Abstract
To date, several activating mutations have been discovered in the common signal-transducing subunit (hβc) of the receptors for human granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Two of these, FIΔ and I374N, result in a 37 amino acid duplication and a single amino acid substitution in the extracellular domain of hβc, respectively. A third, V449E, results in a single amino acid substitution in the transmembrane domain. Previous studies comparing the activity of these mutants in different hematopoietic cell lines imply that the transmembrane and extracellular mutations act by different mechanisms and suggest the requirement for cell type-specific molecules in signalling. To characterize the ability of these mutant hβc subunits to mediate growth and differentiation of primary cells and hence investigate their oncogenic potential, we have expressed all three mutants in primary murine hematopoietic cells using retroviral transduction. It is shown that, whereas expression of either extracellular hβc mutant confers factor-independent proliferation and differentiation on cells of the neutrophil and monocyte lineages only, expression of the transmembrane mutant does so on these lineages as well as the eosinophil, basophil, megakaryocyte, and erythroid lineages. Factor-independent myeloid precursors expressing the transmembrane mutant display extended proliferation in liquid culture and in some cases yielded immortalized cell lines.
INTERLEUKIN-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF ) are cytokines that enhance the survival, proliferation, differentiation, and functional activation of cells of the hematopoietic system. Whereas IL-5 exerts these effects almost exclusively on cells of the eosinophil lineage, GM-CSF effects primarily neutrophilic and monocytic cells and IL-3 effects hematopoietic cells from all five myeloid lineages, erythroid cells, and their progenitors.1
The high-affinity receptors for human IL-3, IL-5, and GM-CSF are composed of two membrane-spanning subunits: a specific α chain that binds each ligand with low affinity, but cannot signal, and a common β subunit (hβc) that is required for high-affinity binding and signalling through the receptor.2-4 Both α and β subunits are members of the cytokine receptor superfamily, the members of which are characterized by an extracellular cytokine receptor module of about 200 amino acids containing several conserved motifs, including the hallmark WSXWS (Trp-Ser-Xaa-Trp-Ser) motif.5 No known enzymatic domains are present in the intracellular domains of members of this family. Rather, the tyrosine phosphorylation induced by binding of IL-3, IL-5, and GM-CSF is due, at least in part, to activation of the Jak2 tyrosine kinase, which is constitutively associated with a membrane-proximal region of the intracellular domain of hβc.6,7 Upon activation, Jak2 phosphorylates several substrates, including the STAT5 transcription factor and the membrane-distal portion of hβc.8 Tyrosine phosphorylation of hβc allows binding of proteins containing src homology 2 (SH2) domains, including hematopoietic cell phosphatase and Shc,9,10 the binding of which leads to activation of the Ras/MAPK pathway.11 12
It is now well documented that abrogation of the growth factor dependence of hematopoietic cells can be a step in leukemogenesis. All genes so far tested that abrogate the growth factor requirement of hematopoietic cell lines such as FDC-P1 confer tumorigenicity on such cells.13 Moreover, there is abundant clinical evidence to support this concept. Blast cells in acute myeloblastic leukemia frequently produce and respond to GM-CSF, reducing or eliminating their requirement for exogenous growth factors.14 Similar observations have been made concerning juvenile chronic myelogenous leukemia and acute lymphoid leukemia.15 16 It is possible that constitutively active mutant cytokine receptors could similarly contribute to such leukemic states through a nonautocrine mechanism.
It has been shown previously that activated forms of cytokine receptors can be leukemogenic. A point mutation in the erythropoietin (Epo) receptor, converting arginine-129 to cysteine, results in a constitutively active form17 that is leukemogenic when expressed retrovirally in mice.18 Furthermore, v-mpl, an amino truncated form of the thrombopoietin receptor c-Mpl, has been transduced by the murine myeloproliferative leukemia virus, which causes a broad spectrum of myeloid and erythroid leukemias.19-21
It is therefore possible that constitutively active hβc mutants could contribute to myeloid leukemias. Indeed, several activating mutations have recently been discovered in hβc that enable it to transmit proliferative signals in the absence of cytokine. One of these mutations, termed FIΔ, results in a 37 amino acid duplication in the extracellular domain, including the WSXWS motif and an adjacent conserved basic region.22 A second mutation, I374N, results in substitution of an extracellular isoleucine residue with asparagine. A third mutation, V449E, results in substitution of a valine residue in the transmembrane domain with glutamic acid.23 These mutants were all discovered via their ability to confer growth factor independence and tumorigenicity on the otherwise GM-CSF– or IL-3–dependent, nontumorigenic murine myeloid cell line, FDC-P1. Interestingly, when expressed at comparable levels in the IL-3–dependent murine pro-B – cell line, BAF-B03, the transmembrane but neither of the extracellular mutants could confer factor independence.23 This implies that the extracellular and transmembrane hβc mutants signal via different mechanisms and that the extracellular mutants require other, perhaps myeloid-specific signalling molecules to deliver a mitogenic signal. Moreover, when expressed in the murine IL-2–dependent T-cell line CTLL-2, none of the mutants could confer factor independence; however, CTLL-2 cells expressing the high-affinity human GM-CSF receptor (hGM-CSFR) could be induced to proliferate on addition of human GM-CSF.23 Hence, V449E also fails to signal in a cell type that responds to the high-affinity hGM-CSFR, implying that this mutant also requires cell-type specific molecules for function.
In light of the apparent differences in signalling between the different hβc mutants, we wished to examine and compare the effects of constitutively active hβc mutants on the growth and differentiation of primary hematopoietic cells and hence gain insight into their leukemogenic potential. We show here that, whereas retroviral expression of either extracellular hβc mutant confers factor-independent proliferation and differentiation on cells of the neutrophil and monocyte lineages only, expression of the transmembrane mutant does so on all five myeloid lineages as well as on erythroid cells and can lead to immortalization.
MATERIALS AND METHODS
Cytokines.Recombinant murine GM-CSF (mGM-CSF ) was obtained and used as crude yeast supernatant, kindly supplied by Dr Tracy Wilson (Walter and Eliza Hall Institute, Melbourne, Australia). Recombinant murine IL-3 (mIL-3) produced from a baculovirus vector was kindly supplied by Dr Andrew Hapel (John Curtin School of Medical Research, Canberra, Australia). Recombinant human Epo (hEpo) was purchased from Janssen Cilag (Lane Cove, New South Wales, Australia).
Cells lines and cDNAs.Ψ2 ecotropic retrovirus packaging cells24 were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Transfected pools were maintained in the above medium plus 200 μg/mL G418 (geneticin; GIBCO, Grand Island, NY). Factor-independent cell lines derived from fetal liver liquid cultures were maintained in Iscove's modified Dulbecco's medium (IMDM) supplemented with 15% FCS.
The hβc cDNA used here was that described by Barry et al.25 The FIΔ,22 V449E,23 and I374N23 hβc mutants have been described previously.
Expression of hβc cDNAs in primary hematopoietic cells.Ψ2 retrovirus packaging cells were transfected with the RufNeo retroviral vector26 containing wild-type or mutant hβc cDNAs. Transfected Ψ2 cells were selected in 400 μg/mL G418 and pooled. The resultant pools were selected for cell surface expression of hβc by flow cytometry, as described below, and used to infect murine fetal liver cells obtained from 14-day pregnant CBA mice. This was achieved by cocultivating 3 × 106 fetal liver cells with 3 × 106 irradiated (30 Gy) Ψ2 producer cells for 48 hours in 75-cm2 flasks in IMDM containing 15% FCS, 500 U/mL mIL-3, 500 U/mL mGM-CSF, and 2 U/mL hEpo. The fetal liver cells were harvested, washed three times, and placed in liquid culture medium or semisolid methyl-cellulose medium. Where indicated, 500 U/mL IL-3, 500 U/mL GM-CSF, 2 U/mL hEpo, and 1 mg/mL G418 were added.
Liquid culture of fetal liver cells.Fetal liver cells were cultured in IMDM containing 15% FCS with growth factors and G418 as described above. For total cell counts, nonadherent cells were decanted and adherent cells were washed in DMEM and incubated in DMEM containing 0.4% Lidocaine (Sigma, St Louis, MO) for 3 to 5 minutes. Cells were then agitated, FCS was added, and the cells were washed twice in DMEM containing 10% FCS. These cells were then combined with the nonadherent cells and cell counts were performed.
Colony assays.Fetal liver cells (2 × 105) were cultured in IMDM containing 1.4% α-methyl-cellulose, 25% FCS, and 1% bovine serum albumin. Colonies containing greater than 50 cells were scored at day 7 of culture. To assess colony types, individual colonies were removed at days 7 through 11 of culture, air spread on glass slides as described by Metcalf,26a and stained with May-Grünwald-Giemsa and the colony types were determined microscopically.
Cell sorting and surface marker analysis by flow cytometry.Transfected Ψ2 pools were selected for hβc expression by cell sorting on a FACStarPLUS flow cytometer (Becton Dickinson, San Jose, CA). Briefly, cells were incubated with the anti-hβc monoclonal antibody (MoAb) 4F327 for 20 minutes on ice, washed, and subsequently incubated with a fluorescein isothiocyanate (FITC)-conjugated sheep antimouse IgG antibody (Silenus Laboratories, Hawthorn, Victoria, Australia) for 20 minutes on ice. Cells were then washed and resuspended in DMEM 2% FCS and hβc-positive cells were collected.
Rat antimouse Mac-1,28 Gr-1,29 Thy-1.2,30 and F4/8031 monoclonal antibodies are those described previously. Cell surface expression of lineage markers on infected fetal liver cells was determined by flow cytometric analysis using an Epics-Profile II analyzer (Coulter, Hialeah, FL). Adherent and nonadherent cells were harvested as described above at day 24 of culture, incubated with the above-mentioned antibodies as described, and then incubated with an FITC-conjugated antirat IgG antibody (Silenus). Expression of hβc mutants on the cell surface fetal liver cells was confirmed by staining with the MoAb 4F3 as described above.
Polymerase chain reaction (PCR) amplification of mutant hβc cDNA fragments from factor-independent cells.PCR reactions were performed directly from whole cell lysates essentially as described by Cassel et al.32 Briefly, 5 × 103 cells were washed in phosphate-buffered saline and resuspended in 50 μL H2O. Samples were heated at 100°C for 10 minutes, 0.4 mg proteinase K was added, and the samples were incubated at 37°C for 1 hour. Samples were incubated at 100°C for 10 minutes and 10 μL of this mix was used in a PCR reaction. PCR was performed with primers spanning the regions of hβc affected by the mutations studied as described by Jenkins et al.23
RESULTS
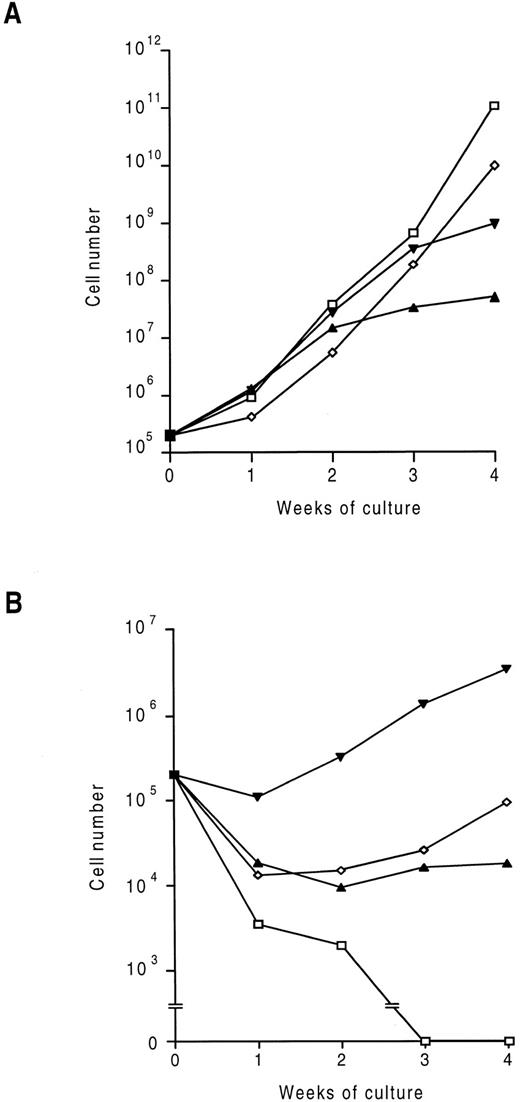
Generation of factor-independent fetal liver cells.To investigate the effect of expression of activated hβc mutants on hematopoietic cell growth and differentiation, three separate constitutively active mutant hβc subunits were expressed in murine fetal liver cells using the RufNeo retrovirus.26 As described in the Materials and Methods, fetal liver cells were cocultivated with ecotropic retrovirus packaging cells producing RufNeo containing either a wild-type or mutant hβc cDNA (as a control, parallel cocultivations were performed with untransfected Ψ2 cells). These cocultivations were performed in the presence of saturating concentrations of IL-3, GM-CSF, and Epo to maximally stimulate survival and growth of a broad range of progenitor cells. When subsequently placed in liquid culture without exogenous growth factors, fetal liver cells infected with a retroviral construct containing wild-type hβc rapidly died (Fig 1), as did mock-infected controls (data not shown). When cells infected with viruses encoding any of the three mutant hβc subunits were placed in culture without exogenous growth factors, some cell death was observed (Fig 1). This was presumably due to some cells not being infected or not expressing sufficient levels of mutant hβc subunits to allow factor-independent proliferation. However, a subset of each of these populations was able to proliferate in the absence of growth factors. The factor-independent response was greatest from cells infected with the V449E vector, but was less in all cases than that obtained with added GM-CSF, IL-3, and Epo.
Time-course of proliferation of infected fetal liver cells in liquid culture. Cells were infected with RufNeo retroviral constructs containing the indicated hβc subunits as described in the Materials and Methods. Cells were then washed and 2 × 105 cells were cultured with (A) and without (B) 500 U/mL mIL-3 and mGM-CSF and 2 U/mL hEpo. Cell counts were performed at weekly intervals. (□) hβc; (▴) FIΔ; (▾) V449E; (⋄) I374N.
Time-course of proliferation of infected fetal liver cells in liquid culture. Cells were infected with RufNeo retroviral constructs containing the indicated hβc subunits as described in the Materials and Methods. Cells were then washed and 2 × 105 cells were cultured with (A) and without (B) 500 U/mL mIL-3 and mGM-CSF and 2 U/mL hEpo. Cell counts were performed at weekly intervals. (□) hβc; (▴) FIΔ; (▾) V449E; (⋄) I374N.
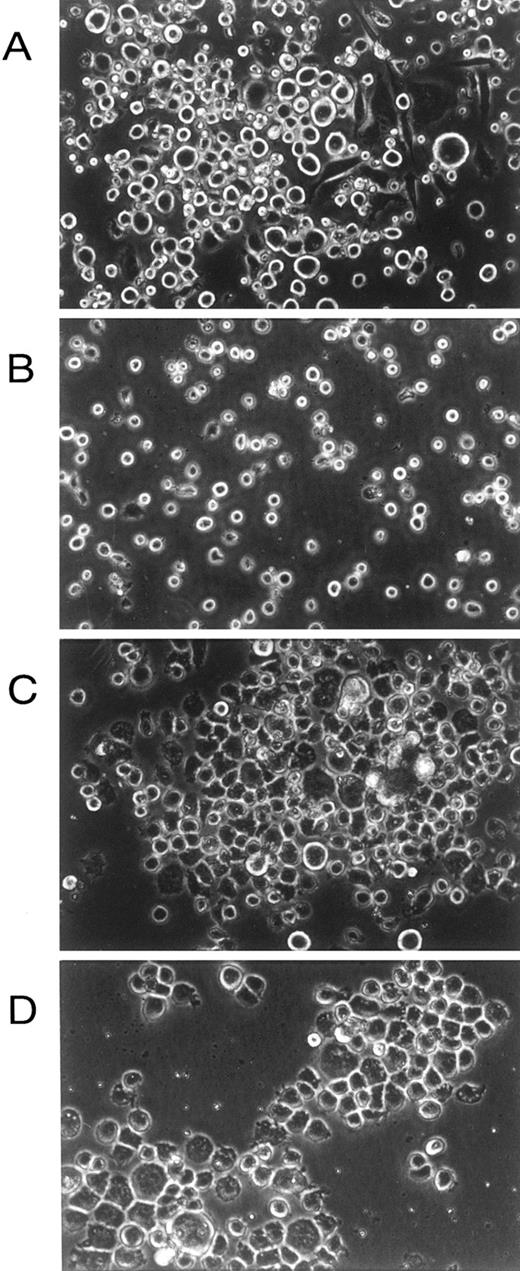
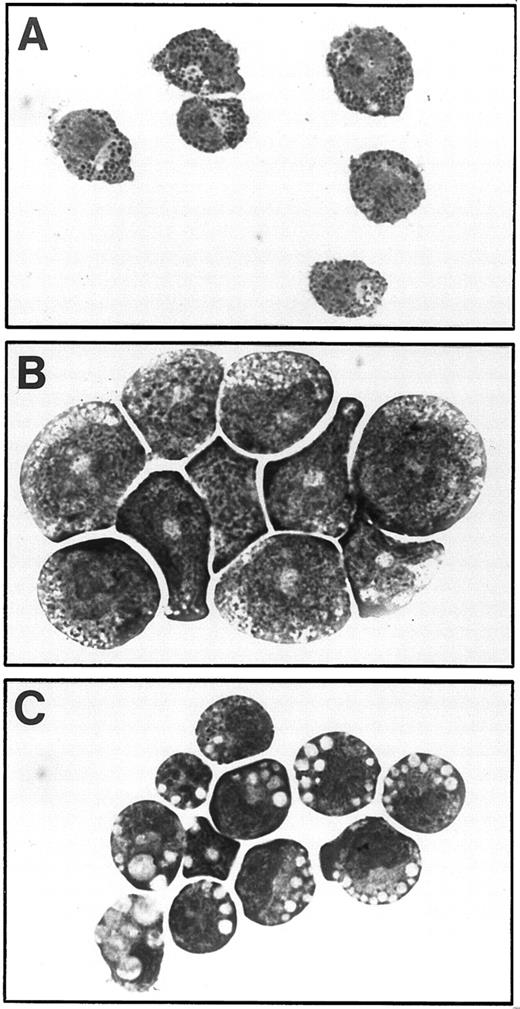
Examination in situ of the factor-dependent and -independent cells showed that, whereas cells grown in the presence of growth factors contained a mixture of adherent and nonadherent cells of various morphologies (as expected; Fig 2A), factor-independent cells containing the transmembrane V449E mutant were almost exclusively small, nonadherent cells (Fig 2B). In contrast, cultures infected with either extracellular hβc mutant (FIΔ and I374N) showed an initial small flourish of nonadherent cell growth; however, these cells died after approximately 11 days, leaving an exclusively adherent cell population that grew in colonies and morphologically resembled macrophages (Fig 2C and D). Hence, an apparently mutually exclusive growth pattern was obtained from primary cells infected with constitutively active transmembrane and extracellular hβc mutants.
In situ morphology of fetal liver liquid cultures. (A) Cells infected with wild-type hβc retroviruses grown in the presence of growth factors (mIL-3, mGM-CSF, and hEpo). (B) Cells infected with V449E retroviruses grown in the absence of growth factors. (C) Cells infected with FIΔ retroviruses grown in the absence of growth factors. (D) Cells infected with I374N retroviruses grown in the absence of growth factors. Photographs are at 300× original magnification and were taken at day 14 of culture.
In situ morphology of fetal liver liquid cultures. (A) Cells infected with wild-type hβc retroviruses grown in the presence of growth factors (mIL-3, mGM-CSF, and hEpo). (B) Cells infected with V449E retroviruses grown in the absence of growth factors. (C) Cells infected with FIΔ retroviruses grown in the absence of growth factors. (D) Cells infected with I374N retroviruses grown in the absence of growth factors. Photographs are at 300× original magnification and were taken at day 14 of culture.
To confirm that the factor-independent cells obtained in these experiments did in fact express mutant hβc subunits, these cells were stained with the anti-hβc MoAb 4F327 and flow cytometric analyses were performed. As shown in Fig 3A, hβc was detected on the surface of all factor-independent cells infected with the hβc mutants. To verify that the hβc subunits expressed on the factor-independent cells were the intended mutants, PCR products spanning the mutated region were obtained from the proviral hβc cDNAs in these cells and, where appropriate, digested with diagnostic restriction enzymes.23 As shown in Fig 3C, the V449E and I374N mutants were verified by their inherent additional Bgl II and lost BstYI sites, respectively, whereas the FIΔ mutant was distinguished by its increased size.
Expression and confirmation of identity of hβc mutants in factor-independent fetal liver cells. (A) Flow cytometric analyses of mutant hβc expression. Dashed lines represent staining with an irrelevant isotype control antibody. Solid lines represent staining with an anti-hβc MoAb. (B) Map of hβc cDNA showing Nco I (N), BstYI (Bs), and Bgl II (Bg) restriction sites used to authenticate each form of hβc, as well as the region duplicated in FIΔ (indicated by boxes). The restriction sites affected by the point mutations are indicated as Bg+ (gained in V449E) and Bs− (lost in I374N). Arrows indicate the positions of PCR primers used to amplify hβc fragments from genomic DNA. (C) Electrophoretic analysis of PCR products generated from genomic DNA of factor-independent fetal liver cells infected with constructs containing the indicated hβc mutants. As a negative control, a reaction was performed containing no DNA (−). Lanes M contain DNA size standards (SPP-1 phage DNA digested with EcoRI [Bresatec Ltd, Adelaide, South Australia]). For comparison, PCR products were generated from RufNeo-hβc plasmids (labeled wild-type). PCR products were either undigested (lanes 1), digested with BglII (lanes 2), or digested with BstYI (lanes 3). Bands in each digest that differ between the mutants and the wild-type are indicated by asterisks.
Expression and confirmation of identity of hβc mutants in factor-independent fetal liver cells. (A) Flow cytometric analyses of mutant hβc expression. Dashed lines represent staining with an irrelevant isotype control antibody. Solid lines represent staining with an anti-hβc MoAb. (B) Map of hβc cDNA showing Nco I (N), BstYI (Bs), and Bgl II (Bg) restriction sites used to authenticate each form of hβc, as well as the region duplicated in FIΔ (indicated by boxes). The restriction sites affected by the point mutations are indicated as Bg+ (gained in V449E) and Bs− (lost in I374N). Arrows indicate the positions of PCR primers used to amplify hβc fragments from genomic DNA. (C) Electrophoretic analysis of PCR products generated from genomic DNA of factor-independent fetal liver cells infected with constructs containing the indicated hβc mutants. As a negative control, a reaction was performed containing no DNA (−). Lanes M contain DNA size standards (SPP-1 phage DNA digested with EcoRI [Bresatec Ltd, Adelaide, South Australia]). For comparison, PCR products were generated from RufNeo-hβc plasmids (labeled wild-type). PCR products were either undigested (lanes 1), digested with BglII (lanes 2), or digested with BstYI (lanes 3). Bands in each digest that differ between the mutants and the wild-type are indicated by asterisks.
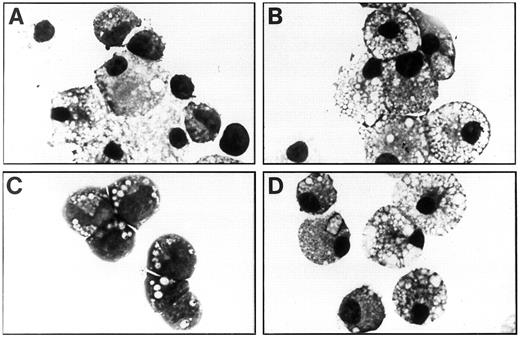
Characterization of factor-independent fetal liver cells.To ascertain the factor-dependent and -independent cell types present in the liquid cultures, these cultures were agitated and cytocentrifuge preparations were made at weekly intervals. As shown in Fig 4A and Table 1, cells grown in IL-3, GM-CSF, and Epo consisted of all five myeloid lineages as well as erythroid cells and immature blast, promyelocyte, and myelocyte cells. However, the factor-independent cells containing the FIΔ and I374N mutants were exclusively mature macrophages and neutrophils at day 7 of culture, the latter of which died after approximately 11 days of culture, leaving a monoculture of mature macrophages at day 21 (Fig 4B and D and Table 1). Whereas all of the cell types obtained with added growth factors (GM-CSF, IL-3, and Epo) and G418 were present in factor-independent cell populations generated with V449E (without Epo), there was a significant decrease in the proportion of monocytic cells in the latter population at day 7 and day 21 (χ2 tests, P < .005 in each case). Conversely, V449E induced superior proliferation of immature cells types compared with growth factors plus G418, with a significant increase in the proportion of myelocytes at day 7 (χ2 test, P < .005) and both blast cells and myelocytes at day 21 (χ2 tests, P < .005 in each case), which at this stage constituted the majority of cells (Fig 4C and Table 1). Interestingly, factor-independent erythroid cells were obtained in the absence of Epo from cells containing V449E. The addition of Epo to factor-independent cultures containing V449E significantly increased the erythroid content at day 7 (χ2 test, P < .005). However, Epo did not significantly increase the numbers of erythroid cells in factor-independent cultures containing FIΔ or I374N above a small background that was obtained when Epo was added to mock-infected or RufNeo-hβc–infected cultures (data not shown).
Morphology of factor-dependent and -independent fetal liver cells at day 21 of liquid suspension culture. (A) Cells infected with RufNeo-hβc grown in IL-3, GM-CSF, and Epo. (B) Cells infected with RufNeo-FIΔ grown in the absence of growth factors. (C) Cells infected with RufNeo-V449E grown in the absence of growth factors. (D) Cells infected with RufNeo-I374N grown in the absence of growth factors. Photographs are at 780× original magnification.
Morphology of factor-dependent and -independent fetal liver cells at day 21 of liquid suspension culture. (A) Cells infected with RufNeo-hβc grown in IL-3, GM-CSF, and Epo. (B) Cells infected with RufNeo-FIΔ grown in the absence of growth factors. (C) Cells infected with RufNeo-V449E grown in the absence of growth factors. (D) Cells infected with RufNeo-I374N grown in the absence of growth factors. Photographs are at 780× original magnification.
Differential Cell Counts of Factor-Dependent and Factor-Independent Fetal Liver Cells
| RufNeo Construct . | Additions . | Cell Type (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Eos . | Mast . | Neut . | MK . | M . | Eryth . | Myel . | Blast . |
| Day 7 | |||||||||
| hβc | IL-3, GM, Epo | 0.6 | 2.9 | 32.8 | 0.6 | 27.0 | 4.6 | 26.4 | 5.2 |
| IL3, GM, Epo, G418 | 1.0 | — | 45.5 | 0.3 | 18.3 | 9.3 | 20.6 | 5.0 | |
| FIΔ | Epo | — | — | 67.3 | — | 28.7 | 2.7 | 1.3 | — |
| — | — | — | 69.7 | — | 30.3 | — | — | — | |
| V449E | Epo | 0.3 | 1.3 | 47.8 | 0.3 | 4.3 | 10.0 | 32.6 | 3.3 |
| — | 2.0 | 0.7 | 48.8 | 0.7 | 0.7 | 2.3 | 39.9 | 5.0 | |
| I374N | Epo | — | — | 4.0 | — | 92.9 | 3.2 | — | — |
| — | — | — | 11.2 | — | 88.8 | — | — | — | |
| Day 21 | |||||||||
| hβc | IL-3, GM, Epo | — | 9.0 | — | — | 41.0 | 41.0 | 9.0 | — |
| IL-3, GM, Epo, G418 | — | 1.0 | 0.3 | — | 79.3 | 13.3 | 6.0 | — | |
| FIΔ | Epo | — | — | — | — | 99.3 | 0.7 | — | — |
| — | — | — | — | — | 100.0 | — | — | — | |
| V449E | Epo | — | 8.3 | 6.0 | — | — | 1.3 | 72.0 | 12.3 |
| — | — | 0.7 | 2.7 | — | — | — | 93.3 | 3.3 | |
| I374N | Epo | — | — | — | — | 100.0 | — | — | — |
| — | — | — | — | — | 100.0 | — | — | — | |
| RufNeo Construct . | Additions . | Cell Type (%) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Eos . | Mast . | Neut . | MK . | M . | Eryth . | Myel . | Blast . |
| Day 7 | |||||||||
| hβc | IL-3, GM, Epo | 0.6 | 2.9 | 32.8 | 0.6 | 27.0 | 4.6 | 26.4 | 5.2 |
| IL3, GM, Epo, G418 | 1.0 | — | 45.5 | 0.3 | 18.3 | 9.3 | 20.6 | 5.0 | |
| FIΔ | Epo | — | — | 67.3 | — | 28.7 | 2.7 | 1.3 | — |
| — | — | — | 69.7 | — | 30.3 | — | — | — | |
| V449E | Epo | 0.3 | 1.3 | 47.8 | 0.3 | 4.3 | 10.0 | 32.6 | 3.3 |
| — | 2.0 | 0.7 | 48.8 | 0.7 | 0.7 | 2.3 | 39.9 | 5.0 | |
| I374N | Epo | — | — | 4.0 | — | 92.9 | 3.2 | — | — |
| — | — | — | 11.2 | — | 88.8 | — | — | — | |
| Day 21 | |||||||||
| hβc | IL-3, GM, Epo | — | 9.0 | — | — | 41.0 | 41.0 | 9.0 | — |
| IL-3, GM, Epo, G418 | — | 1.0 | 0.3 | — | 79.3 | 13.3 | 6.0 | — | |
| FIΔ | Epo | — | — | — | — | 99.3 | 0.7 | — | — |
| — | — | — | — | — | 100.0 | — | — | — | |
| V449E | Epo | — | 8.3 | 6.0 | — | — | 1.3 | 72.0 | 12.3 |
| — | — | 0.7 | 2.7 | — | — | — | 93.3 | 3.3 | |
| I374N | Epo | — | — | — | — | 100.0 | — | — | — |
| — | — | — | — | — | 100.0 | — | — | — | |
Data are representative of three separate experiments.
Abbreviations: Eos, eosinophils; Mast, mast cells; Neut, neutrophils; MK, megakaryocytes; M, monocytes/macrophages; Eryth, erythroid cells; Myel, promyelocytes/myelocytes; Blast, blast cells; GM, GM-CSF.
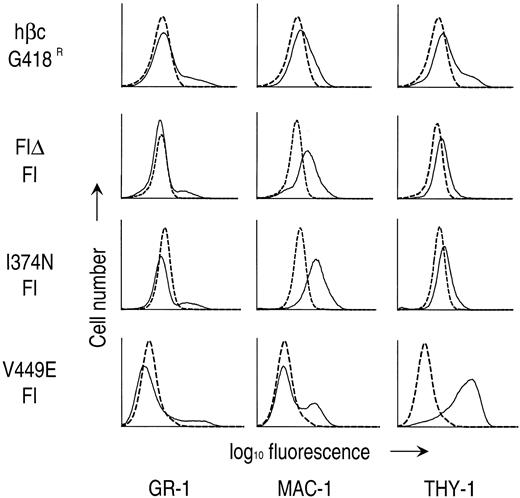
Next, to verify the identities of the various cell types observed, the expression of hematopoietic cell lineage markers was examined on day 24 of culture on factor-dependent and -independent cells by flow cytometry. As shown in Fig 5, a small fraction of G418-resistant cells infected with RufNeo-hβc and factor-independent cells containing any of the hβc mutants expressed the Gr-1 marker that is found on mature granulocytes and some granulocyte-macrophage precursors. Factor-independent cells containing either extracellular hβc mutant expressed the granulocyte-macrophage–specific marker Mac-1, which is concordant with the mature macrophage morphology of these cells. However, only a fraction of factor-independent cells containing V449E expressed Mac-1, indicating that monocytic and neutrophilic cells make up only a fraction of this population (Fig 5). Accordingly, whereas all factor-independent cells containing I374N expressed the macrophage-specific cell marker F4/80, only a small fraction of cells containing V449E were positive for this marker (data not shown). Factor-independent cells containing V449E expressed the Thy-1 marker at high levels, unlike cells grown in growth factors or factor-independent cells containing extracellular hβc mutants (Fig 5). Thy-1 is found on populations enriched for stem and early progenitor cells, hence its expression confirms the immature nature of these cells.33-35
Expression of lineage-specific cell surface antigens on G418-resistant (G418R) and factor-independent (FI) fetal liver cells. Flow cytometric analyses were performed as described in the Materials and Methods. Dashed lines represent staining with an irrelevant isotype control antibody. Solid lines represent staining with MoAbs directed to the indicated cell surface markers.
Expression of lineage-specific cell surface antigens on G418-resistant (G418R) and factor-independent (FI) fetal liver cells. Flow cytometric analyses were performed as described in the Materials and Methods. Dashed lines represent staining with an irrelevant isotype control antibody. Solid lines represent staining with MoAbs directed to the indicated cell surface markers.
Taken together, these results indicate that the activated extracellular hβc mutants FIΔ and I374N can give rise to factor-independent neutrophil and macrophage cells only. In contrast, the transmembrane hβc mutant V449E can induce proliferation and differentiation of all the lineages stimulated by its cognate ligands, IL-3, IL-5, and GM-CSF. Notably, however, V449E gives rise to a significantly lower proportion of macrophages and a higher proportion of immature cells than does a combination of IL-3, GM-CSF, and Epo.
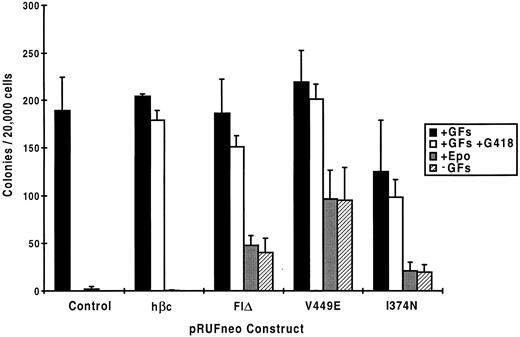
Clonal analysis of factor-independent fetal liver cells.To further characterize the factor-independent response of fetal liver cells bearing mutant hβc subunits, we examined the proliferation of individual progenitors in colony assays. As shown in Fig 6, when fetal liver cells exposed to retroviruses encoding the various hβc mutants were cultured in semisolid medium, 79% to 92% of progenitors were infected as assessed by the fraction of G418-resistant colonies obtained in the presence of growth factors. When cultured in the absence of growth factors, V449E induced colony formation by approximately half of the infected progenitors, whereas for FIΔ and I374N, approximately one quarter and one fifth of infected progenitors were factor-independent, respectively. The addition of Epo to cells infected with activated hβc mutants did not increase colony numbers beyond a small number of background colonies obtained when mock-infected or RufNeo-hβc–infected cells were cultured in Epo alone, which is concordant with results obtained from liquid cultures (see above).
Colony formation by mock-infected and infected fetal liver cells in methyl-cellulose. Mock-infected and infected fetal liver cells were plated in the indicated conditions in semisolid methyl-cellulose medium and the resultant colonies were scored after 7 days. The data shown are a combination of two separate experiments. In each experiment, duplicate dishes were counted in the case of those containing growth factors, whereas in the case of dishes containing Epo alone or without added growth factors, eight dishes were counted. GFs, growth factors (IL-3, GM-CSF, and Epo).
Colony formation by mock-infected and infected fetal liver cells in methyl-cellulose. Mock-infected and infected fetal liver cells were plated in the indicated conditions in semisolid methyl-cellulose medium and the resultant colonies were scored after 7 days. The data shown are a combination of two separate experiments. In each experiment, duplicate dishes were counted in the case of those containing growth factors, whereas in the case of dishes containing Epo alone or without added growth factors, eight dishes were counted. GFs, growth factors (IL-3, GM-CSF, and Epo).
Factor-independent colonies containing V449E were of various types, including neutrophil (G), eosinophil (Eo), mast cell (Mast), macrophage (M), granulocyte-macrophage (GM), mixed myeloid/erythroid (Mix) colonies containing granulocytes, erythroid cells, megakaryocytes and macrophages, and blast cell colonies (Table 2). In contrast, FIΔ and I374N gave rise to only G, M, and GM colonies in the absence of growth factors. The addition of Epo did not increase numbers of burst-forming unit-erythroid (BFU-E) colonies above a small background that was obtained with mock-infected or RufNeo-hβc–infected cells cultured in Epo alone (data not shown). However, Epo did increase the proportion of erythroid cells in factor-independent mixed myeloid/erythroid colonies containing V449E (data not shown), which is consistent with the fact that a significant increase in V449E-induced erythroid growth was obtained in liquid cultures containing Epo (see above). The lack of factor-independent BFU-E colonies induced by expression of V449E suggests that the factor-independent erythroid growth supported by this mutant in liquid cultures (Table 1) was derived from mixed myeloid/erythroid progenitors.
Factor-Dependent and -Independent Colonies Formed by Fetal Liver Cells Infected With Wild-Type and Mutant hβc Subunits
| RufNeo Construct . | Additions . | Colony types (%) . | Colonies Examined . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | G . | Eo . | Mast . | M . | GM . | Mix . | BFU-E . | Blast . | . |
| hβc | IL-3, GM, Epo | 12 | 1 | 4 | 39 | 33 | 8 | 4 | — | 103 |
| IL-3, GM, Epo, G418 | 18 | 3 | 6 | 38 | 26 | 5 | 5 | — | 109 | |
| FIΔ | Epo | 34 | — | — | 24 | 36 | — | 6 | — | 80 |
| — | 17 | — | — | 45 | 38 | — | — | — | 84 | |
| V449E | Epo | 9 | 1 | 12 | 26 | 17 | 29 | 3 | 3 | 69 |
| — | 13 | 8 | 7 | 30 | 20 | 18 | — | 5 | 61 | |
| I374N | Epo | 4 | — | — | 79 | 15 | — | 1 | — | 73 |
| — | 1 | — | — | 89 | 10 | — | — | — | 93 | |
| RufNeo Construct . | Additions . | Colony types (%) . | Colonies Examined . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | G . | Eo . | Mast . | M . | GM . | Mix . | BFU-E . | Blast . | . |
| hβc | IL-3, GM, Epo | 12 | 1 | 4 | 39 | 33 | 8 | 4 | — | 103 |
| IL-3, GM, Epo, G418 | 18 | 3 | 6 | 38 | 26 | 5 | 5 | — | 109 | |
| FIΔ | Epo | 34 | — | — | 24 | 36 | — | 6 | — | 80 |
| — | 17 | — | — | 45 | 38 | — | — | — | 84 | |
| V449E | Epo | 9 | 1 | 12 | 26 | 17 | 29 | 3 | 3 | 69 |
| — | 13 | 8 | 7 | 30 | 20 | 18 | — | 5 | 61 | |
| I374N | Epo | 4 | — | — | 79 | 15 | — | 1 | — | 73 |
| — | 1 | — | — | 89 | 10 | — | — | — | 93 | |
Data are a combination of two separate experiments. Colony types were determined at day 7 of culture.
Abbreviations: G, neutrophilic granulocyte; Eo, eosinophil; Mast, mast cell; M, macrophage; GM, granulocyte-macrophage; Mix, mixed myeloid/erythroid; BFU-E, erythroid burst-forming unit; Blast, blast cell; GM, GM-CSF.
These results are concordant with those obtained in liquid culture and confirm the observations that, whereas V449E can induce proliferation and differentiation of all the cell types examined in this study, FIΔ and I374N can do so to neutrophil and macrophage precursors only.
Isolation and characterization of factor-independent cell lines.Cultures containing growth factors eventually gave rise to mast cell lines, as is frequently the case when murine hematopoietic cell populations are grown in the presence of IL-3 (Fig 7A).36-38 However, from two populations of factor-independent cells containing the V449E mutant (of 6 studied), continuously proliferating cell lines were established, termed RTVE1 and RTVE2.
Morphology of factor-independent fetal liver cell lines. (A) For comparison, mast cells obtained from fetal liver cultures infected with RufNeo-hβc and cultured for 6 weeks in the presence of growth factors (IL-3, GM-CSF, and Epo). (B and C) RTVE1 and RTVE2 cell lines, respectively, obtained after infection of fetal liver cells with RufNeo-V449E. Photographs are at 780× original magnification.
Morphology of factor-independent fetal liver cell lines. (A) For comparison, mast cells obtained from fetal liver cultures infected with RufNeo-hβc and cultured for 6 weeks in the presence of growth factors (IL-3, GM-CSF, and Epo). (B and C) RTVE1 and RTVE2 cell lines, respectively, obtained after infection of fetal liver cells with RufNeo-V449E. Photographs are at 780× original magnification.
Cells of the RTVE1 line grew in clumps and required cell contact for growth; when clumps were disaggregated, the resulting single cells rapidly died. These cells displayed ring nuclei characteristic of neutrophilic myelocytes (Fig 7B). Treatment with a myeloperoxidase stain showed large primary granules expressing this enzyme, confirming that these cells are partially differentiated (data not shown).
Cells of the RTVE2 line were cytologically less mature, with their development ostensibly arrested at the blast cell stage (Fig 7C), and did not require cell contact for growth. This line displayed some spontaneous neutrophil and macrophage differentiation, implying that it contains precursors for both of these lineages. Cells of this line did not express myeloperoxidase (data not shown), which is consistent with their myeloblast morphology, because this enzyme is induced at the promyelocyte stage.39 40
DISCUSSION
To investigate the signalling and leukemogenic potential of three constitutively active mutants of the common β subunit for the human IL-3, IL-5, and GM-CSF receptors, we have introduced these mutants into primary murine hematopoietic cells. We show that activated forms of hβc can signal in primary hematopoietic cells and highlight differences in the biological effects of extracellular and transmembrane activated hβc mutants.
Implications for hβc signalling.Whereas the factor-independent transmembrane hβc mutant V449E could deliver a mitogenic signal in all the cell types in which hβc normally signals, two extracellular mutants showed a restricted cell specificity, signalling only in granulocyte-macrophage progenitors and their progeny. The different factor-independent colony and cell types generated by the transmembrane and extracellular mutants suggests signalling differences between these mutants. Preliminary studies indicating differences in the tyrosine phosphorylation of extracellular and transmembrane mutants are supportive of this notion (B. Jenkins, T. Blake, and T.J.G., manuscript in preparation). Furthermore, preliminary mouse bone marrow reconstitution experiments suggest that these two classes of hβc mutants elicit different biological effects in vivo (M.P.M. and T.J.G., unpublished data). Signalling differences between the transmembrane and extracellular hβc mutants could be quantitative (ie, reflect different signal strengths) or qualitative (ie, reflect use of different signal transduction pathways).
The cellular proliferation caused by expression of V449E was far greater than that caused by expression of the extracellular mutants (Fig 1). This finding raises the question of whether the ability of V449E to signal in a greater variety of cell types than FIΔ or I374N is an effect of greater stimulation of common signalling pathways. Indeed, stimulation of murine bone marrow cells with GM-CSF induces, in order of increasing concentration, macrophage, neutrophil, granulocyte-macrophage, eosinophil, and megakaryocyte colonies from murine bone marrow.41 42 Hence, whereas small doses of this factor produce exclusively macrophage, neutrophil, and granulocyte-macrophage colonies, as do the extracellular hβc mutants (Table 2), larger doses induce formation of other colony types, as does the V449E mutant. However, the growth patterns of factor-independent fetal liver cells in liquid culture are incongruent with this possibility. There is an almost complete lack of macrophage development in factor-free fetal liver liquid cultures infected with the V449E mutant, whereas similar cultures infected with extracellular hβc mutants were exclusively of this cell type at day 21 of culture (Table 1). Thus, the spectrum of cell types induced by each class of hβc mutant are separate and complementary, rather than one encompassing and expanding upon the other.
In contrast to the extracellular hβc mutants, the V449E mutant caused extensive proliferation of early myeloid precursors (myeloblasts, promyelocytes, and myelocytes). These precursors comprised a much larger proportion of factor-independent fetal liver cultures containing V449E relative to those grown in growth factors (IL-3, GM-CSF, and Epo; Table 1). There are at least two possible and nonexclusive explanations for this phenotype. Retroviral expression of V449E could lead to stimulation of those early progenitors that do not normally express receptors for murine IL-3 or GM-CSF. This would lead to an increased proportion of immature cells at late stages of culture relative to cells grown in these growth factors. In fact, a similar result was obtained from transgenic mice expressing the high-affinity hGM-CSFR.43 When bone marrow cells derived from these mice were stimulated with hGM-CSF, the numbers of mixed and blast cell colonies obtained were twofold to threefold higher than those obtained with mIL-3, suggesting that the hGM-CSFRs were expressed in progenitors insensitive to mIL-3. Consistent with this finding, the proportion of mixed colonies formed by V449E was threefold greater than that from cells stimulated with mIL-3 plus mGM-CSF (plus Epo), and blast colonies were only detected in factor-free cultures containing V449E. This may explain the persistence of immature cell types in factor-free liquid cultures containing V449E.
Alternatively, V449E may be deficient in some aspects of differentiative signalling to early myeloid precursors and/or more effective in proliferative signalling, biasing their development towards self-renewal. However, because mature cells of all myeloid lineages, as well as erythroid cells, were obtained with this mutant, any differences in differentiative signalling between V449E and the wild-type IL-3/GM-CSF receptors are likely to be quantitative.
The extracellular hβc mutants FIΔ and I374N could signal only to granulocyte-macrophage progenitors and their progeny, suggesting that components of the mitogenic signal transduction pathways that these mutants use may only be present in these cell types. Previous work in our laboratory showed that, of the three mutants used in this study, only V449E could signal to the IL-3 responsive pro-B–cell line BAF-B03.23 The lack of function of extracellular hβc mutants in BAF-B03 cells was explained by postulating that they require another, perhaps myeloid-specific molecule(s) for signalling. The granulocyte-macrophage–specific signalling of the extracellular hβc mutants presented here suggests that expression of such a molecule(s) may be restricted further to the granulocyte-macrophage lineages.
Implications for the tumorigenic potential of hβc.The ability of constitutively active hβc mutants to deliver mitogenic signals to primary myeloid progenitors as well as to render the murine cell line FDC-P1 tumorigenic raises the possibility that such mutants can contribute to human leukemias, as does autocrine production of GM-CSF.14 The results presented here suggest that, of the three mutants studied, V449E would be predicted to have the greatest leukemogenic potential. This mutant caused extensive proliferation of early myeloid precursors (myeloblasts, promyelocytes, and myelocytes), whereas extracellular hβc mutants did not (Table 1). Cells from myeloid leukemias such as acute myeloblastic leukemia often resemble blast cells, the proliferation of which was only observed with V449E (Table 1).14 Moreover, as discussed above, V449E may bias the development of these precursors towards self-renewal. This would be likely to increase the chances of a second mutational event blocking cell differentiation, which is required for leukemogensis.
Indeed, in this study, two immortalized cell lines, termed RTVE1 and RTVE2, were derived from factor-independent fetal liver liquid cultures bearing the V449E mutant (Fig 7). It is possible that the retroviral infection per se of the fetal liver cells increased the frequency of immortalizing events in these cultures. This has generally occurred as a consequence of a retrovirus integrating near proto-oncogenes, leading to increased expression, or by insertional mutagenesis of proto-oncogenes, activating their leukemogenic potential.44 In fact, during the course of this study, two factor-dependent cell lines were obtained from RufNeo-hβc–infected fetal liver populations grown in growth factors (data not shown), implying that immortalizing events were not peculiar to factor-independent fetal liver cultures containing V449E.
We have attempted to assess the tumorigenicity of these lines by injecting mice subcutaneously with 5 × 106 cells. Only two of eight mice injected with RTVE1 cells developed tumors, whereas none of four mice injected with RTVE2 cells developed tumors (data not shown). These data imply that additional mutations are required to confer tumorigenicity on these lines. This is similar to the case for v-Mpl, in that cell lines immortalized by the myeloproliferative leukemia virus require extended periods of culture for oncogenicity, implying the requirement of additional mutations.19 Nevertheless, the limited tumorigenicity of the RTVE1 cell line implies that the V449E mutant has oncogenic potential and that this potential is not limited to previously immortalized growth factor-dependent lines such as FDC-P1.23
Further study is required to characterize at the biochemical level the particular signalling mechanisms used by each class of hβc mutant and to identify putative cell-type–specific signalling molecules. Comparison of these mechanisms with the biologic effects demonstrated in this report promises to correlate growth and differentiation of different hematopoietic cell types with the induction of specific signalling pathways.
ACKNOWLEDGMENT
The authors thank Drs Leonie Ashman and Richard D'Andrea for critically reading the manuscript. Brendan Jenkins is thanked for providing ecotropic retrovirus producing cell lines.
Supported in part by grants (to T.J.G.) from the National Health and Medical Research Council of Australia (NH&MRC) and the Anti-Cancer Foundation of the Universities of South Australia. M.P.M. is a recipient of an Australian Postgraduate Research Award from the University of Adelaide, and T.J.G. is a Senior Research Fellow of the NH&MRC.
Address reprint requests to Thomas J. Gonda, PhD, The Hanson Centre for Cancer Research, Institute for Medical and Veterinary Science, Frome Road, Adelaide, SA 5000, Australia.

![Fig. 3. Expression and confirmation of identity of hβc mutants in factor-independent fetal liver cells. (A) Flow cytometric analyses of mutant hβc expression. Dashed lines represent staining with an irrelevant isotype control antibody. Solid lines represent staining with an anti-hβc MoAb. (B) Map of hβc cDNA showing Nco I (N), BstYI (Bs), and Bgl II (Bg) restriction sites used to authenticate each form of hβc, as well as the region duplicated in FIΔ (indicated by boxes). The restriction sites affected by the point mutations are indicated as Bg+ (gained in V449E) and Bs− (lost in I374N). Arrows indicate the positions of PCR primers used to amplify hβc fragments from genomic DNA. (C) Electrophoretic analysis of PCR products generated from genomic DNA of factor-independent fetal liver cells infected with constructs containing the indicated hβc mutants. As a negative control, a reaction was performed containing no DNA (−). Lanes M contain DNA size standards (SPP-1 phage DNA digested with EcoRI [Bresatec Ltd, Adelaide, South Australia]). For comparison, PCR products were generated from RufNeo-hβc plasmids (labeled wild-type). PCR products were either undigested (lanes 1), digested with BglII (lanes 2), or digested with BstYI (lanes 3). Bands in each digest that differ between the mutants and the wild-type are indicated by asterisks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1471/3/m_bl_0033f3.jpeg?Expires=1770388627&Signature=tt-dOaDU01xdDIsY-DbBdliTQ1EpThlyx1uF6L3Vp4K8dY3~5OEiVj96-ZBu9KuVofl-wPkxPJDRip5L1byYb-NUnd1bPWd6OnTj1RqjlRoCNJWE4DjwdV1hQS~KG3kxEvaDcrRBcaWZ~OtjD7Ca0NFBLO8Aktq0sqz0YVIYQlea8qXXKE6uaZ2-YRw4xARSxmd0hqCgw9csOzBs6~t9kt2akV9U3EARcjKo~C3kMGNMVKiBhujKjp8Jlp4iDimMCt5OUf8UmGerH~WkpgxJtsq4zDjmQnj-E6GMvM3sRvGMrsLF40JwC3KPLyU8N~Szgof8zluFYunfTrkjq58E~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal