Abstract
Lipoprotein(a) [Lp(a)], which has been shown to interact with fibrin(ogen) and other components of the blood clotting cascade, is a major independent risk factor for atherothrombotic disease in humans. The physiological function(s) of Lp(a), as well as the precise mechanism(s) by which high plasma levels of Lp(a) increase risk are unknown. Identification of further potential apo(a)-protein ligands may be crucial to illuminate apo(a)'s function(s) and pathophysiological properties. We used the repetitive apo(a) kringle IV type 2, which is variable in number in apo(a), to screen a human liver cDNA library by the yeast two-hybrid interaction trap system. Among 11 positive clones that emerged from the screen, eight clones were identified as β-2 glycoprotein I and one as fibronectin. Coimmunoprecipitation experiments confirmed that β-2 glycoprotein I and apo(a)/Lp(a) interact in human plasma and in cell culture supernatants of COS-1 cells, which ectopically expressed apo(a). The apo(a)-β2-glycoprotein I interaction indicates new potential roles for Lp(a) in fibrinolysis and autoimmunity.
LIPOPROTEIN(a) [Lp(a)] from human plasma is composed of one low-density lipoprotein (LDL) and a glycoprotein, called apolipoprotein(a) [apo(a)]. The apo(a) gene has been localized to chromosome 6q2.6-q2.7 in close vicinity to the plasminogen (PLG) gene with which it shares extensive sequence homology.1,2 One of the PLG-related KIV modules in apo(a) is kringle IV type 2, which is present in a variable number in the apo(a) gene resulting in an extensive size polymorphism of the apo(a) gene, mRNA and protein.3-5 The number of kringle IV repeats in apo(a) is inversely correlated with plasma concentrations of Lp(a), which range from less than 0.2 to over 200 mg/dl.3,6 There is a also a strong correlation between the number of kringle IV repeats and the risk for coronary heart disease (CHD).7 Numerous studies have shown that high Lp(a) is a risk factor for atherothrombotic disease (reviewed in Utermann8 ), suggesting that the concentration of Lp(a) in plasma, or the number of kringle IV repeats, predict risk.
Several mechanisms by which high levels of Lp(a) may promote increased risks for atherothrombotic vascular disease have been suggested by the sequence homology between apo(a) and plasminogen,9-16 an important factor in the dissolution of blood clots. The physiological function of Lp(a), however, is still unclear. In vitro interactions of apo(a) with different protein ligands have suggested physiological functions for apo(a)/Lp(a), some of which have been confirmed in transgenic mice, including antifibrinolytic activity11 and inhibition of plasmin-dependent activation of transforming growth factor-β (TGF-β),12 but none is known to play a role in humans in vivo. Understanding apo(a)'s physiological function(s) may be instrumental for development of new strategies for reducing (or counteracting) high plasma Lp(a) levels, eventually leading to novel ways to block apo(a)'s pathophysiological function in CHD and stroke.
Because next to the known apo(a) protein ligands (eg, fibrin(ogen),13-15 PLG-receptors,9,16,17 fibronectin18 ) additional hitherto unknown apo(a) protein ligands may exist, which are not predicted by the homology of apo(a) with plasminogen, we have attempted to identify such potential target proteins, employing the GAL4 Two-Hybrid Library Interaction Trap System. This experimental approach exploits the specific protein-protein interactions in intact yeast cells for “fishing” of putative target proteins out of a cDNA library.19 Because we reasoned that the repetitive apo(a) kringle IV type 2 domain, which extends from the particle into solution, might be an “anchor” used to attach the Lp(a) particle to a matrix/ligand, we used in this study a single apo(a) kringle IV type 2 domain as bait. To our surprise, we identified (among already known protein ligands) β-2 glycoprotein I from human plasma as a novel and physiological apo(a) protein ligand.
MATERIALS AND METHODS
Yeast strains and media.The genotype of the Saccharomyces cerevisiae reporter strain HF7c used for the two-hybrid screening, is MATa, ura3-52, his3-200, ade2-101, lys2-801, trp1-901, leu2-3, 112, gal4-542, gal80-538, LYS2::GAL1-HIS3, URA3:: (GAL4 17-mers)3 -CYC1-lacZ. The genotype of SFY526, used to test interactions between GAL4-BD and GAL4-AD fusions, is MATa, ura3-52, his3-200, ade2-101, lys2-801, trp1-901, leu2-3, 112, canr, gal4-542, gal80-538, URA3::GAL1-lacZ (Clontech Laboratories, Inc, Palo Alto, CA). Stains were grown under standard conditions in rich or synthetic medium with appropriate supplements at 30°C.
Two-hybrid screening and ligand-cDNA isolation.For the yeast two-hybrid screening, apo(a)KIV2 was cotransformed with the human liver cDNA Matchmaker library in the pGAD10 vector (Clontech Laboratories, Inc) into the HF7c yeast strain, as described by the manufacturer, and the transformants were plated to synthetic dropout selection medium (lacking leucine, histidine, and tryptophan), containing 5 mmol/L 3-amino-1,2,4-triazole, and incubated at 30°C up to 7 days.
β-galactosidase reporter activity.His+ colonies were assayed for β-galactosidase activity by transferring individual colonies on filters placed on selection medium. The plates were incubated for 2 days at 30°C, the filters lifted, and immersed in liquid nitrogen for 10 seconds. After thawing at room temperature, the filters were placed on filter circles saturated with X-Gal-solution in a petri-dish (permeabilized cells up) and incubated overnight as indicated.
Plasmids.apo(a)KIV2 and apo(a)KIV6 were cloned from full-length apo(a) cDNA into the GAL4-BD vector pGBT9 using polymerase chain reaction (PCR) and recombinant PCR primers (apo(a)KIV2 : 5′-CCAAGCGAATTCGGTGGCGGTGGATCCGCACCGACTGAGCAAAGGCC TG-3′ and 5′-CCTTTGGTCGACTCATCATTGTTCGGAAGGAGCCTCT AGGCT-3′; apo(a)KIV6 : 5′-CCAAGCGAATTCGGTGGCGGTGGATCC GCACCAACGGAGCAAAGCCCCG-3′ and 5′-GCTTTGGTCGACTCATCATT GTTCAGAAACAGCCGTGGACGT-3′) engineered to create proper compatible ends and correct reading frame for expression as fusion proteins in the context of the GAL4 DNA binding domain. Correct constructs have been identified and confirmed by restriction analysis and partial DNA sequencing, using pGBT9 specific primers (GAL4 BD 5′: 5′-CATCGGAAGAGAGTAG-3′ and GAL4 BD 3′: 5′-CCTAAGAGTCACTTTAAAA-3′).
Immunoblotting of GAL4 DBD-fusion baits in yeast extracts.A total of 5 mL of transformed yeast cells grown overnight in selective medium lacking tryptophan were used to inoculate 15 mL yeast extract peptone dextrose (YPD) medium. At an optical density (600 nm) of 0.5, the cells were pelleted, washed, resuspended at 5 × 108 cells/mL in ice-cold lysis buffer (50 mmol/L TrisHCl, pH 7.5; 150 mmol/L NaCl; 1% NP-40; 0.25% sodium-deoxycholate; 1 mmol/L EDTA; 1 μg/mL aprotinin/leupeptin and 1 mmol/L phenylmethylsulfonyl fluoride) and frozen at −20°C. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%), transferred to polyvinylidene fluoride (PVDF ) membrane (Millipore, Vienna, Austria) and fusion proteins were detected using a GAL4-BD specific antibody (Santa Cruz Inc, Santa Cruz, CA), followed by a rabbit antimouse IgG-peroxidase conjugate and a chemiluminescence detection kit (ECL reagent; Amersham Corp, Arlington Heights, IL).
Transient transfection.The SV40-transformed African green monkey kidney cell line COS-1 or the human liver HepG2 cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured as recommended by ATCC. For transient transfections, cells were seeded at a density of 0.5 × 106 cells/well in 6-well plates in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37°C. Twenty-four hours later, the cells were transfected with 2 μg of circular plasmid DNA/dish by lipofectamin (GIBCO-BRL, Gaithersburg, MD) as described by the manufacturer. Sixty to 65 hours after transfection, cell culture supernatants were harvested and adjusted to 1 mmol/L phenylmethylsulfonyl fluoride.
Coimmunoprecipitation.Cell culture supernatants, as well as undiluted plasma samples (1 mL each), were precleared by incubating with 50 μL protein G-Sepharose beads for 2 hours at 4°C and then immunoprecipitated overnight at 4°C with the indicated antibodies at 5 μg/mL final antibody concentration, followed by addition of 50 μL protein G-Sepharose beads for the last 2 hours. Immunoprecipitates were collected by centrifugation for 1 minute at 13,000 rpm and 4°C in an Eppendorf microfuge and washed six times with phosphate-buffered saline (PBS)/0.02% Tween buffer.
Western blot analysis.Immunoprecipitates were resuspended in SDS-PAGE sample buffer, boiled for 5 minutes at 95°C, and separated on 10% or 4% to 12% Tris-glycine gels (Novex, San Diego, CA). Immunoblot analysis, using apo(a)-specific monoclonal antibody (MoAb) 1A2 was performed as described.8 Specific polyclonal antibodies to β-2 glycoprotein I were a kind gift from Dr G. Müncher (Behring Werke, Marburg, Germany) and used at 5 μL serum/mL with protein-G sepharose as secondary agent in immunoprecipitation analysis. Anti-β–2 glycoprotein I MoAbs F7 and F10 were purchased from Biodesign (Kennebunk, ME) and used overnight at 5 μg/mL at 4°C.
Enzyme-linked immunosorbent assay (ELISA)-based determination of β-2 glycoprotein I-apo(a)/Lp(a) interaction.Apo(a) and Lp(a) have been prepared by ultracentrifugation as described.5 Microtiter plates (Nunc, Roskilde, Denmark) were coated with 100 μL/well of apo(a) (10 μg/mL), Lp(a) (equivalent to 10 μg/mL apo[a]) and LDL (equivalent to the LDL-portion of Lp[a]) in bicarbonate coating buffer (50 mmol/L NaHCO3 , pH 9.6) and incubated overnight at 4°C. Nonspecific sites were blocked with PBS, 0.2% Casein (300 μL/well) for 30 minutes at 37°C. Plates were washed three times each with PBS/0.02% Tween between the different incubation steps. Immunodetection of bound β-2 glycoprotein I was obtained by successive incubations with 150 μL/well of MoAb F7 or F10, respectively (5 μg/mL final antibody concentration, 2 hours at 37°C), followed by antimouse IgG-peroxidase conjugate (1 hour at 37°C) and substrate solution O-phenylenediamine dihydrochloride (0.4 mg/mL).
RESULTS
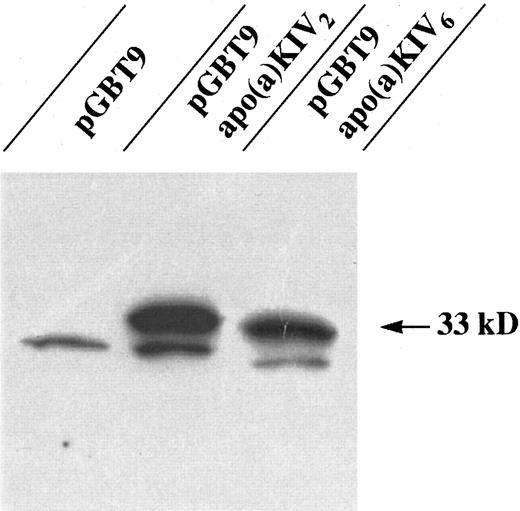
Screening for apo(a) binding proteins using the Two-Hybrid System.We have attempted to identify novel apo(a)/Lp(a) binding proteins employing the “GAL4 Two-Hybrid Interaction Trap Liver Library” approach and using a single apo(a) domain (the highly repetitive kringle IV type 2) as bait. Human apo(a) substructures, ie, the repetitive kringle IV type 2, as well as a unique kringle IV type 6 cDNAs, were cloned into pGBT9 by PCR using recombinant PCR primers, engineered to create proper compatible ends and correct reading frame for apo(a) kringle IV expression as a fusion protein in the context of the GAL4 DNA binding domain (DBD). A flexible pentamer linker sequence Gly-Gly-Gly-Gly-Ser was inserted between the two fusion partner domains to allow independent and authentic folding of GAL4-DBD and kringle IV, respectively. Correct constructs were identified and confirmed by restriction analysis and DNA sequencing, using vector-specific sequencing primers. To demonstrate expression of the recombinant GAL4 apo(a) kringle IV fusion proteins, yeast cells were transformed with the respective pGBT9-kringle IV expression plasmid DNAs or empty vector control. Transformed cells were harvested after 24 hours, and the recombinant protein products were subjected to electrophoresis and detected by immunoblotting using a GAL4 DBD-specific antibody (Fig 1). This experiment showed the expression of recombinant apo(a) kringle IV-fusion proteins with an apparent molecular weight of 33 kD in the yeast H7Fc host cells.
Western blot analysis of GAL4-BD fusion bait proteins expressed in yeast strain HF7c. The two GAL4 BD hybrid constructs pGBT9-apo(a)KIV2 and pGBT9-apo(a)KIV6 together with pGBT9 vector control were transformed into the yeast strain HF7c and detected by an immunobloting using a MoAb specific for GAL4-BD. The arrow indicates the position of the GAL4-BD fusion proteins.
Western blot analysis of GAL4-BD fusion bait proteins expressed in yeast strain HF7c. The two GAL4 BD hybrid constructs pGBT9-apo(a)KIV2 and pGBT9-apo(a)KIV6 together with pGBT9 vector control were transformed into the yeast strain HF7c and detected by an immunobloting using a MoAb specific for GAL4-BD. The arrow indicates the position of the GAL4-BD fusion proteins.
To identify cDNA clones encoding proteins that interact with kringle IV type 2 from apo(a), we transformed the yeast host strain HF7c, carrying a GAL4-HIS3 selection and GAL4-β-galactosidase reporter gene with the kringle IV type 2-expression plasmid as a bait and a human liver cDNA library in which cDNA was fused to the GAL4 transcription activation domain. If proteins [eg, apo(a)-bait and apo(a) ligand] interact with each other by specific protein-protein interaction, the DNA-binding domain will be tethered to its transcriptional activation domain, and the proper function of the transcriptional activator will be reconstituted. A total of 2.4 × 106 transformants were subjected to positive genetic growth selection on His−Leu−Trp− plates, containing 3 mmol/L 3-AT. This yielded 21 colonies, 12 of which stained intensively for β-galactosidase activity within 60 minutes. To determine whether activation of the GAL4-dependent reporter genes by the above clones reflect a specific interaction of the encoded proteins with the kringle IV type 2 bait, each cDNA clone was rescued from yeast colonies and was retransformed into the same yeast strain in the absence or presence of the GAL4 kringle IV2 bait-expression plasmid. Additionally, we transformed each of the putative ligand expression plasmids with an expression plasmid in which the GAL4 DNA binding domain was fused to a nonspecific protein (p53), which is not expected to interact with proteins that bind to apo(a). Kringle IV type 2 bait alone or together with SV40 control ligand did not activate the promoter, as well (data not shown). One of these 12 clones was able to activate expression of the reporter genes in the absence of the GAL4-kringle IV2 bait or in the presence of GAL4-p53, identifying this clone as false positive.20 The remaining 11 colonies were all specific in that they activated β-galactosidase expression only in the presence of the GAL4-kringle IV2 bait. Subsequently, these positive clones were sequenced using primers in the pGAD10-flanking sequence. Computer-aided homology alignment searches using the EMBL/GenBank DNA sequence data base identified one clone as fibronectin and novel cDNA sequences (two clones) of, as yet, unknown identity.
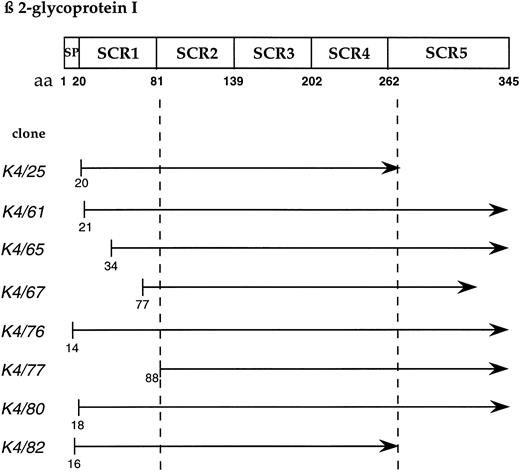
Interaction of β-2 glycoprotein I clones with apo(a)KIV2 bait in the two hybrid genetic screen. Positive clones obtained on screening of a human liver cDNA library are shown. The distinct domains of β-2 glycoprotein I are shown on the top. The minimal consensus binding domain for apo(a)KIV2 is indicated by dotted lines. SP, signal peptide; SCR, short consensus repeat.
Interaction of β-2 glycoprotein I clones with apo(a)KIV2 bait in the two hybrid genetic screen. Positive clones obtained on screening of a human liver cDNA library are shown. The distinct domains of β-2 glycoprotein I are shown on the top. The minimal consensus binding domain for apo(a)KIV2 is indicated by dotted lines. SP, signal peptide; SCR, short consensus repeat.
Eight clones contained overlapping sequences from the plasma protein β-2 glycoprotein I. Each clone was fused in-frame to the GAL4 activation domain. From the minimal region of overlap, we conclude that the interaction of apo(a) with β-2 glycoprotein I requires a sequence in a stretch of only 181 aa, which is contained in the SCR2 to SCR4 domain of β-2 glycoprotein I (Fig 2).
To test whether the association of apo(a) with β2-glycoprotein I is exclusively mediated by the repetitive kringle IV type 2, we performed a Two-Hybrid assay with β2-glycoprotein I using the “unique” kringle apo(a) kringle IV type 6 as bait. This assay showed that apo(a) kringle IV type 6 is also able to bind to β2-glycoprotein I (data not shown).
These results suggest that β-2 glycoprotein I may interact with distinct kringle IV domains of apo(a) in a specific manner and may represent a novel physiological apo(a) protein ligand in human plasma.
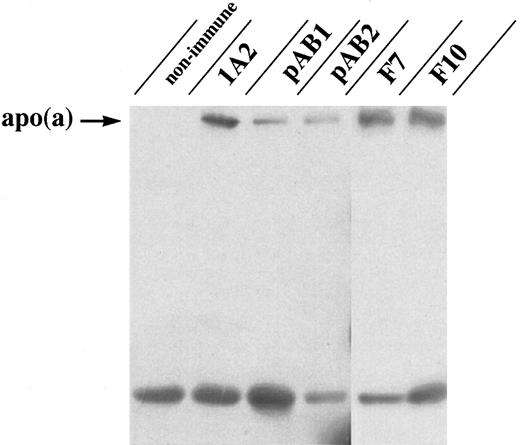
Biochemical confirmation of Lp(a)/apo(a)-β–2 glycoprotein I interaction using coimmunoprecipitation assays.To confirm the putative apo(a) and β-2 glycoprotein I interaction in an independent system, β-2 glycoprotein I was immunoprecipitated from human plasma, and the resultant precipitates were analyzed by immunoblotting with antisera specific to apo(a). As shown in Fig 3, apo(a) coimmunoprecipitates from human plasma with β-2 glycoprotein I using two different polyclonal and two different monoclonal anti–β-2 glycoprotein I antibodies, indicating an interaction of these two proteins in human plasma.
Coimmunoprecipitation of apo(a)/Lp(a) with β-2 glycoprotein I from human plasma. Immunoprecipitates were formed in plasma by using two different polyclonal (pAB1, pAB2) and two different monoclonal (F7, F10) antibodies raised against β-2 glycoprotein I or nonimmune serum, respectively. Immunodetection for the presence of apo(a) was performed using anti-apo(a) MoAb 1A2. Immunoprecipitation of apo(a) with the apo(a)-specific MoAb 1A2 served as positive control. The band seen at 50 kD represents the reduced IgG heavy chain.
Coimmunoprecipitation of apo(a)/Lp(a) with β-2 glycoprotein I from human plasma. Immunoprecipitates were formed in plasma by using two different polyclonal (pAB1, pAB2) and two different monoclonal (F7, F10) antibodies raised against β-2 glycoprotein I or nonimmune serum, respectively. Immunodetection for the presence of apo(a) was performed using anti-apo(a) MoAb 1A2. Immunoprecipitation of apo(a) with the apo(a)-specific MoAb 1A2 served as positive control. The band seen at 50 kD represents the reduced IgG heavy chain.
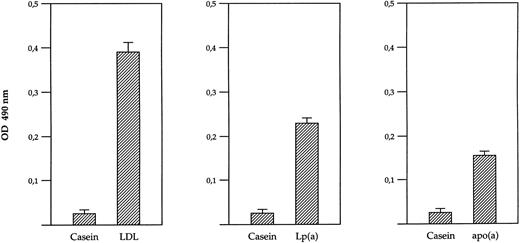
Direct binding of β-2 glycoprotein I to immobilized Lp(a), apo(a), and LDL. Microtiter plate wells coated with purified LDL, Lp(a) and free apo(a) or Casein were incubated with purified β-2 glycoprotein I and binding was detected by ELISA as described in Materials and Methods.
Direct binding of β-2 glycoprotein I to immobilized Lp(a), apo(a), and LDL. Microtiter plate wells coated with purified LDL, Lp(a) and free apo(a) or Casein were incubated with purified β-2 glycoprotein I and binding was detected by ELISA as described in Materials and Methods.
ELISA-based determination of β-2 glycoprotein I binding to solid phase Lp(a)/apo(a) and LDL.The two-hybrid results indicated a direct interaction of β-2 glycoprotein I with the apo(a) moiety of Lp(a), independent from the LDL moiety in Lp(a). To confirm this direct interaction, we established an ELISA-based system. Purified Lp(a), purified LDL, and purified free apo(a) were adsorbed to microtiter wells and incubated with β-2 glycoprotein I. Binding was detected by incubation of bound β-2 glycoprotein I with two anti-β–2 glycoprotein I MoAbs, F7 or F10, respectively, followed by addition of a peroxidase-conjugated secondary antibody (goat antimouse IgG). Both MoAbs obtained similar results, eg, specific binding of β-2 glycoprotein I to immobilized apo(a), Lp(a), and LDL could be observed. This ELISA-based assay system has been used in a strictly nonquantitative manner due to discrepancies in Lp(a), LDL, and apo(a) coating efficiencies. However, it still can be used as a qualitative measure of protein-protein interaction. As β-2 glycoprotein I was already reported to be associated with LDL in human plasma,21 this interaction served as positive control. No significant binding of β-2 glycoprotein I to casein was observed, nor did MoAbs, F7 and F10, detect signals in the wells coated with Lp(a), LDL, and apo(a) without prior incubation with β-2 glycoprotein I (data not shown). The results of these ELISA experiments are summarized in Fig 4, showing that β-2–glycoprotein interacts directly via apo(a) and not only indirectly with apo(a) via the LDL-moiety of Lp(a).
Analysis of the ability of free apo(a) to bind to β-2 glycoprotein I using ectopic expression of apo(a) in COS-1 cells.To further confirm the interaction of β-2 glycoprotein I to free apo(a) (uncomplexed to LDL) under more physiological conditions, we used COS-1 cells, which do not express apoB and hence are unable to form Lp(a), for transient transfection with two recombinant apo(a) isoforms differing in the number of identical repetitive KIV2 repeats (r-apo(a)K11, one repetitive KIV2 repeat, and r-apo(a)K26, 16 repetitive KIV2 repeats), respectively. Following transfection, purified β-2 glycoprotein I was added to the COS-1 cell supernatants and binding of β-2 glycoprotein I to free apo(a) was shown using coimmunoprecipitation assays as described above. As shown in Fig 5, coimmunoprecipitation of apo(a) with β-2 glycoprotein I could be observed using two independent anti-β–2 glycoprotein I MoAbs. In conclusion, β-2 glycoprotein I seems to be able to bind Lp(a) either on the LDL or the apo(a) moiety.
Coimmunoprecipitation of apo(a) and β-2 glycoprotein I from COS-1 supernatants. COS-1 cells were transiently transfected with empty vector control (upper panel) or two recombinant apo(a) isoforms [r-apo(a)K11, middle panel; r-apo(a)K26, lower panel]. Following transfection, purified β-2 glycoprotein I was added to COS-1 cellular supernatants and binding of β-2 glycoprotein I to the two different r-apo(a)s was shown using a coimmunoprecipitation assay. Immunoprecipitates were formed by using two different MoAbs (F7, F10) against β-2-glycoprotein I or nonimmune serum. Immunoprecipitation of apo(a) with the apo(a) specific MoAb, 1A2, served as positive control. Immunoprecipitates were resolved by 4% to 12% Tris-Glycin gel electrophoresis and immunodetected by immunoblot analysis using anti-apo(a) MoAb 1A2.
Coimmunoprecipitation of apo(a) and β-2 glycoprotein I from COS-1 supernatants. COS-1 cells were transiently transfected with empty vector control (upper panel) or two recombinant apo(a) isoforms [r-apo(a)K11, middle panel; r-apo(a)K26, lower panel]. Following transfection, purified β-2 glycoprotein I was added to COS-1 cellular supernatants and binding of β-2 glycoprotein I to the two different r-apo(a)s was shown using a coimmunoprecipitation assay. Immunoprecipitates were formed by using two different MoAbs (F7, F10) against β-2-glycoprotein I or nonimmune serum. Immunoprecipitation of apo(a) with the apo(a) specific MoAb, 1A2, served as positive control. Immunoprecipitates were resolved by 4% to 12% Tris-Glycin gel electrophoresis and immunodetected by immunoblot analysis using anti-apo(a) MoAb 1A2.
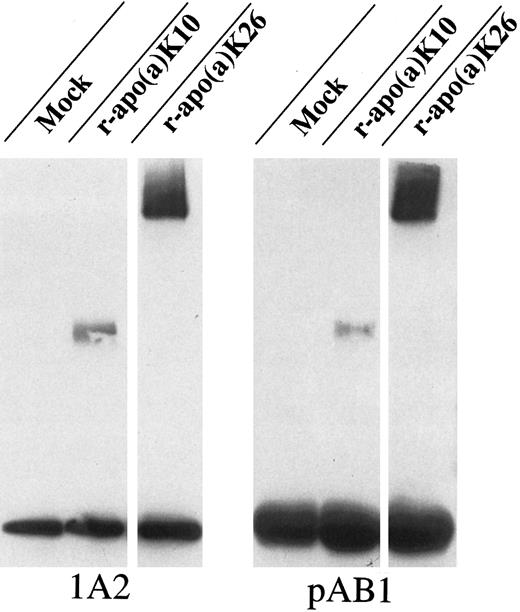
De novo-assembled Lp(a) particle was found to be bound to β-2 glycoprotein I in human liver cell line HepG2 supernatants.Human Hep-G2 liver cells, which are known to secrete β-2 glycoprotein I and LDL, but not apo(a) (data not shown), have been transfected with two different apo(a) isoform expression plasmids [r-apo(a)K10, no repetitive KIV2 repeat, and r-apo(a)K26, 16 repetitive KIV2 repeats], and the de novo-assembled Lp(a) particle, which forms in the media, was found to be bound to Hep-G2 cell secreted β-2 glycoprotein I by coimmunoprecipitation studies. This further indicates a physiological association of Lp(a) and β-2 glycoprotein I (Fig 6).
Coimmunoprecipitation of apo(a) and β-2 glycoprotein I in HepG2 supernatants. HepG2 cells, endogenously expressing β-2 glycoprotein I, were transiently transfected with different apo(a) isoforms (r-apo(a)K10 and K26) or empty vector control. Supernatants were harvested 60 hours postransfection and coimmunoprecipitated using polyclonal antibody against β-2 glycoprotein I (pAB1). Immunoprecipitation of apo(a) with the apo(a)-specific MoAb, 1A2, served as positive control. Immunoprecipitates were resolved by 4% to 12% Tris-Glycin gel electrophoresis and immunodetected by immunoblot analysis using anti-apo(a) MoAb 1A2.
Coimmunoprecipitation of apo(a) and β-2 glycoprotein I in HepG2 supernatants. HepG2 cells, endogenously expressing β-2 glycoprotein I, were transiently transfected with different apo(a) isoforms (r-apo(a)K10 and K26) or empty vector control. Supernatants were harvested 60 hours postransfection and coimmunoprecipitated using polyclonal antibody against β-2 glycoprotein I (pAB1). Immunoprecipitation of apo(a) with the apo(a)-specific MoAb, 1A2, served as positive control. Immunoprecipitates were resolved by 4% to 12% Tris-Glycin gel electrophoresis and immunodetected by immunoblot analysis using anti-apo(a) MoAb 1A2.
DISCUSSION
Apo(a), the characteristic component of the Lp(a) particle has been invented twice during mammalian evolution, once in the hedgehog (or an ancestor thereof ) and once in primates,22 but the physiological function(s) of this evolutionary new compound in human blood is presently unknown, as is the precise mechanism(s) by which high levels of Lp(a) increase risk for atherothrombotic events. Reported Lp(a) activities include competition for plasminogen binding to fibrinogen and fibrin,13,15,23 competition for plasminogen activation by tissue-type plasmin activator (t-PA),14,23,24 competition of plasminogen binding to cellular binding sites,9,16 competition of plasminogen binding to tetranectin,25 and enhancement of plasminogen activator inhibitor 1 (PAI-1) activity.26 Because of these multiple interactions, Lp(a) has been described as an interloper into the fibrinolytic system.27 Lp(a) also increases smooth muscle cell migration and proliferation by inhibition of TGF-β activation by plasmin.12,28,29 Other than fibrin and tetranectin, proteins involved in blood clotting, fibronectin,18 an extracellular matrix protein, was shown to bind to apo(a). It has been postulated that Lp(a) transports cholesterol to sites of injury and wound healing that may be beneficial, but as a side effect, might also trigger deposition of cholesterol in growing atherosclerotic plaques and inhibit fibrinolysis.30 Deposition of apo(a)/apo B complexes in human atherosclerotic plaques31 and in lesions from apo(a) transgenic mice32 has indeed been shown.
In the present study, we have searched for additional hitherto unknown apo(a) protein ligands. Their identification may be crucial to further illuminate apo(a)'s function(s) and/or pathophysiological properties. We have attempted to find novel interacting proteins by employing the GAL4 Matchmaker Two-Hybrid Interaction Trap System and a human liver cDNA library. We chose apo(a) kringle IV type 2 as target to “fish” for interacting proteins because this kringle-domain occurs in multiple copies, which presumably do not interact with LDL, but form a free “tail” as potential site for interaction(s) eg, it could anchor Lp(a) to a matrix. After screening 2.4 × 106 transformants, we finally identified 11 positive, true interacting clones. Among the clones that emerged from the screen were fibronectin (one clone) and two clones of unknown identity. Fibronectin is known from in vitro studies to bind to apo(a)/Lp(a).18 33 Our result, therefore, not only extends this finding to an in vivo situation, but moreover shows the potential of the Two-Hybrid approach to identify ligands for apo(a), which are secretory proteins. To the best of our knowledge, this is the first time that the yeast Two-Hybrid System has been successfully used for secretory proteins.
The remaining eight clones were all 100% homologous to sequences of β2-glycoprotein I, a plasma protein also known as apolipoprotein H.34 The interaction between β2-glycoprotein I and apo(a)/Lp(a) was confirmed both in vitro by protein-protein binding studies and ex vivo by coimmunoprecipitation experiments using plasma and cellular supernatants ectopically expressing apo(a), indicating a stable interaction in vivo.
Identical results were obtained using two polyclonal, as well as two different monoclonal β-2 glycoprotein I specific antibodies, which makes any unspecific interaction or cross-reactivity unlikely. Our ELISA data and the COS-1 transfection experiments also show that there is a direct interaction between β2-glycoprotein I and apo(a), which is not mediated by LDL. LDL and other lipoproteins are long known to bind β2-glycoprotein I, but this interaction is believed to reflect the affinity of β2-glycoprotein I for phospholipids in the lipoproteins.21 The ELISA approach showed significant binding of β2-glycoprotein I to free apo(a), Lp(a), as well as LDL.
Together our data leave little doubt that β2-glycoprotein I physiologically interacts with apo(a)/Lp(a) in human plasma. The results from the Two-Hybrid assay using apo(a)KIV2 and apo(a)KIV6 indicate that the interaction of apo(a) and β2-glycoprotein I is not restricted to the repetitive kringle apo(a)KIV2 , but can also be mediated by the unique kringle apo(a)KIV6 . It is, therefore, presently unclear whether in vivo the interaction of apo(a) with β2-glycoprotein I is mediated by apo(a)KIV2 , apo(a)KIV6 , or both or also involves further kringle substructures.
The most obvious question raised by our results is what the physiological role of this interaction might be and whether it helps to explain the pathophysiological properties of Lp(a). Unfortunately, the physiological role of β2-glycoprotein I is at least as unclear as for apo(a). β2-glycoprotein I, which was first isolated by Schultze et al35 and has a unique amino acid sequence rich in proline and cysteine residues and is composed of five repeating modules, belong to the complement control protein (CCP) superfamily.36 The major expression site of human β2-glycoprotein I is the liver.37 Our analysis of overlapping clones shows that the binding of β2-glycoprotein I to apo(a) is mediated by the CCP (also called short consensus repeat [SCR]) domains 2-4. Interestingly, apo(a) was shown to interact with complement activation factor iC3b, a protein that itself is bound by several proteins of the CCP superfamily, although not by β2-glycoprotein I.38 39
Although a role for β2-glycoprotein I has been proposed in a variety of pathological pathways, no precise metabolic function has yet been assigned to this protein. As a constituent of lipoproteins of various densities,21 a function in lipid metabolism has been considered and it has been suggested that β2-glycoprotein I may play a role in triglyceride metabolism.40-42 Furthermore, β2-glycoprotein I has been shown to exhibit anticoagulant properties such as inhibition of contact activation in the intrinsic coagulation pathway,43 adenosine diphosphate (ADP)-dependent platelet aggregation,44 and prothrombinase activity of platelets.45 These functions may be modulated by interaction with apo(a)/Lp(a), providing still another potential mechanism for interloping of apo(a)/Lp(a) into blood clotting. There exists further intriguing scenarios for the role of the β2-glycoprotein I-apo(a) interaction, all of which are speculative at present.
To date, the site and mechanism by which Lp(a) particles are removed from plasma are unclear. Binding to β2-glycoprotein I could represent a possible route by which Lp(a) is cleared from plasma. It has been suggested that the entry of hepatitis B virus (HBV) particles into liver cells could be mediated via an interaction of HBV particles with lipoprotein- or liver membrane-associated β2-glycoprotein I.46 47 In analogy to the postulated HBV uptake, Lp(a) could enter liver cells by such a pathway.
Alternatively, regarding the putative role of β2-glycoprotein I in the immune clearance of “nonself” particles,48 Lp(a) particles could be taken up by macrophages via a receptor for β2-glycoprotein I or as a multimeric complex with β2-glycoprotein I. Receptor-mediated uptake of Lp(a) by macrophages has indeed been demonstrated.49
One of the best studied properties of β2-glycoprotein I is the binding to negatively charged phospholipids such as cardiolipin (CL) or phosphatidylserine (PS), which creates novel antigenic epitopes recognized by certain antiphospholipid antibodies.50-52 Autoantibodies produced against β2-glycoprotein I may interfere with the in vivo function of this plasma protein, thereby predisposing individuals to a procoagulant state, which may lead to the development of thrombotic disorders often seen in association with CL in systemic lupus erythematosus (SLE)53 and rheumatoid arthritis (RA).54,55 Because there is a strong association between significantly increased Lp(a) levels and the occurrence of thrombotic events in patients affected by these immunomediated diseases,56-58 which is not yet understood, one may speculate that the β2-glycoprotein I-Lp(a) complex could be the trigger for the observed complications. Our findings thus may link Lp(a) to autoimmunity.
Our results indicate that the yeast Two-Hybrid System provides means to identify novel proteins that may interact with apolipoprotein(a)/Lp(a). β-2 glycoprotein I appears to represent one of those proteins. The functional implication of the Lp(a)-β–2 glycoprotein I interaction mediated by the apo(a) kringle IV-domain and the relevance for the in vivo situation remains, for the moment, unclear. Undoubtedly, more work has to be done to elucidate the full functional relevance of this interaction. But our data may open new avenues for the understanding of Lp(a)'s functions and pathophysiological properties.
ACKNOWLEDGMENT
We are grateful to Dr J. Müller (Boehringer Mannheim, Mannheim, Germany) and Dr G. Müncher (Behringwerke, Marburg, Germany) for providing r-apo(a) constructs and β-2 glycoprotein I polyclonal antisera, respectively.
S.K. and F.F. contributed equally to this study and, therefore, share first authorship.
Supported in part by grants from the EC-Biomed 2 shared cost project P95-0898, the Austrian “Fond zur Förderung der wissenschaftlichen Forschung” S7109, the Austrian “Legerlotz-Stiftung”, and the Austrian Federal Bank (Nr. 5312).
Address reprint requests Professor Gerd Utermann, Institute for Medical Biology and Human Genetics, University of Innsbruck, Schoepfstr. 41, A-6020 Innsbruck, Austria.

![Fig. 5. Coimmunoprecipitation of apo(a) and β-2 glycoprotein I from COS-1 supernatants. COS-1 cells were transiently transfected with empty vector control (upper panel) or two recombinant apo(a) isoforms [r-apo(a)K11, middle panel; r-apo(a)K26, lower panel]. Following transfection, purified β-2 glycoprotein I was added to COS-1 cellular supernatants and binding of β-2 glycoprotein I to the two different r-apo(a)s was shown using a coimmunoprecipitation assay. Immunoprecipitates were formed by using two different MoAbs (F7, F10) against β-2-glycoprotein I or nonimmune serum. Immunoprecipitation of apo(a) with the apo(a) specific MoAb, 1A2, served as positive control. Immunoprecipitates were resolved by 4% to 12% Tris-Glycin gel electrophoresis and immunodetected by immunoblot analysis using anti-apo(a) MoAb 1A2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1482/3/m_bl_0002f5.jpeg?Expires=1767734827&Signature=3SZeO5-zGsPAPx8sRn3qBmJ2KFcoX-2oIMEuuVdRDDTj6bSrI6hxV1uyqYXqj9vUefzWJ-SEsHn2AKqzwxMShzBG7Kqc08onDdystFID7YjnMdUMo~lPKuKmCwe~2Vy17l5dYn11vVlQHTL1U8FKTe3IL9~uP2lv-pL3MgccyG3BDLqnw7dNb5JBI177u1hES3XFfv8DcJx~F6ZW6hZKMxhHjEskeLkBQHC1O7oarF~Zl6uyJ9apr-sn6W-Dl4YUAOeQuBOTHpCOIwsibIPf8qdgoJ7vg03VInQeeL9xYMbT1N4KsTqj7bSYpkVXLTFBTeNIB88B3sAcEVuVYMkO8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal