Abstract
To determine the kinetics of tissue macrophage and microglial engraftment after bone marrow (BM) transplantation, we have developed a model using the ROSA 26 mouse. Transplanted ROSA 26 cells can be precisely identified in recipient animals because they constitutively express β-galactosidase (β-gal) and neomycin resistance. B6/129 F2 mice were irradiated and transplanted with BM from ROSA 26 donors and their tissues (spleen, marrow, brain, liver, and lung) examined at various time points to determine the kinetics of engraftment. Frozen sections from transplanted animals were stained histochemically for β-gal to identify donor cells. At 1, 2, 6, and 12 months posttransplantation, 98% to 100% of granulocyte-macrophage colonies were of donor (ROSA 26) origin determined by β-gal staining and by neomycin resistance. Splenic monocytes/macrophages were 89% donor origin by 1 month confirming quick and complete engraftment of hematopoietic tissues. At this time, only rare ROSA 26 tissue macrophages or microglia were observed. Alveolar macrophage engraftment was evident by 2 months and had increased to 61% of total tissue macrophages at 1 year posttransplantation. The kinetics of liver Kupffer cell engraftment were similar to those seen in the lung. However, donor microglial engraftment remained only 23% of total microglia at 6 months and increased to only 30% by 1 year. Also, donor microglia were predominantly seen at perivascular and leptomeningeal, and not parenchymal, sites. The data show that microglia derive from BM precursors but turn over at a significantly slower rate than other tissue macrophages. No clinical or histological graft-versus-host disease was observed in the recipients of ROSA 26 BM. These kinetics may impact strategies for the gene therapy of lysosomal storage diseases. Because individual donor cells can be identified in situ, the ROSA 26 model should have many applications in transplantation biology including studies of homing and differentiation.
LYSOSOMAL STORAGE diseases such as Gaucher's disease and Hurler's syndrome are autosomal-recessive disorders resulting from the absence of an enzyme critical for the metabolism of a mucopolysaccharide or mucolipid.1-5 Lack of the enzymatic activity leads to accumulation of metabolic intermediates in the reticuloendothelial system often with devastating, progressive disease. Affected individuals usually develop massive organomegally as well as skeletal and neurological abnormalities. Enzyme replacement therapy can halt disease progression but it is costly and not curative.6-8 Bone marrow transplantation (BMT) has been used to treat these diseases in both human and animal studies.9-12 After transplantation, BM-derived tissue macrophages engraft in host tissues and provide a tissue-based source of normal enzyme. This allows degradation of metabolic intermediates resulting in a reduction of organomegally and halting progression of the disease. These individuals' life expectancy is increased despite the fact that many skeletal and neurological defects are not reversed by transplantation.13 14
Macrophages appear to differ from other hematopoietic cells in that they form resident populations in many of the body's tissues.15,16 The phenotypic and functional characteristics of these cells are influenced by the local microenvironment in the tissues. Thus, it is believed that monocytes derived from BM circulate in the blood from where they enter tissues and differentiate into resident macrophages such as Kupffer cells in the liver and alveolar macrophages in the lung.17-23
Microglia resemble tissue macrophages in their response to signals, secretion of cytokines, and in their surface receptors and antigens.24-26 Although some investigators suggest that microglia originate from neopallial astroglia or resident stem cells,27-30 the predominant evidence supports BM derivation.
In animal studies using carbon labeling techniques, blood monocytes have been shown to enter the early postnatal brain and become amoeboid microglia. These cells later differentiate into more ramified microglia.31 Microglia have been shown to divide in situ (as have tissue macrophages in other tissues) as well as be recruited from the blood.32-34 Male-derived donor mononuclear cells (presumably microglia) have been shown in the brains of human female BM transplant recipients.35 Thus, it appears that microglia are derived from a precursor cell which is related to the monocytic lineage.36 37
The goals of this study were to determine the kinetics of tissue macrophage and microglial engraftment after BMT. An improved understanding of the biology of these cells should help in the development of therapies, including gene therapy, for lysosomal storage diseases. Prior studies of these kinetic issues have been limited by methodology. For example, studies using retroviral-labeled BM are limited by inability to label all cells or by downregulation of marker expression during differentiation.38 In addition, some allogeneic BMT models have required manipulation of the recipient animal (eg, the induction of graft-versus-host disease (GVHD) major histocompatibility complex (MHC) to detect donor class I antigens),39 40 thereby making the interpretation of the data difficult.
We chose to develop a BMT model using the ROSA 26 mouse. The ROSA 26 mouse is a transgenic animal produced by a promoter trap insertion of β-geo which encodes for a protein that contains both Escherichia coli β-gal activity and neomycin resistance activities.41 Friedrich and Soriano42 showed that all cells of the ROSA 26 mouse express β-galactosidase (β-gal) staining throughout embryonic life. We hypothesized that all cells of an adult ROSA 26 mouse, including hematopoietic cells, would also express β-gal activity and neomycin resistance in a constituative manner. If so, marrow from the ROSA 26 mouse would provide a unique source of unmodified marrow cells to study the kinetics of macrophage and microglia engraftment after BMT.
MATERIALS AND METHODS
Animals.ROSA 26 breeding pairs were a gift from Dr Philipe Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA). Litters were weaned at 3 to 4 weeks of age. Animals 6 to 10 weeks of age were used as donors for BMT. The ROSA 26 mouse was produced using a promoter trap strategy.32 Briefly, 129Sv AB1 embryonic stem (ES) cells were infected with a retroviral vector containing the β-geo sequence. Mouse chimeras were produced by injecting ES cells into C57BL/6J mice. Chimeras were bred with C57 BL/6J females. Once germ line transmission was observed, the chimeras were bred to 129Sv mice. Thus, an appropriate control animal for transplantation studies is a hybrid of these strains. B6/129 F1 breeding pairs (a hybrid of the C57BL/6J and 129Sv strains) were obtained from the Jackson Laboratory (Bar Harbor, ME). B6/129 F2 animals, the product of these matings, served as a source of control donor cells and as recipient animals in BMTs. Young adults (6 to 10 weeks of age) were used as recipient animals. All animals were maintained under standard conditions.
Preparation of BM.Donor mice were killed and their femurs and tibias removed aseptically. Marrow cavities were flushed with Ca,Mg-free Hanks' Balanced Salt Solution (HBSS) (GIBCO-BRL, Grand Island, NY) using a 25-gauge needle attached to a syringe. Single cell suspensions were prepared by repeat pipetting and the cell preparations passed through a nylon mesh to remove particulate matter. Cells were washed two times in HBSS, counted using a hemocytometer, and are resuspended at 107 cells/mL before transplantation.
Mouse transplantation protocol.Beginning 2 weeks before transplantation, B6/129 F2 were given drinking water containing Polymixin 106 U/mL and Neomycin 1.1 g/L (Sigma, St Louis, MO) in tap water. Immediately before transplantation, mice were placed in a pie-shaped restrainer and received 1,050 cGy radiation from a dual Cesium source. After radiation, recipient mice received 5 × 106 BM (either ROSA 26 or B6/129/F2 BM) cells via tail vein injection using 1-mL syringes with 27-gauge needles. Irradiated, transplanted animals continued to receive antibiotic containing drinking water for 4 weeks after transplantation.
Preparation of frozen sections.Animals were killed at various times posttransplant for analysis of tissue macrophage engraftment. BM, spleen, liver, lung, and brain were removed. In some cases, animals were perfused with phosphate-buffered saline (PBS) followed by fixative before tissue removal. Tissues were placed in OCT Compound (Tissue-Tek, Elkhart, IN) and snap frozen in isopentane chilled with liquid nitrogen. Frozen samples were stored at −70°C until further use. Six micrometer tissue sections were cut using a cryostat and placed on positively charged slides (Fisher Scientific, Pittsburgh, PA). Sections were either stained immediately or stored at −70°C until use.
β-Gal detection.Cytospin preparations or frozen tissue sections were allowed to air dry. The slides were fixed for 10 minutes with 0.5% Gluteraldehyde (Fisher Scientific) or 4% paraformaldehyde in PBS at 4°C and washed three timed with PBS. Specimens were then placed in PBS containing 10 mmol/L K3Fe(CN)6 and 10 mmol/L K4Fe(CN)6 along with the β-gal substrate, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) (1 mg/mL) (Molecular Probes, Eugene, OR). Specimens were incubated at 37°C for 8 hours, washed well in tap water, and counterstained with safranin-O or Mayer's Hematoxylin Solution (Sigma). Specimens for immunocytochemical (ICC) staining were not counterstained. Specimens were dehydrated by serial passage through ethanol: 80%, 95%, 100%, and 100% followed by xylene. Permount (Fisher Scientific) was used to place coverslips on the specimens. Once dried, the samples were examined for the presence of the blue β-gal reaction product by light microscopy. Hematopoietic colonies (see below for methods) were stained to detect β-gal in a similar fashion. Colonies grown on an Agar base were fixed and washed as above by gentle layering of fixative and PBS washes over the agar plate using a Pasteur pipette. Staining buffer was then layered over the plates to cover the colonies and the samples placed at 37°C for 8 hours. Plates were examined using an inverted microscope. Positive colonies were stained bright blue and contrasted the unstained, β-gal− colonies.
Immunocytochemical detection of cellular antigens.ICC methods were used to determine the phenotype of β-gal expressing cells present in frozen tissue sections of transplanted animals. Tissue sections stained for β-galactosidase were washed three times with PBS, refixed in cold acetone or 4% paraformaldehyde for 10 minutes, and postfixed in formyl calcium for 10 seconds. After three more washes with PBS, endogenous biotin was blocked using the Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). Sections were then washed in PBS and incubated with rat-antimouse monoclonal antibodies (MoAbs) at a concentration of 1 to 20 μg/mL in PBS with 0.1% crystalline bovine serum albumin (BSA) overnight at 4°C. The sections were incubated with affinity purified biotinylated antirat IgG for 30 minutes at room temperature (Vector Laboratories). After washing three times, secondary antibodies coupled to alkaline phosphatase were then added (Vector Laboratories) (30 minutes, room temperature). Development of the red alkaline phosphatase substrate (Vector Laboratories) allowed immunocytochemical detection of cell-surface antigens, and contrasted well with the blue cytoplasmic β-gal product. Slides were then washed, dehydrated, and coverslips mounted as described above. Double-positive cells, with blue cytoplasm and red cytoplasmic membranes, were then enumerated using light microscopy. In some cases, β-gal+ cells were detected by incubating sections with an MoAb directed against β-gal (Sigma) followed by a secondary antibody coupled to peroxidase (Vector Elite). In these cases, stained cells were detected using a 3-3′ diaminobenzidine (DAB) substrate (Vector).
Hematopoietic colony assays.Freshly isolated BM cells from control or transplanted animals were examined for production of hematopoietic colonies. Cells were resuspended at 2 × 105/mL in Iscove's modified Dulbecco's medium (IMDM; GIBCO-BRL) supplemented with 20% fetal calf serum (Hyclone Laboratories, Logan, UT), 2 × 10−5 mol/L β-mercaptoethanol, and an Antibiotic-Antimycotic (GIBCO-BRL) with concentrations of 200 U/mL penicillin G sodium, 200 μg/mL streptomycin sulfate, and 500 ng/mL amphotericin B. These cells are warmed to 37°C in a water bath and mixed with an equal volume of 0.7% Agar Noble (Difco Laboratories, Detroit, MI) in IMDM without supplements. The cell suspension was mixed well and 1 mL placed in triplicate 35-mm tissue culture plates. For enumeration of granulocyte-macrophage (GM) colonies, 5 μL of supernatant containing recombinant murine GM colony-stimulating factor (GM-CSF ) (provided by Dr Kenneth Kaushansky, University of Washington, Seattle) was added to each plate.43 Plates were incubated for 6 days in a humidified chamber containing 5% CO2 at 37°C. Colonies (>50 cells) were counted with the use of an inverted microscope and expressed as GM colonies per 105 cells. Neomycin-resistant colonies were detected by adding the neomycin analogue G418 (GIBCO-BRL) to the colony assay at a concentration of 1,500 μg/mL. Prior control studies have shown that neomycin at 1,000 μg/mL or higher was capable of killing all normal hematopoietic colonies. Colonies expressing the transgene for neomycin were not affected by neomycin at this concentration.
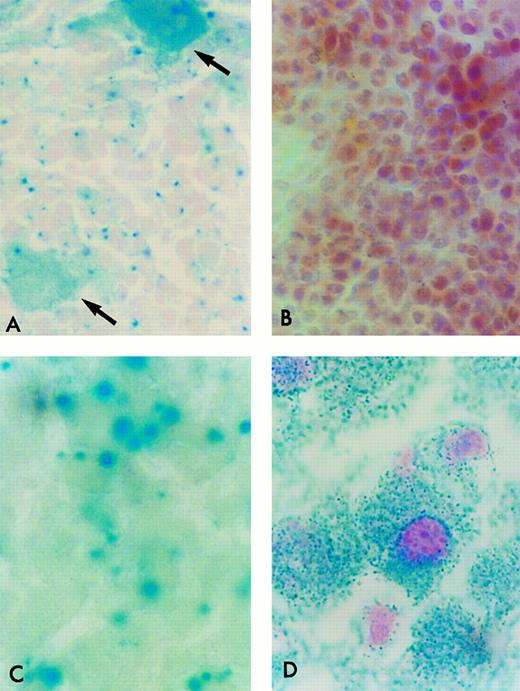
β-Gal Histochemical staining of cryostat sections of tissues from ROSA 26 animals. Frozen sections of tissues were incubated in the presence of X-gal and counterstained with safranin-O. All nucleated ROSA 26 BM cells stain blue, indicating the presence of β-gal activity (A). Most ROSA 26 cells stain with a bright blue perinuclear area. Other cells, such as the megakaryocyte (open arrow) stain with a more diffuse pattern. No cells in the control B6/129 F2 marrow stain blue, indicating the absence of β-gal activity (B). All nucleated cells in the adult ROSA 26 brain (C) and liver (D) also stain blue demonstrating β-gal activity. (B) is counterstained more intensely to allow the identification of individual cells.
β-Gal Histochemical staining of cryostat sections of tissues from ROSA 26 animals. Frozen sections of tissues were incubated in the presence of X-gal and counterstained with safranin-O. All nucleated ROSA 26 BM cells stain blue, indicating the presence of β-gal activity (A). Most ROSA 26 cells stain with a bright blue perinuclear area. Other cells, such as the megakaryocyte (open arrow) stain with a more diffuse pattern. No cells in the control B6/129 F2 marrow stain blue, indicating the absence of β-gal activity (B). All nucleated cells in the adult ROSA 26 brain (C) and liver (D) also stain blue demonstrating β-gal activity. (B) is counterstained more intensely to allow the identification of individual cells.
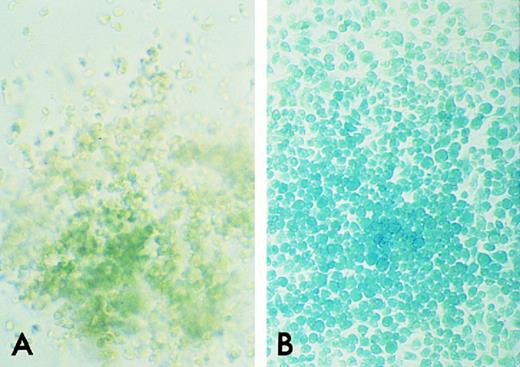
β-gal Histochemical staining of GM colonies from ROSA 26 and B6/129 F2 BM. GM colonies on an agar base were incubated in the presence of X-gal. A GM colony from B6/129 F2 marrow shows no blue staining, indicating the absence of β-gal activity (A). A GM colony from ROSA 26 marrow stains uniformly bright blue (B).
β-gal Histochemical staining of GM colonies from ROSA 26 and B6/129 F2 BM. GM colonies on an agar base were incubated in the presence of X-gal. A GM colony from B6/129 F2 marrow shows no blue staining, indicating the absence of β-gal activity (A). A GM colony from ROSA 26 marrow stains uniformly bright blue (B).
β-Gal histochemical staining of cryostat sections of spleens from B6/129 F2 animals 1 month after transplantation with ROSA 26 or B6/129 F2 BM. None of the cells in the spleen of a B6/129 F2 mouse transplanted with control B6/129 F2 marrow stain blue indicating the absence of β-gal activity (A). Nearly all cells in the spleen of a B6/129 F2 animal transplanted with ROSA 26 marrow stain blue indicating engraftment with β-gal expressing ROSA 26 BM (B). Some positive cells are highlighted by arrows.
β-Gal histochemical staining of cryostat sections of spleens from B6/129 F2 animals 1 month after transplantation with ROSA 26 or B6/129 F2 BM. None of the cells in the spleen of a B6/129 F2 mouse transplanted with control B6/129 F2 marrow stain blue indicating the absence of β-gal activity (A). Nearly all cells in the spleen of a B6/129 F2 animal transplanted with ROSA 26 marrow stain blue indicating engraftment with β-gal expressing ROSA 26 BM (B). Some positive cells are highlighted by arrows.
MoAbs.The rat-antimouse MoAbs directed against Mac-1 (Clone M1/70), Thy-1 (Clone G-7), as well as isotype controls for rat and mouse IgG were purchased from Pharmingen (San Diego, CA). Rat-antimouse F4/80 (Clone A3-1) was purchased from Biosource International (Camarillo, CA). Affinity-purified biotinylated antirat Ig was purchased from Vector Laboratories.
RESULTS
β-Gal expression by tissues from ROSA 26 and B6/129 F2 animals.To investigate whether adult ROSA 26 animals continued the ubiquitous pattern of β-gal expression present during embryonic development, tissues were removed from adult animals and frozen sections were prepared and stained in the presence of X-gal, a substrate for β-gal. As shown in Fig 1A, every nucleated marrow cell stained blue, indicating the presence of β-gal activity. Most stained with a bright blue, punctate perinuclear pattern, although some cells, such as megakaryocytes and platelets, stained in a more diffuse pattern as shown (arrows) in Fig 1A. BM from the control strain B6/129 F2 animal did not stain blue, indicating the absence of β-gal activity (Fig 1B). All nucleated cells studied from the adult ROSA 26 mouse, including those from brain (Fig 1C), lung, spleen, pancreas, liver (Fig 1D), and lymph nodes stained blue indicating the presence of β-gal activity in these tissues, whereas none of these tissues from B6/129 F2 animals stained blue (data not shown).
β-Gal expression by GM colonies from ROSA 26 and B6/129 F2 animals.To investigate whether ROSA 26 hematopoietic colonies grown in vitro continued to express β-gal, GM colonies cultured from ROSA 26 and B6/129 F2 BM were stained in the presence of X-gal. As shown in Fig 2B, GM colonies from ROSA 26 BM uniformly stain bright blue. In contrast, GM colonies from B6/129 F2 BM did not stain blue, indicating the absence of β-gal activity (Fig 2A).
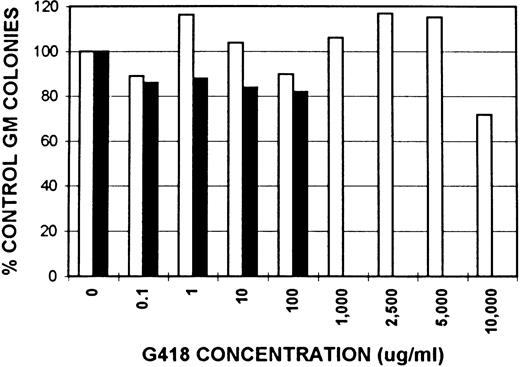
Neomycin resistance activity in GM colonies from ROSA 26 and B6/129 F2 animals.The expression of neomycin resistance by ROSA 26 and control animals was studied by culturing BM in the presence of increasing concentrations of G418, a neomycin analogue. The results of one of three similar experiments are shown in Fig 3. GM colonies from ROSA 26 BM grow at extremely high concentrations of G418. In contrast, GM colonies from control B6/129 F2 BM grow only at concentrations less than 1,000 μg/mL in all experiments.
Effect of neomycin on GM colony growth from ROSA 26 (□) and B6/129 F2 (▪) marrow. GM colonies were grown in the presence of increasing concentrations of G418, a neomycin analogue. The number of colonies that grew in the presence of G418 was compared with the number of colonies that grew in media alone and expressed as a percentage. ROSA 26 GM colonies grow at extremely high concentrations of G418. In contrast, GM colonies from B6/129 F2 marrow grow only at G418 concentrations less than 1,000 μg/mL. Concentrations given are of total drug. The specific activity of the lot of G418 used in all experiments was 705 μg/mg.
Effect of neomycin on GM colony growth from ROSA 26 (□) and B6/129 F2 (▪) marrow. GM colonies were grown in the presence of increasing concentrations of G418, a neomycin analogue. The number of colonies that grew in the presence of G418 was compared with the number of colonies that grew in media alone and expressed as a percentage. ROSA 26 GM colonies grow at extremely high concentrations of G418. In contrast, GM colonies from B6/129 F2 marrow grow only at G418 concentrations less than 1,000 μg/mL. Concentrations given are of total drug. The specific activity of the lot of G418 used in all experiments was 705 μg/mg.
ROSA 26 BM rapidly engrafts in hematopoietic tissues.Frozen sections from spleens of B6/129 F2 animals transplanted with either ROSA 26 or B6/129 F2 BM were studied at 1 month after transplantation. Nearly all of the hematopoietic cells seen in the spleens of animals transplanted with ROSA 26 BM stain blue in the presence of X-gal, indicating engraftment with ROSA 26 marrow (Fig 4B). In contrast, none of the cells in the spleens of animals transplanted with B6/129 F2 marrow stain blue (Fig 4A). GM colonies from animals transplanted with ROSA 26 BM are 98% ROSA 26 origin by 1 month after transplantation as demonstrated by comparing both β-gal staining and by neomycin-resistant colony numbers (data not shown).
Only rare ROSA 26 origin tissue macrophages and microglia engraft by 1 month after transplantation.In contrast to the rapid engraftment seen in hematopoietic tissues, only rare ROSA 26 origin tissue macrophages or microglia are seen in animals 1 month after transplantation. Rare ROSA origin cells are seen in the lungs of recipient animals (Fig 5A). The ROSA 26 origin microglia seen in recipient animals soon after transplantation are located in perivascular or leptomeningeal sites. Two ROSA 26 origin cells stained for β-gal are shown in the leptomeninges of a B6/129 F2 animal 1 month after transplantation with ROSA 26 BM (Fig 5B). No β-gal+ cells are seen in the brains of B6/129 F2 animals transplanted with B6/129 F2 BM (data not shown).
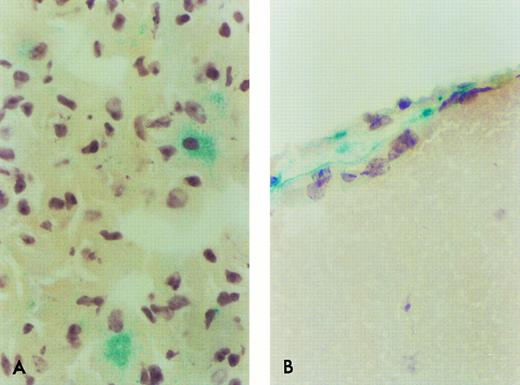
β-Gal expressing ROSA 26 donor cell engraftment in the lung and brain of B6/129 F2 animals 1 month after transplantation. Only rare β-gal–expressing cells are found in the lung (A) or brain (B) of B6/129 F2 animals by 1 month following transplantation with ROSA 26 BM. The positive cells shown in (B) are seen in a leptomeningeal site. No positive cell was seen in any site in tissues from animals transplanted with control BM. These pictures were selected to document the few positive cells at these sites.
β-Gal expressing ROSA 26 donor cell engraftment in the lung and brain of B6/129 F2 animals 1 month after transplantation. Only rare β-gal–expressing cells are found in the lung (A) or brain (B) of B6/129 F2 animals by 1 month following transplantation with ROSA 26 BM. The positive cells shown in (B) are seen in a leptomeningeal site. No positive cell was seen in any site in tissues from animals transplanted with control BM. These pictures were selected to document the few positive cells at these sites.
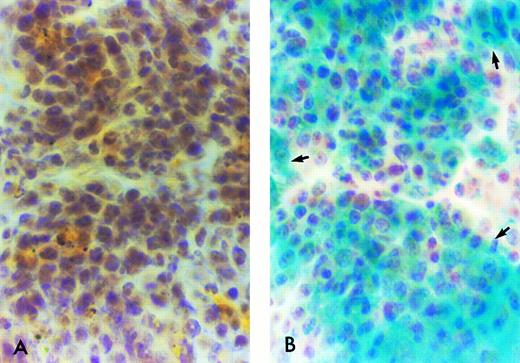
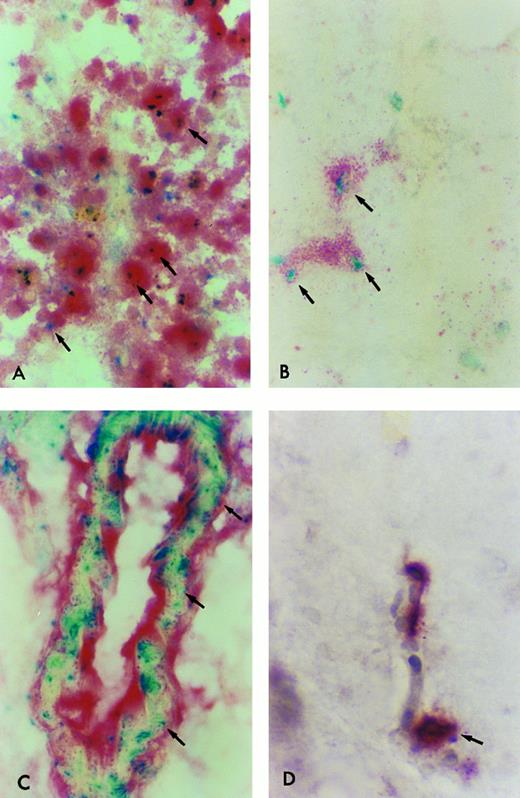
β-Gal expressing tissue macrophage and microglia engraftment in B6/129F2 animals 1 year after transplantation. Cryostat sections of spleen, lung, liver, and brain from B6/129 F2 animals transplanted with ROSA 26 BM were histochemically stained blue to demonstrate the presence of β-gal expressing ROSA 26 origin cells followed by immunocytochemical staining red with the antibodies F4/80 and M1/70 directed against mouse macrophage markers. Double-positive cells (arrows) are shown in the spleen (A), lung (B), and perivascular areas of the brain (C). Only rare double-positive cells are found in the parenchyma of the brain (D). All double-positive cells in (B) and (D) are indicated by arrows. Some double-positive cells in (A) and (C) are highlighted by arrows.
β-Gal expressing tissue macrophage and microglia engraftment in B6/129F2 animals 1 year after transplantation. Cryostat sections of spleen, lung, liver, and brain from B6/129 F2 animals transplanted with ROSA 26 BM were histochemically stained blue to demonstrate the presence of β-gal expressing ROSA 26 origin cells followed by immunocytochemical staining red with the antibodies F4/80 and M1/70 directed against mouse macrophage markers. Double-positive cells (arrows) are shown in the spleen (A), lung (B), and perivascular areas of the brain (C). Only rare double-positive cells are found in the parenchyma of the brain (D). All double-positive cells in (B) and (D) are indicated by arrows. Some double-positive cells in (A) and (C) are highlighted by arrows.
ROSA 26–derived tissue macrophages and microglia can be identified in situ.To determine the phenotype of the ROSA 26 origin cells, an immunocytochemical technique was developed to follow the histochemical stain for β-gal. Tissue macrophages and microglia were characterized using antibodies directed against the F4/80 and Mac-1 antigens.44 45 F4/80, which identifies macrophages, monocytes and some progenitors, was used to identify macrophages in all tissues studied. For identification of microglia, an MoAb directed against Mac-1 was used in combination with F4/80 to increase sensitivity. Mac-1 is expressed on macrophages, monocytes, granulocytes, natural killer (NK) cells, and some B cells. Figure 6 shows double-positive (F4/80 and β-gal expressing) cells in tissues of B6/129 F2 mice 1 year after transplantation with ROSA 26 BM. Numerous double-positive cells are seen in the red pulp of the spleen at 1 year (Fig 6A). Double-positive cells are scattered throughout the parenchyma of the liver (data not shown) and lung (Fig 6B) at 1 year. Double-positive perivascular and leptomeningeal microglia are found at 1 year (Fig 6C) but only rare double-positive cells are seen in the parenchyma of the brain (Fig 6D).
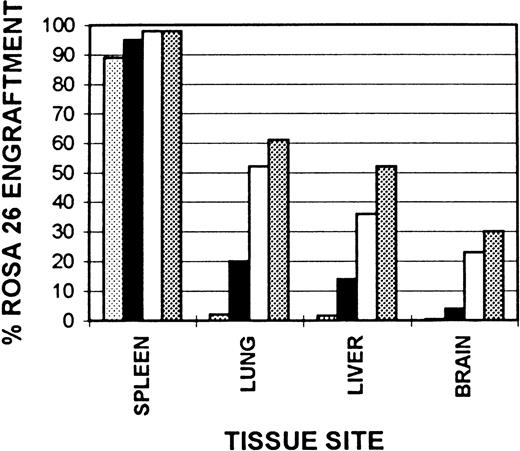
Kinetics of tissue macrophage and microglial engraftment.Cummulative ROSA 26 engraftment data are shown in Fig 7. Splenic monocyte/macrophage engraftment begins early following transplantation with nearly 90% ROSA 26 engraftment by 1 month. This progresses to 98% by 6 months and remains at this level at 1 year after transplantation. Although only rare alveolar macrophages are of ROSA 26 origin at 1 month after transplantation, by 6 months 52% are of ROSA 26 origin and by 1 year this number has increased to 61%. The kinetics of Kupffer cell engraftment are similar to those in the lung with 36% ROSA 26 origin macrophages seen at 6 months, increasing to 52% by 1 year. In contrast, the kinetics of microglial engraftment appear to be much slower. Less than 1% of ROSA 26 origin microglia are seen at 1 month after transplantation. At 2 months 4% are of ROSA 26 origin, at 6 months, 23%, and by 1 year only 30% are of ROSA 26 origin.
Kinetics of tissue macrophage and microglial engraftment. Cryostat sections of spleen, lung, liver, and brain from B6/129 F2 animals transplanted with ROSA 26 BM were histochemically stained to show the presence of β-gal expressing ROSA 26 origin cells followed by immunocytochemical staining with the antibodies F4/80 and M1/70 directed against mouse macrophage markers. A percent engraftment with ROSA 26 origin cells was calculated using the formula: % Engraftment = β-Gal+ Macrophages/Total Macrophages. Macrophages were enumerated on three representative sections each from three transplanted animals at every time point. (▧), 1 month, (▪), 2 months; (□), 6 months; (▧), 1 year.
Kinetics of tissue macrophage and microglial engraftment. Cryostat sections of spleen, lung, liver, and brain from B6/129 F2 animals transplanted with ROSA 26 BM were histochemically stained to show the presence of β-gal expressing ROSA 26 origin cells followed by immunocytochemical staining with the antibodies F4/80 and M1/70 directed against mouse macrophage markers. A percent engraftment with ROSA 26 origin cells was calculated using the formula: % Engraftment = β-Gal+ Macrophages/Total Macrophages. Macrophages were enumerated on three representative sections each from three transplanted animals at every time point. (▧), 1 month, (▪), 2 months; (□), 6 months; (▧), 1 year.
DISCUSSION
We have studied the kinetics of tissue macrophage and microglia engraftment after BMT using the ROSA 26 transplantation model. Individual cells have been shown in situ by β-gal staining of tissues. In addition, ROSA 26 BM cells have been cultured in vitro and demonstrate high level neomycin resistance as well as β-gal activity. Using this model we have found that hematopoietic tissue engraftment with donor ROSA 26 cells is both rapid and persistent. In contrast to the rapid engraftment seen in hematopoietic tissues, the replacement of tissue macrophages and central nervous system (CNS) microglia appears to occur at a slower pace.
Our studies confirm that CNS macrophages and microglia in perivascular and leptomeningeal sites are derived from BM. These cells engraft slowly following transplantation and 30% replacement is seen at 1 year after transplantation. However, only rare ROSA 26–derived parenchymal microglia were seen at 1 year after transplantation. Possible explanations for this finding are that parenchymal cells can arise from a cell other than a BM-derived cell,27-30 or more likely, that they are of hematopoietic origin but enter the CNS before birth34 and turn over at an extremely slow rate.
BMT models have previously been used to study the origin of CNS macrophages and microglia. Hickey and Kimura36 examined rat BMT chimeras 2 months after transplantation. Their studies required the induction of GVHD to upregulate expression of MHC class I markers allowing identification of donor origin cells. These experiments demonstrated 50% to 70% replacement of perivascular macrophages and thus they found a more rapid engraftment of perivascular microglia than is seen in our studies or in the investigations by others. It is possible that induction of GVHD increased the turnover of host perivascular microglia or the ability of donor cells to engraft at perivascular sites. Other models have required upregulation of donor MHC class I molecules with cytokines or by the induction of experimental autoimmune encephalitis to detect donor microglia.46 47
Additional studies have examined microglial engraftment using a Ly 5.1/5.2 congenic BMT system.48 Analysis of the mononuclear cell preparation from digested CNS tissue showed that 34% of Mac-1+ CNS microglia were donor origin at 120 days after transplantation. A disadvantage to this type of analysis is that individual cells cannot be shown in situ, thus, the contribution of donor cells to perivascular versus parenchymal sites cannot be assessed.
A bacteriophage λ transgenic mouse model has been used to study microglia engraftment in neonatal and adult BMT recipients.49 However, only 80% to 90% of donor marrow cells could be detected with the λ DNA probe in this model. Donor macrophages were identified with in situ hybridization for the λ bacteriophage along with immunocytochemical detection of the macrophage markers MOMA-1, Mac-1, and F4/80. This study suggests the utility of using a transgenic model and the importance of in situ analysis. However, in situ hybridization techniques were not sensitive enough to detect all cells containing the relevant gene. Also, kinetic data were not presented.
A recent study used retroviral labeling of BM from 5-fluorouracil-treated mice.38 This study examined the kinetics of macrophage and microglia engraftment in detail using ICC demonstration of CD11b/CD18+ microglia that expressed the transferred gene, glucocerebrosidase. Twenty percent of microglia (primarily perivascular) were found to be donor origin at 3 to 4 months. This engraftment remained stable at 6 to 8 months (21%). No further time points were assessed. These data are similar to our finding that 23% of microglia are donor origin at 6 months after transplantation, with 30% of donor origin at 1 year. They also found that 67% of lung macrophages were replaced by donor cells at 6 to 8 months after transplantation, consistent with our data. However, in contrast with our results, only 10% of liver macrophages were donor origin at 6 to 8 months and the percentage of splenic macrophages of donor origin declined between 1 and 6 months. Higher engraftment was seen with polymerase chain reaction (PCR) determinations, suggesting decreased glucocerebrosidase expression limited the ability to detect donor cells in situ. It is also of concern that T lymphocytes and most B lymphocytes were not of donor origin by either ICC or PCR analyses. This suggests that few pluripotent stem cells had been infected by the retroviral vector, an issue which is not relevant when donor cells are derived from transgenic mice.
A potential concern with the ROSA 26 transplantation model is the effects of MHC barriers on engraftment kinetics. Donor ROSA 26 mice were produced in 129Sv AB1 ES cells, bred with C57BL/6J mice, and then back bred onto the 129Sv strain. Recipient mice (B6/129 F2) are the product of B6/129 F1 breeding pairs and thus are a hybrid of the 129 Sv and C57BL/6J strains of mice. Because we have seen both rapid and persistent engraftment of the hematopoietic compartment in all transplanted animals studied, and have observed no clinical or pathologic evidence of GVHD in any transplanted animal, we suspect that minor MHC incompatibility is not a significant concern. A ROSA 26 genotype backcrossed onto the C57BL stain is now available through Jackson Laboratories (Bar Harbor, ME)50 and can be used in future studies.
In both animals and humans with lysosomal storage diseases, hepatosplenomegaly has been corrected rapidly after allogeneic BMT. Thus, a small number of genetically correct donor origin cells may have a significant biological effect in these disease states. Unfortunately, the neurological defects in affected individuals generally persist.9-14 This finding is consistent with our data which show that microglia, especially parenchymal microglia, turn over at a significantly slower rate than other tissue macrophages.
Several strategies might improve these results. First, it remains unclear what the relative contribution of parenchymal versus perivascular and leptomeningeal microglia are in correcting CNS defects after transplantation for lysosomal storage diseases. Studies by others suggest that parenchymal microglial precursors enter the CNS antenatally.34 Therefore, in utero transplantation with genetically correct cells may more completely correct the lysosomal storage defect in identified individuals by allowing donor cells to engraft at CNS parenchymal sites. As a second approach, one could change the radiation dosage and schedule to optimize radiation induced changes in the blood brain barrier51,52 and facilitate the entry of cells into tissues. Alternatively, one could induce endothelial cell and monocyte selectins and integrins that play an important role in the adhesion of monocytes to endothelium.53-60 The ROSA 26 model will allow completion of these cell turnover studies. Because individual donor cells can be identified in situ, this model should have many applications in transplantation biology, including studies of homing and differentiation.
ACKNOWLEDGMENT
The authors thank Dr Allen Gown and Marilyn Skelly for helpful discussions.
Supported by National Institutes of Health Grants No. R01 DK 49652, T32 HL 07093, and K08 DK 02443. J.L.A. is the recipient of a Faculty Research Award from the American Cancer Society. D.W.K. is the recipient of a fellowship from the Cure For Lymphoma Foundation.
Address reprint requests to David W. Kennedy, MD, PhD, Division of Hematology, Box 357710, University of Washington, Seattle, WA 98195.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal