with fibroblasts expressing either the neo gene as a control or CD40L, A20 cells were incubated for 10 hours with 50 ng/mL fas-binding antibody Jo-2 (Pharmingen) or an isotype control Ab in the absence or presence of 30 μg/mL cycloheximide. Dead cells were quantified by FACS analysis after staining with 20 μg/mL propidium-iodide.
Tumor FACS analysis.For APC analysis, the MoAbs specific for CD11c, CD11b (Mac-1), Mac-3, MHC class II (I-Ad/I-Ed), B7.2, and B7.1 were used. For T-lymphocyte analysis, the antibodies used were CD3 and MHC class II and for A20 tumor cell analysis, B220, MHC class I and fas. Appropriate isotype controls were used in all experiments and all antibodies were used as direct conjugates to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) (Pharmingen).
Briefly, tumors at the site of transgenic CD40L expression were surgically removed and single cell suspensions prepared. These were washed three times in PBS containing 1% fetal calf serum (FCS) and 0.01% sodium azide (FACS medium). Approximately 1 to 2 × 106 cells were placed into FACS tubes and incubated with rabbit immunoglobulins for 20 minutes at room temperature. The appropriate MoAbs were then added to the tubes (5 to 10 μL per antibody) and incubated in the dark at room temperature for 10 minutes. Finally, the cells were washed in FACS medium three times before analysis by flow cytometry.
RESULTS
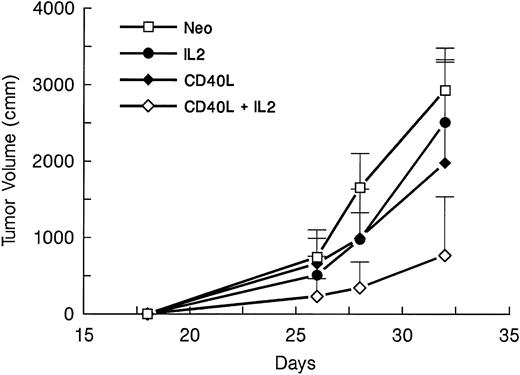
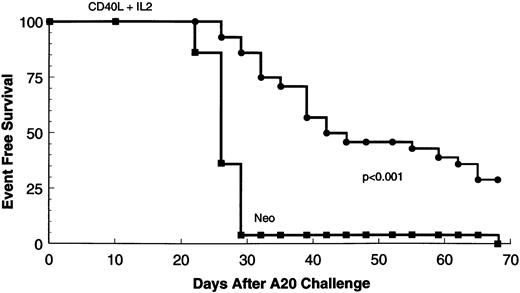
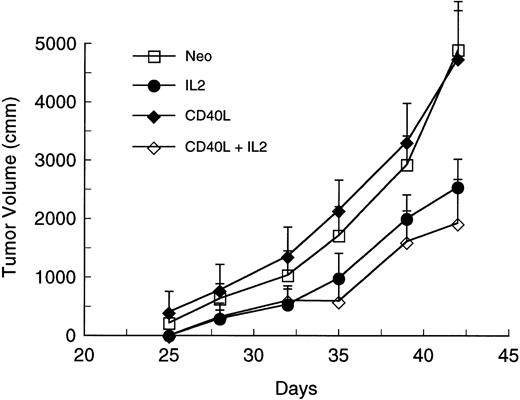
Because most clinical applications of tumor immunogens will be in patients with preexisting disease, we determined whether CD40L alone or in conjunction with IL-2 could elicit an antitumor response against a preexisting tumor at a distal site. Mice were injected with 105 A20 leukemic cells. Four days later, they received a mixture of irradiated 105 parental-type A20 tumor cells and 2 × 105 transduced fibroblasts expressing CD40L or IL-2 alone or in combination. Immunization was repeated after 10 days. Tumor volumes were determined from three dimensional caliper measurements, and mice were killed when the tumor mass exceeded 30% of body weight or when there was evidence of ulceration or distress. Figure 1 shows tumor volumes for each mouse at various times after challenge with A20 cells. The results show that in comparison to the neo control group, tumor growth is delayed in mice receiving gene-modified fibroblasts expressing either CD40L (P < .04) or IL-2 (P < .002). Addition of CD40L expressing fibroblasts to IL-2–secreting cells produces significant additional suppression of tumor growth (Fig 1 and Table 1) (P < .005 for comparison with the IL-2 only group). Figure 2 is a tumor-free survival curve presenting results from a larger group of animals (28/group) and shows that the IL-2/CD40L combination significantly increases long-term tumor-free survival (P < .001) compared with controls.
Effect of irradiated A20 cells mixed with gene-modified fibroblasts expressing CD40L or IL-2 on the growth of preexisting tumor. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, mice received SQ injection of 105 A20 cells mixed with 2 × 105 transduced CL7.1 fibroblasts expressing either the CD40L or IL-2 gene, or neo (control). Before injection, the cell mixture was irradiated to 1,000 cGy. The fourth group of mice was treated with irradiated A20 cells and a combination of fibroblasts expressing either CD40L or IL-2. Tumor volumes are shown as the mean mm3 ± standard error of mean (SEM).
Effect of irradiated A20 cells mixed with gene-modified fibroblasts expressing CD40L or IL-2 on the growth of preexisting tumor. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, mice received SQ injection of 105 A20 cells mixed with 2 × 105 transduced CL7.1 fibroblasts expressing either the CD40L or IL-2 gene, or neo (control). Before injection, the cell mixture was irradiated to 1,000 cGy. The fourth group of mice was treated with irradiated A20 cells and a combination of fibroblasts expressing either CD40L or IL-2. Tumor volumes are shown as the mean mm3 ± standard error of mean (SEM).
Effects of CD40L and IL-2 on Tumor Development and Survival at Day 42
| . | Neo . | CD40L . | IL-2 . | CD40L + IL-2 . |
|---|---|---|---|---|
| Mice with tumor | 7 | 6 | 6 | 3 |
| Mice surviving | 0 | 1 | 2 | 6 |
| Mice inoculated | 7 | 7 | 7 | 7 |
| . | Neo . | CD40L . | IL-2 . | CD40L + IL-2 . |
|---|---|---|---|---|
| Mice with tumor | 7 | 6 | 6 | 3 |
| Mice surviving | 0 | 1 | 2 | 6 |
| Mice inoculated | 7 | 7 | 7 | 7 |
Tumor-free survival of mice receiving neo-transduced fibroblasts (n = 28) or CD40L and IL-2 transduced cells (n = 28). Only in the CD40L and IL-2 group was there discernible long-term tumor-free survival and absence of tumor at necropsy.
Tumor-free survival of mice receiving neo-transduced fibroblasts (n = 28) or CD40L and IL-2 transduced cells (n = 28). Only in the CD40L and IL-2 group was there discernible long-term tumor-free survival and absence of tumor at necropsy.
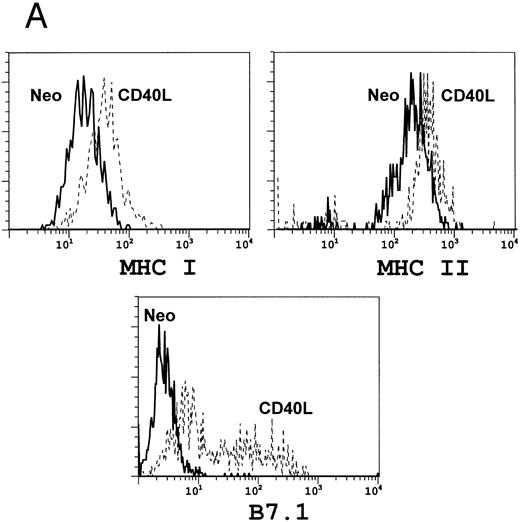
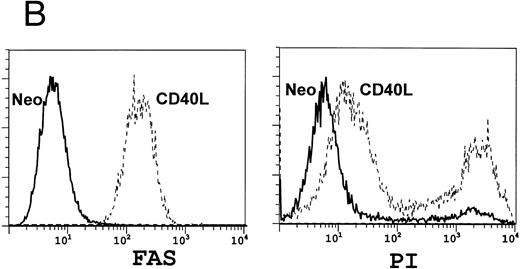
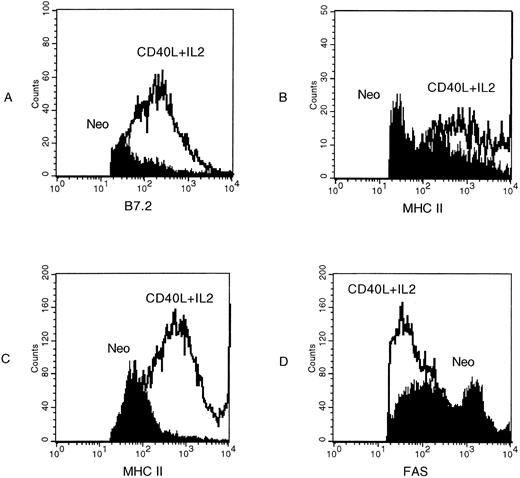
We next analyzed the effects of CD40L expression on the A20 tumor cells. Figure 3A shows that after 3 days of culture in the presence of CD40L (but not neo ) expressing fibroblasts, there is marked upregulation of B7.1 and an increase in MHC Class I and II molecules expression by A20 tumor cells. These phenotypic modifications will favor successful T-cell stimulation and antigen presentation by the tumor cells.27 Figure 3B shows that there is also an increase in fas expression after exposure of the tumor cells to CD40L. Figure 3B also shows that cross-linking of Fas with the MoAb Jo-2 induced cell death, as measured by propidium iodide (PI) incorporation. Addition of cycloheximide increased cell death by an additional 25% to 232%, a characteristic of Fas-mediated apoptosis.28-30 Such predisposition of tumor cells to death signals has been shown to increase the processing and presentation of their antigens by professional antigen presenting cells.31 32
Coculture of A20 cells with fibroblasts expressing CD40L upregulates antigen expression. A total of 106 lymphoblastoid A20 cells were cocultured with 2 × 105 fibroblasts expressing either the neo gene as a control (solid line) or CD40L (broken line). After 3 days of culture, A20 cells were examined for surface antigen expression using FACS analysis. (A) Shows upregulation of B7.1 and of MHC Class I and II, while (B) shows upregulation of FAS (left panel) with a consequent increase in A20 cell death on addition of the FAS-binding antibody Jo-2 (right panel).
Coculture of A20 cells with fibroblasts expressing CD40L upregulates antigen expression. A total of 106 lymphoblastoid A20 cells were cocultured with 2 × 105 fibroblasts expressing either the neo gene as a control (solid line) or CD40L (broken line). After 3 days of culture, A20 cells were examined for surface antigen expression using FACS analysis. (A) Shows upregulation of B7.1 and of MHC Class I and II, while (B) shows upregulation of FAS (left panel) with a consequent increase in A20 cell death on addition of the FAS-binding antibody Jo-2 (right panel).
To determine whether activation of tumor cells through the CD40 pathway was critical for the events observed in vivo, we performed two additional studies. We used fluorescence analysis to determine the phenotype of cells at the site of injected tumor and CD40L expressing cells. These studies showed that transgenic expression of CD40L produced an increase in the proportions of activated APC and T cells (Fig 4A, B, and C). However, in contrast to the ex vivo effects of CD40L stimulation, we found no evidence of in vivo activation of the leukemia cells. Expression of fas (Fig 4D), B7.1, and class I and class II MHC molecules (not shown) were identical to the neo transduced controls. This discrepancy between the ex vivo and in vivo effects of CD40L may indicate that CD40 activation of tumor cells contributes trivially to in vivo antileukemic activity and that activation of CD40+ APC and T lymphocytes are the critical components. Alternatively, it may simply show that CD40 activated, fas positive, tumor cells are rapidly eliminated. The results of our second set of studies lead us to favor the latter explanation. Figure 5 shows that when we use the CD40− WEHI-3 leukemia line in an otherwise identical tumor eradication protocol, transgenic expression of CD40L alone provides no protection and does not add significantly to the protection afforded by transgenic IL-2.
FACS analysis of tumor cells from control (neo ) mice compared with cells from mice treated with gene-modified CL7.1 fibroblasts expressing CD40L. (A) and (B) Show upregulation of B7.2 and MHC class II on CD11a+ antigen presenting cells, while (C) shows upregulation of MHC class II on infiltrating CD3+ T lymphocytes. (D) Shows that there is no upregulation of fas on the surface of B220+ A20 cells in the presence of CD40L.
FACS analysis of tumor cells from control (neo ) mice compared with cells from mice treated with gene-modified CL7.1 fibroblasts expressing CD40L. (A) and (B) Show upregulation of B7.2 and MHC class II on CD11a+ antigen presenting cells, while (C) shows upregulation of MHC class II on infiltrating CD3+ T lymphocytes. (D) Shows that there is no upregulation of fas on the surface of B220+ A20 cells in the presence of CD40L.
Irradiated WEHI-3 cells mixed with gene-modified fibroblasts expressing CD40L have no effect on the growth of preexisting tumor. Four groups of mice (seven per group) were challenged with 105 WEHI cells on day 1. On days 4 and 14 mice received SQ injection of 105 WEHI cells mixed with 2 × 105 transduced CL7.1 fibroblasts expressing either the CD40L or IL-2 gene, or neo (control). Before injection, the cell mixture was irradiated to 1,000 cGy. The fourth group of mice was treated with irradiated WEHI cells and a combination of fibroblasts expressing either CD40L or IL-2. Tumor volumes are shown as the mean mm3 ± SEM.
Irradiated WEHI-3 cells mixed with gene-modified fibroblasts expressing CD40L have no effect on the growth of preexisting tumor. Four groups of mice (seven per group) were challenged with 105 WEHI cells on day 1. On days 4 and 14 mice received SQ injection of 105 WEHI cells mixed with 2 × 105 transduced CL7.1 fibroblasts expressing either the CD40L or IL-2 gene, or neo (control). Before injection, the cell mixture was irradiated to 1,000 cGy. The fourth group of mice was treated with irradiated WEHI cells and a combination of fibroblasts expressing either CD40L or IL-2. Tumor volumes are shown as the mean mm3 ± SEM.
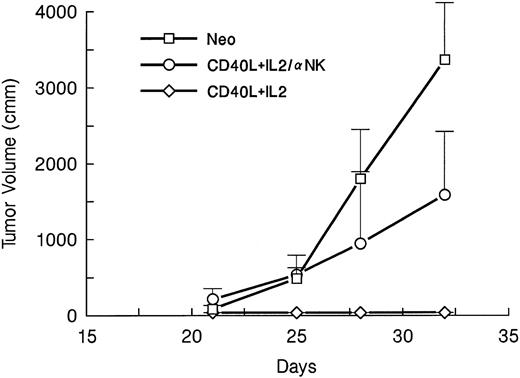
The effector mechanisms responsible for the observed antileukemia effects of the optimal CD40L and IL-2 combination were analyzed by MoAb depletion of effector cell subsets. The animals were treated with depleting doses of either CD4, CD8, or NK-cell antibodies. Mice were then injected with wild-type A20 tumor, followed on days 4 and 14 by irradiated tumor cells and gene-modified fibroblasts expressing CD40L and IL-2. Depletion of either T-cell subset (Fig 6) or of NK cells (Fig 7) reduced the protection afforded by the gene modified cells, so that by day 35, there were no tumor-free survivors in any depletion group, compared with a 71% tumor-free survival in the nondepleted controls. Hence, an intact CD4 and CD8 T lymphocyte and NK cell system is required for optimal immunotherapy in this murine model.
CD4+ and CD8+ T cells contribute to the inhibition of preexisting leukemia following CD40L/IL-2 costimulation. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, the animals were treated with irradiated A20 cells mixed with fibroblasts expressing either the CD40L or IL-2 gene or neo (control). Mice were depleted of T lymphocytes by IP injection of the Gk1.5 (anti-CD4) or 2.43 (anti-CD8) MoAbs 1 day before tumor challenge and again on alternate days for 1 week, and then weekly for 4 weeks. The data are reported as mean mm3 ± SE.
CD4+ and CD8+ T cells contribute to the inhibition of preexisting leukemia following CD40L/IL-2 costimulation. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, the animals were treated with irradiated A20 cells mixed with fibroblasts expressing either the CD40L or IL-2 gene or neo (control). Mice were depleted of T lymphocytes by IP injection of the Gk1.5 (anti-CD4) or 2.43 (anti-CD8) MoAbs 1 day before tumor challenge and again on alternate days for 1 week, and then weekly for 4 weeks. The data are reported as mean mm3 ± SE.
NK cells also contribute to the inhibition of preexisting leukemia following CD40L/IL-2 costimulation. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, the animals were treated with irradiated A20 cells mixed with fibroblasts expressing either the CD40L or IL-2 gene or neo (control). Mice were depleted of NK cells by IV injection of the antiasialo GM-1 MoAbs 1 day before tumor challenge and again on 2 days per week for 2 weeks. The data are reported as mean mm3 ± SE.
NK cells also contribute to the inhibition of preexisting leukemia following CD40L/IL-2 costimulation. Four groups of mice (seven per group) were challenged with 105 A20 cells on day 1. On days 4 and 14, the animals were treated with irradiated A20 cells mixed with fibroblasts expressing either the CD40L or IL-2 gene or neo (control). Mice were depleted of NK cells by IV injection of the antiasialo GM-1 MoAbs 1 day before tumor challenge and again on 2 days per week for 2 weeks. The data are reported as mean mm3 ± SE.
DISCUSSION
There have been many attempts to compensate for the defective antigen presentation process in tumor cells. Tumor cells genetically modified to express immunostimulatory molecules have been shown to induce potent antitumor responses not only against the gene-modified tumor, but also against parental tumor cells. Initial efforts to increase the immunogenicity of tumor cells focused on transfer of genes for many different cytokines,33 including IL-2.12-14 More recently expression of the costimulator molecule, B7.1, has been shown to be protective in a number of animal tumor models.3,34-36 In addition to enhancing the antigen-presenting capacity of tumor cells themselves, other approaches have aimed to improve presentation of tumor antigens by professional APCs, either locally or in the draining lymph nodes.37 Most recently, activation of cultured CD40+ leukemia and lymphoma cells by CD40L has been shown to enable these cells to generate an antitumor response ex vivo.38-40 Our results show that the immunostimulatory effects of transgenic CD40L may also be observed in vivo.
Tumor cells may escape immune surveillance by their lack of costimulatory surface molecules and by downregulation of MHC expression.41 CD40L may overcome both these deficits by promoting tumor antigen-presentation by the malignant cells themselves or by stimulation of professional APCs. Triggering of CD40 on normal B cells promotes their antigen-presenting capacity by upregulating the expression of intercellular adhesion molecules as well as the costimulatory molecule, B7.1 and MHC class I and II molecules.38-40 The A20 pre-B lymphoblastic leukemia cells used in this study show similar changes, so that their coculture with fibroblasts expressing CD40L strongly upregulates B7.1 and produces lesser changes in expression of MHC class I and II antigens. Thus coinjection of nonmodified leukemic cells and fibroblasts expressing CD40L could potentially enhance the immunogenicity of the injected A20 cells by increasing their capacity to present antigen effectively and allowing them to overcome the anergy induced when antigen-specific T cells encounter antigen in the absence of appropriate costimulation.38-40
Interaction of CD40 with the CD40 antigen on A20 cells has additional effects that may further enhance presentation of tumor antigens. In normal B cells, CD40L induces a signal that may either result in cell activation and division or in the upregulation of Apo-1/fas, and increased susceptibility to the delivery of death signals.42 Whether cells divide or die after CD40L stimulation appears critically dependent on whether or not sIg receptors are engaged. It is not yet clear if this same functional dichotomy applies to human ALL blasts, but upregulation of fas by CD40-crosslinking has been described for a human B-cell lymphoma cell line.43 This effect is consistent with our observations in the murine B-leukemia cells studied here. In terms of generating antileukemia activity, fas dependent apoptosis may render tumor cell antigens more readily available for processing and presentation by professional APC40,42,44 and thereby boost their immunogenicity.31 32 We do not know for certain whether the consequences of CD40 activation of leukemia cells contribute significantly to the overall antitumor activity of the ligand in vivo. Examination of CD40+ leukemia cells at the site of transgenic CD40L expression shows no evidence for activation or increased fas expression. However, the relationship of CD40-mediated activation of the leukemia itself to the antitumor effects observed is supported by the observation that transgenic CD40L cannot protect against a myeloid leukemia line (WEHI-3) that is CD40−. Hence, our failure to detect in vivo CD40 activated A20 cells may simply reflect the rapid destruction of these fas-positive cells.
The additional immunostimulatory actions of CD40L may still contribute critically to the antileukemia response, even if they are not in themselves sufficient for significant protection. These additional actions may result in part from direct stimulation of professional APCs, on which CD40 is expressed at high densities.5 Engagement of CD40 upregulates dendritic cell expression of adhesion molecules and of B7.1, maintains high levels of MHC class II expression,6 and prolongs cell survival. Binding of CD40L to dendritic cells also promotes IL-12 release, increasing the function of IL-2 activated cytotoxic T cells and expanding NK cell populations.11 In addition, CD40L may directly stimulate the effector cells of the immune response. Under physiologic conditions, T-cell responses can be amplified by CD40-CD40L interactions between antigen activated T cells that express both receptor and ligand.7,9 10 In the experimental setting we describe, CD40L transduced fibroblasts may substitute as a partner for CD40+ tumor-specific T cells in this stimulatory loop. The presence of greatly increased expression of activation molecules on APC and T lymphocytes in vivo in the presence of transgenic CD40L certainly suggest a contribution from these mechanisms and indicate that cellular interactions at the local tumor/transgenic CD40L inoculation site help initiate the systemic immune response that follows.
The CD40L-dependent response generated to preexisting malignancy is significantly increased by coinjection of cells secreting IL-2 to produce antileukemic activity greater than obtained with either molecule alone. We propose that the ability of CD40L to enhance antigen presentation, to act as a costimulator molecule, and to induce IL-12 release all serve to recruit both T cell and NK cell antitumor effector mechanisms, which IL-2 is then able to further amplify. Certainly, activation of T cells by CD40L favors the growth of CD4+ Th1 cells7-10 able to generate helper function for cytotoxic precursors. This effect will complement the action of IL-2, which allows other cytotoxic precursor cells to bypass their requirement for Th1-derived helper signals in the generation of a cytotoxic T-lymphocyte response.12 Consistent with this suggestion is the observation that an optimal antitumor response requires the presence of CD4+ and CD8+ T lymphocytes, as well as NK cells.
Finally, our results show the feasibility of obtaining an antileukemia response when two complementary immunostimulatory molecules are individually expressed on accessory cells. While preparation of transduced autologous fibroblasts for use in human tumor vaccines is not a minor task,45-47 these cells are significantly easier to grow and transduce than primary tumor cells from many malignancies, including ALL. Hence, this feature should greatly simplify the design and the logistic demands of clinical protocols intended to test the approach in human leukemia.
ACKNOWLEDGMENT
We acknowledge Nancy Parnell for word processing. We also thank the personnel from the Animal Resource Center for technical assistance.
Supported in part by National Institutes of Health (NIH) Grant No. CA 58211, Cancer Center Support Core Grant No. CA 21765, the American Lebanese Syrian Associated Charities (ALSAC), the Assisi Foundation, and the German National Research Foundation (Deutsche Forschungsgemeinschaft) Di 583/1-1, Munich, Germany.
Address reprint requests to Malcolm Brenner, MD, PhD, FRCP, FRCPath, Director, Cell and Gene Therapy Program, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal