Abstract
In thalassemia after successful bone marrow transplantation (BMT), iron overload remains an important cause of morbidity. After BMT, patients have normal erythropoiesis capable of producing a hyperplastic response to phlebotomy so that this procedure can be contemplated as a method of mobilizing iron from overloaded tissues. A phlebotomy program (6 mL/kg blood withdrawal at 14-day intervals) was proposed to 48 patients with prolonged follow-up (range, 2 to 7 years) after BMT. Seven patients were not submitted to the program (five because of refusal and two because of reversible side effects). The remaining 41 patients (mean age, 16 ± 2.9 years) were treated for a mean period of 35 ± 18 months. All were evaluated before and after 3 ± 0.6 years of followup. Values are expressed as mean ± standard deviation (SD) or as median with a range (25 to 75 percentile). Serum ferritin decreased from 2,587 (2,129 to 4,817) to 417 (210 to 982) μg/L (P < .0001), total transferrin increased from 2.34 ± 0.37 to 2.7 ± 0.58 g/L (P = .0001), transferrin saturation decreased from 90% ± 14% to 50% ± 29% (P < .0001). Liver iron concentration evaluated on liver biopsy specimens decreased from 20.8 (15.5 to 28.1) to 4.2 (1.6 to 14.6) mg/g dry weight (P < .0001). Aspartate transaminase decreased from 2.7 ± 2 to 1.1 ± 0.6 (P < .0001) and alanine transaminase from 5.2 ± 3.4 to 1.7 ± 1.2 (P < .0001) times the upper level of normality. The Knodell score for liver histological activity decreased from 6.9 ± 3 to 4.9 ± 2.8 (P < .0001). These data indicate that phlebotomy is safe, efficient, and widely applicable to ex-thalassemics after BMT.
BONE MARROW transplantation (BMT) is the only currently available curative treatment for homozygous β-thalassemia.1 We developed a system for assigning patients undergoing BMT to prognostically useful categories. Three risk factors have been identified: the presence of hepatomegaly, the presence of liver fibrosis in the pretransplantation liver biopsy, and a history of inadequate chelation therapy in the years before BMT. These three factors stratify patients into three groups: class 1, none of the risk factors; class 2, one or two of the risk factors; and class 3, all three risk factors. Immediately after cure by BMT, patients still have disease processes acquired before transplantation as a consequence of thalassemia and its treatment. We have reported spontaneous, but slow decreases, in serum ferritin and liver iron and increases in serum total iron binding capacity as the years elapse after BMT.2,3 For some patients without severe iron overload, the rate of unloading was sufficient to reduce tissue iron levels to normal, but this was not achieved in patients who had severe iron overload at the time of transplant. Moreover, prolonged follow-up with monitoring by sequential liver biopsies has shown that patients with severe pretransplant liver damage and high iron stores may have progression of liver fibrosis after BMT.3 4 Some of this disease process may be a consequence of the association of iron overload and hepatitis C infection and therefore susceptible to improvement by removing the iron overload.
After BMT, patients have normal erythropoiesis capable of producing a hyperplastic response to phlebotomy so that this procedure can be contemplated as a method of mobilizing iron from overloaded tissues. This report describes experience with this approach.
MATERIALS AND METHODS
Patient selection.From July 1991 to December 1992, 48 patients who had been regularly followed-up after transplantation in Pesaro for homozygous β-thalassemia were enrolled in a program of regular phlebotomy. They were transplanted August 20, 1984 through September 27, 1990, and the mean time between BMT and start of phlebotomy was 4.5 ± 1.5 years (range, 2 to 7 years). Patients were eligible for this study if they were in prognostic classes 2 or 3 before transplantation,1 the 2-year follow-up examinations showed serum ferritin levels greater than 2,000 μg/L and the 2-year posttransplant liver biopsies showed moderate or severe iron overload.3 Patients with mixed chimerism5 or active evidence of chronic graft-versus-host disease were excluded. Informed consent was obtained from all patients and parents after the procedure and risks had been explained in detail.
Regimen.The posttransplant phlebotomy treatment for these patients was performed at several centers throughout Italy (see Appendix 1). A protocol was agreed with collaborating centers that specified phlebotomy of 6 mL/kg at 14-day intervals. When the serum ferritin level fell below 300 μg/L, a less intensive regimen was used with a 21-day interval. Guidelines also included replacement of volume of blood withdrawn with normal saline or with autologous plasma, complete blood count before any venesection, physical examination and blood pressure measurement before and after any venesection. Centers were advised not to perform venesection if the hemoglobin was <90 g/L or the systolic blood pressure was less than 90 mm Hg. Liver function tests and indirect assessment of iron overload (serum ferritin) were recommended after the fourth venesection and at any venesection after the achievement of a serum ferritin <300 μg/L. Six patients treated in Torino received monthly erythrocytapheresis. Of a total of 1,968 phlebotomies, 1,958 (99.5%) generated a physician-signed report sent to Pesaro every 4 to 6 months detailing blood volume removed, complete blood count, amount of iron removed (1 g of hemoglobin = 3.4 mg of iron) and any adverse effects.
Evaluation.Annual follow-up in Pesaro (to December 31, 1995) included physical examination, complete blood count, serum ferritin (enzyme-linked immunosorbent assay [Eurogenetics, Turin, Italy] reference range, 20 to 200 μg/L) total transferrin and unbound transferrin (expressed as g/L, normal values respectively 2.5 to 4 and 1.5 to 3.4), serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (expressed as multiples of the upper limit of the reference range). Hepatitis C virus (HCV) status was assessed with the latest generation antibody-detection technique and with the polymerase chain reaction (PCR) using commercially available kits (Amplicor Hepatitis C virus test, Roche Diagnostic System Inc, Basel, Switzerland) and the technique recommended by the manufacturer. A biopsy of the center of right lobe of the liver with ultrasound guidance was performed annually. Liver biopsies were assessed by a coloration technique3,6 and reviewed by one of us (P.M.) who was blinded to the therapy and patients' clinical condition. They were graded for inflammation according to the Knodell Index7 (Appendix 2). Two patients who received interferon therapy for HCV disease were evaluated for iron depletion, but not for inflammatory lesions.
The liver iron concentration (LIC) was assayed by atomic absorption spectrophotometry6,8 and expressed as mg/g dry weight (dw), which may be converted to μmol of iron per gm by multiplying by 17.9 and to wet weight by dividing the concentration by 3.33, assuming that liver is 70% water9 (normal value <1.6 mg/g dw10 11 ). Specimens with a total dry weight of 0.49 mg or less were not evaluated for iron content.
Statistics.Treatment adherence was calculated as the ratio of phlebotomies performed to phlebotomies scheduled. Values were expressed as medians with a range (25 to 75 percentile) or as means ± standard deviations. The paired Student's t-test for means was used to assess the significance of difference between comparison, and the Wilcoxon test was used for comparison of ordered values.
RESULTS
Patient characteristics.Table 1 reports the pretransplant characteristics of the 41 patients treated. Only seven patients had received regular chelation before transplantation; 20 were class 2 and 21 were class 3. Thirty-five (85%) were hepatitis C seropositive and 29 had HCV-RNA detectable in serum by PCR; no patient was hepatitis B surface antigen positive. The mean time between the start of the program and last follow-up was 3 ± 0.6 years. The following parameters were registered before the beginning of the program and at last follow up: age (years) 16 ± 2.9 and 19 ± 3; height (centimeters) 150 ± 9 and 157 ± 7; body weight (kilograms) 43 ± 9 and 51 ± 10, hemoglobin (g/L) 126 ± 15 and 127 ± 16. A total of 170 annual follow-up examinations were conducted in Pesaro, 146 liver biopsies were performed, and there was one complication related to liver biopsy (intraabdominal bleeding requiring conservative management with 5 days of hospital stay and one transfusion).
Patient Characteristics Before BMT (n = 41)
| Male/female | 19/22 |
| Age (yr) | 12 ± 3.1 |
| Number of red blood cells units transfused before BMT | 188 ± 91 |
| Years of transfusion-dependent life before BMT | 11 ± 1 |
| Thalassemia status of BM donors (normal/trait) | 15/26 |
| Serum ferritin ( μg/L) | 3,964 (2,761-5,246) |
| Transferrin saturation (%) | 89 ± 17 |
| AST* | 2.5 ± 1.7 |
| ALT* | 4.4 ± 3.3 |
| Knodell Index7 (41 liver biopsies) | 7.3 ± 3.3 |
| Liver iron concentration mg/g dw (21 liver biopsies) | 24.3 (18.8–27.6) |
| Male/female | 19/22 |
| Age (yr) | 12 ± 3.1 |
| Number of red blood cells units transfused before BMT | 188 ± 91 |
| Years of transfusion-dependent life before BMT | 11 ± 1 |
| Thalassemia status of BM donors (normal/trait) | 15/26 |
| Serum ferritin ( μg/L) | 3,964 (2,761-5,246) |
| Transferrin saturation (%) | 89 ± 17 |
| AST* | 2.5 ± 1.7 |
| ALT* | 4.4 ± 3.3 |
| Knodell Index7 (41 liver biopsies) | 7.3 ± 3.3 |
| Liver iron concentration mg/g dw (21 liver biopsies) | 24.3 (18.8–27.6) |
Multiple of upper limit of reference range. Values are expressed as mean ± SD or as median with a range (25 to 75 percentile).
Adherence to treatment and complications.Five patients were denied treatment. In one case, for problems with venous access; in three cases, for no specific reason; and in one case, because the referring physician refused to submit the patient to the proposed treatment. Two patients experienced symptomatic hypotension. One of these patients had a previously documented adrenal deficiency, and hypotension occurred 24 hours after the third phlebotomy. The episode was considered unrelated to phlebotomy, but the patient was withdrawn from the study and assigned to deferoxamine treatment. The second patient had an episode of hypotension after the second phlebotomy. The treatment was interrupted for 3 months and thereafter restarted without complication. One patient was anemic (hemoglobin [Hb] 70 g/L) after the third phlebotomy (the guidelines had not been followed): after 1 month interruption, the phlebotomy program was continued without further complications for 1 year, at which time it was judged that the patient needed more aggressive iron depletion than could be achieved by a tolerable schedule of phlebotomy, and deferoxamine treatment was instituted.12 This patient had the lowest baseline Hb level of the whole group (Hb 90 g/L). Four patients developed mild reversible thrombocytopenia (with platelet counts of 93, 99, 102, and 113 × 109/L). No other complications were registered. Treatment was proposed to 48 patients: seven were not submitted to the program (four received deferoxamine,12 three refused any form of treatment) and 41 (85%) were treated. Adherence to protocol (phlebotomies scheduled/performed) was 72% ± 23%; eight patients had an adherence rate of 100% and nine less than 50%.
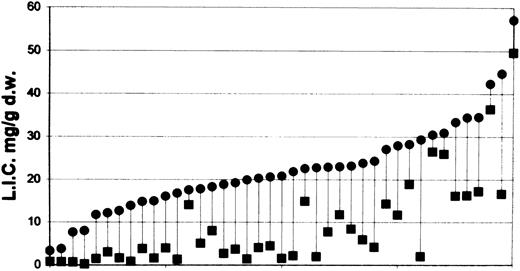
Iron depletion.Table 2 describes the performance of the treatment program and estimates of iron removed by phlebotomy. Table 3 presents the laboratory assessments of iron removal as indicated by the levels of iron and transport proteins in serum and the iron concentrations in the liver before and after 3 years of treatment. Figure 1 shows the level of liver iron concentration before and at last follow-up in all 41 patients.
Adherence to Treatment and Iron Depletion
| Characteristic . | Mean ± SD . |
|---|---|
| Months of therapy | 35 ± 18 |
| Phlebotomies (n) | 48 ± 26 |
| Adherence to treatment (%) | 72 ± 23 |
| Blood withdrawal (L) | 13.4 ± 7.1 |
| Iron removed (g) | 5.6 ± 3 |
| Characteristic . | Mean ± SD . |
|---|---|
| Months of therapy | 35 ± 18 |
| Phlebotomies (n) | 48 ± 26 |
| Adherence to treatment (%) | 72 ± 23 |
| Blood withdrawal (L) | 13.4 ± 7.1 |
| Iron removed (g) | 5.6 ± 3 |
Laboratory Measures of Iron Depletion
| Parameter . | Before Phlebotomy . | Last Follow-up . | Two-tailed P Value . |
|---|---|---|---|
| Serum ferritin ( μg/L) | 2,587 (2,129-4,817) | 417 (210-982) | <.00013-150 |
| Total transferrin (g/L) | 2.34 ± 0.37 | 2.7 ± 0.58 | .00013-151 |
| Unbound transferrin (g/L) | 0.11 (0.02-0.42) | 1.47 (0.63-2.14) | <.00013-150 |
| Transferrin saturation (%) | 90 ± 14 | 50 ± 29 | <.00013-151 |
| Liver iron concentration (mg/g dw) | 20.8 (15.5-28.1) | 4.2 (1.6-14.6) | <.00013-150 |
| Parameter . | Before Phlebotomy . | Last Follow-up . | Two-tailed P Value . |
|---|---|---|---|
| Serum ferritin ( μg/L) | 2,587 (2,129-4,817) | 417 (210-982) | <.00013-150 |
| Total transferrin (g/L) | 2.34 ± 0.37 | 2.7 ± 0.58 | .00013-151 |
| Unbound transferrin (g/L) | 0.11 (0.02-0.42) | 1.47 (0.63-2.14) | <.00013-150 |
| Transferrin saturation (%) | 90 ± 14 | 50 ± 29 | <.00013-151 |
| Liver iron concentration (mg/g dw) | 20.8 (15.5-28.1) | 4.2 (1.6-14.6) | <.00013-150 |
Tests of significance performed with Wilcoxon test for comparison of ordered values (median [25 to 75 percentile]).
Tests of significance were performed with paired Student's t-test for comparison of mean ± SD.
Liver iron concentration (LIC) expressed as mg/g dry weight before the start of phlebotomy program (•) and at last follow-up (▪) of all 41 patients treated. Data have been ordered on the basis of pretreatment liver iron concentration.
Liver iron concentration (LIC) expressed as mg/g dry weight before the start of phlebotomy program (•) and at last follow-up (▪) of all 41 patients treated. Data have been ordered on the basis of pretreatment liver iron concentration.
Liver inflammatory lesions.Table 4 reports inflammatory parameters in the 39 evaluable patients before and after the course of phlebotomy. The scores for inflammation and transaminase values showed a trend towards a uniform decrease in parallel with liver iron content, but this was not the case for the fibrosis score. During the study period, 29 (74%) patients showed no change in the fibrosis score; of the 10 remaining patients, seven (18%) showed an improvement and three (8%) deteriorated. All of these changes have been confirmed by at least two follow-up liver biopsies.
Inflammatory Lesions and Liver Fibrosis
| Parameter . | Before Phlebotomy . | Last Follow-up . | Two-tailed P Value . |
|---|---|---|---|
| AST (times upper level) | 2.7 ± 2 | 1.1 ± 0.6 | <.00014-150 |
| ALT (times upper level) | 5.2 ± 3.4 | 1.7 ± 1.2 | <.00014-150 |
| Liver histology scores7: | |||
| Piecemeal Necrosis | 1 (0-1) | 0 (0-1) | .01264-151 |
| Intralobular degeneration | 1 (1-3) | 0 (0-1) | .00014-151 |
| Portal inflammation | 1 (1-3) | 1 (1-1) | .00634-151 |
| Fibrosis | 3 (3-3) | 3 (1-3) | .184-151 |
| Knodell index‡ | 6.9 ± 3 | 4.9 ± 2.8 | <.00014-150 |
| Parameter . | Before Phlebotomy . | Last Follow-up . | Two-tailed P Value . |
|---|---|---|---|
| AST (times upper level) | 2.7 ± 2 | 1.1 ± 0.6 | <.00014-150 |
| ALT (times upper level) | 5.2 ± 3.4 | 1.7 ± 1.2 | <.00014-150 |
| Liver histology scores7: | |||
| Piecemeal Necrosis | 1 (0-1) | 0 (0-1) | .01264-151 |
| Intralobular degeneration | 1 (1-3) | 0 (0-1) | .00014-151 |
| Portal inflammation | 1 (1-3) | 1 (1-1) | .00634-151 |
| Fibrosis | 3 (3-3) | 3 (1-3) | .184-151 |
| Knodell index‡ | 6.9 ± 3 | 4.9 ± 2.8 | <.00014-150 |
Data were available for 39 patients.
Tests of significance were performed with paired Students' t-test for comparison of mean ± SD.
Wilcoxon test for comparison of ordered values (median [25 to 75 percentile]). Thirty-three patients were anti-HCV seropositive and 27 had HCV viremia as detected by PCR.
See Appendix 2.
By December 1995, eight patients achieved a normal liver iron content: 0.9 ± 0.4 (range, 0.7 to 1.4) mg/g dw compared with pretreatment values of 10.6 ± 6 (range, 3.3 to 19.9). All of the others patients are continuing regular treatment.
DISCUSSION
Although BMT is a rational therapeutic approach for the cure of β-thalassemia major, there is no reason to expect that it will erase the damage resulting from years of thalassemia and its treatment. When transplantation is performed in patients with advanced disease, a halt to disease progression is not sufficient. Health and a normal life expectancy can only be achieved by curing the lesions that already existed at the time of transplant. Moreover, BMT may actually aggravate some of the existing lesions because of procedure-related morbidity related to the patients pretransplant clinical condition.1 4 Foremost among the complications of treated thalassemia are those that result from iron overload.
We have reported a gradual spontaneous decrease of iron overload assessed by indirect and direct techniques2,3 in the years after BMT. In patients with the least degree of iron overload, the rate of this decrease was almost sufficient to normalize iron levels,13 but this did not occur in patients with severe iron overload at the time of transplant, and in these patients, there may be progression of liver damage after BMT.3,4 It has been proposed that normalization of iron overload could be accelerated by natural low intensity iron chelation14 combined with the use of iron for growth in the years after BMT. However, with this approach, the decrease in iron load occurs so slowly that a very long time would be needed to achieve a clinically useful improvement.2 14 In the present series, the slow decrease of serum ferritin observed during the period between transplant and the beginning of the phlebotomy program was not accompained by a concomitant decrease of iron concentration in the liver in the 21 evaluable cases. In addition, many patients have coincidental infection with HCV or other viruses contributing to increased liver damage. Because of the normal erythropoiesis achieved and the very limited excretion of excess iron, phlebotomy appeared to be appropriate to remove excess transfusional iron acquired before BMT.
Patients were enrolled on this program on the basis of an indirect or semiquantitative estimate of iron overload using the serum ferritin level.6,15 16 Thus, patients had very different levels of iron stores at enrollment: liver iron concentration ranged from 3.3 to 57.18 mg/g dw (17.7 to 307 μmol/g wet weight). Some patients achieved normal liver iron concentrations in a few months, while others still have severe iron overload after 3 years of therapy (Fig 1).
In the 41 treated patients, adherence to protocol was only 72% ± 23% despite treatment being designed to maintain an almost completely normal life. Psychological rather than medical difficulties were encountered. No cases of graft failure, prolonged anemia, or protein depletion were seen, while it was possible to achieve normal liver iron concentrations (Fig 1). The speed with which this can be accomplished will also be affected by growth rate, natural low intensity iron chelation,14 variability in iron distribution due to BMT,3 and variability in iron absorption over the years. This study clearly shows that a moderately intensive phlebotomy program is safe for thalassemic patients after BMT. Other reports confirm the feasibility of regular phlebotomy after BMT,17,18 and this form of therapy is likely to be much safer, cheaper, and more widely applicable than alternative iron chelation therapy.19
During the treatment there was a significant decrease in transaminase levels, confirmed by significant improvement of liver histologic inflammatory lesions. There is much debate about the severity and likelihood of progression of HCV disease,20-22 and on the possible effect of iron on virus virulence and response to interferon therapy.23,24 A recent study showed that HCV was not a major contributing factor to morbidity and mortality during the first 10 years after BMT25 for causes other than thalassemia. However, thalassemic patients, after BMT, present a special circumstance because of the persistence of infections that may have been acquired many years before BMT, and the nature and amount of iron overload, which is much higher than the threshold level identified as a predictor of resistance to interferon therapy.23 The role of cofactor in HCV disease progression has been emphasized22 and our experience suggests that iron, at least at the level reported here, could be one of these cofactors for patients with a history of thalassemia.
During the 3 years of follow-up, two patients (both anti-HCV negative) showed disappearance of mild fibrosis, five had an improvement in liver fibrosis score from grade 3 to grade 1 (disappearance of fibrous bridges). Three patients worsened, one from grade 1 to grade 3, two patients developed cirrhosis from severe fibrosis. Because of the possible sampling variation in the diagnosis of cirrhosis and fibrosis,6 26 these modifications must be confirmed by further observations.
When liver architecture is preserved,27 fibrosis is potentially reversible as seen in hemochromatosis and Wilson disease.28 However, cirrhosis is usually believed to be irreversible even if the concept of irreversibility is not proven28 (in two patients with genetic hemochromatosis, serial liver biopsies showed apparent reversal of established cirrhosis29 ). Thalassemic patients differ from those in previous studies in various aspects: the dual liver insult (iron and viral infection) and BMT-related toxicity may be counterbalanced by the possibility of recovery after cure of the thalassemia disease, particularly in children whose organs have still to complete development. Prolonged follow-up with repeated observation will be needed to determine the degree of reversibility of liver disease in this group of patients.
ACKNOWLEDGMENT
We are very grateful to all physicians and nurses involved in the phlebotomy program; their continuous and precious efforts made this report possible. We acknowledge Dr Reginald A. Clift for his criticism during the preparation of the manuscript and Professor Martin J. Pippard for editing the manuscript.
APPENDIX 1
The Cooperative Italian Group for the treatment of transplanted thalassemia includes the following investigators and collaborating centers: Rovelli A, Monguzzi W, Masera G, Clinica Pediatrica Universita' di Milano, Polo di Monza, Ospedale San Gerardo, Monza; Piga A, Garofalo F, Gabutti V, Istituto di Clinica Pediatrica Universita' degli Studi di Torino; DiGregorio F, Romeo MA, Cammella A, Russo G, Clinica Pediatrica Universita' degli Studi di Catania; Gallisai D, Burrai C, Costi C, Marinaro AM, Istituto ‘Amerigo Filia’ Clinica Pediatrica Universita' degli Studi di Sassari; Erbeia M, Mazzani D, Divisione di Pediatria, Unita' Sanitaria Locale 38 Courgne', Torino; Nobili B, Perrotta S, Ferrara, Cutillo S, Clinica Pediatrica I°, Seconda Universita' di Napoli; Mulas G, Careddu F, Centro Trasfusionale di Microcitemia, Ospedale Civile di Olbia, Olbia, Sassari; Mancini E, Centro Trasfusionale ed Immunoematologia, Ospedale Umberto I, Frosinone; Argiolu F, Addari C, Cao A, Istituto di Clinica e Biologia dell' eta' evolutiva, Universita' degli Studi di Cagliari; Ruggiero L, Divisione di Pediatria, Ospedale ‘Card. G Panico,’ Tricase, Lecce; De Nunzio A, Ospedale di Teano; Terzoli S, Clinica Pediatrica Universita' degli Studi di Milano; D'Ascola G, Bruciatelli M, Centro per le microcitemie Ospedale Reggio Calabria; Satta AM, Servizio Immunoematologia e Centro Trasfusionale, Presidio Ospedaliero ‘A Segni,’ Ozieri, Sassari; Borgna-Pignatti C, Marradi P, Clinica Pediatrica, Universita' degli Studi di Verona, Policlinico Borgo Roma, Verona; Puggioni G, Murgia T, Servizio Immunoematologia e Trasfusionale, Ospedale di Nuoro; Porta E, Centro Trasfusionale, Presidio Ospedaliero Viareggio, Viareggio, Lucca; Poggi V, Pinta-Boccalatte MF, Reparto Ematologia-Pediatria, Ospedale Pausilipon, Napoli; Polizzi B, Divisione Pediatria, Ospedale ‘Gravina e Santo Pietro,’ Caltagirone; Maroni P, Chelazzi G, Servizio Immunoematologia e Trasfusionale Ospedale di Circolo e Fondazione Macchi, Varese; Rocco S, Rotoli B, Divisione Ematologia Clinica, Universita' degli Studi di Napoli Federico II, Napoli; Mangiagli G, Bellomo G, Divisione Pediatria, Servizio di Talassemia, Ospedale Umberto I, Siracusa; Minelli P, Divisione Pediatria, Presidio Ospedaliero di Bentivoglio, Bentivoglio, Bologna; Lamantia A, Servizio di Immunoematologia e Trasfusionale, Azienda Ospedaliera di Alessandria; Colarossi M, Centro Trasfusionale Ospedale ‘Santa Maria Goretti,’ Latina; Martinelli L, Sezione Trasfusionale, Ospedale di Villafranca, Villafranca, Verona; Cappellini MD, Istituto di Medicina Interna, Universita' degli Studi di Milano; Fernando V, Tommasi G, Centro Trasfusionale, Presidio Ospedaliero Copertino, Copertino, Lecce; DeRosa C, Bufardi S, Sezione Autonoma Microcitemie, Azienda Ospedaliera ‘A Cardarelli,’ Napoli; Melchiorri G, Divisione Pediatria, Ospedale di Trento; Caruso V, Servizio Thalassemia Ospedale Garibaldi, Catania.
APPENDIX 2
Key points of the Knodell numerical scoring system7:
Piecemeal necrosis: none = 0; mild = 1; moderate (involves less than 50% of circumference of most portal tracts) = 3; marked (involves more than 50% of circumference of most portal tracts) = 4; moderate piecemeal necrosis plus bridging necrosis = 5; marked piecemeal necrosis plus bridging necrosis = 6; multilobular necrosis = 10.
Intralobular degeneration: none = 0; mild (acidophilic bodies, ballooning degeneration and/or scattered foci of hepatocellular necrosis in < one third of lobules or nodules) = 1; moderate (involvement of one third to two thirds of the lobules or nodules) = 3; marked (involvement of > two thirds of the lobules or nodules) = 4.
Portal inflammation: none = 0; mild (sprinkling of inflammatory cells in < one third of portal tracts) = 1, moderate (increased inflammatory cells in one third to two thirds of portal tracts) = 3; marked (dense packing of inflammatory cells in > two thirds or portal tracts) = 4.
Fibrosis: none = 0; fibrous portal expansion = 1; bridging fibrosis = 3; cirrhosis = 4.
Supported by Fondazione Berloni per la lotta contro la Talassemia (Pesaro, Italy).
Address reprint requests to Emanuele Angelucci, MD, Divisione Ematologica e Centro Trapianto Midollo Osseo di Muraglia, Azienda Ospedale di Pesaro, 61100 Pesaro, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal