Abstract
Recent studies performed in mice knocked out for the tumor necrosis factor (TNF ), the lymphotoxin-α, or the type I TNF receptor (R), genes have shown that these animals display gross defects in germinal center (GC) formation, suggesting that members of the TNF and TNFR superfamilies are involved in the control of B-cell migration. Based on these premises, we have here investigated the effects of human recombinant (r) TNF on the polarization and locomotion of tonsillar B cells. rTNF increased the spontaneous polarization and locomotion of unfractionated tonsillar B lymphocytes in a dose-dependent manner by inducing a true chemotactic response. Memory (IgD−, CD38−) and naive (IgD+, CD38−), but not GC (IgD−, CD38+) B cells purified from total tonsillar B lymphocytes, showed a significantly higher locomotion in the presence than in the absence of rTNF. Accordingly, type I and II TNF receptors (TNFRs) were detected by flow cytometry on the surface of memory and naive, but not GC, B lymphocytes. Blocking experiments with monoclonal antibodies to type I or II TNFR showed that rTNF enhanced the spontaneous chemotaxis of memory and naive B cells through the selective engagement of type II TNFR. Finally, the TNF gene was found to be expressed in memory, naive and GC B lymphocytes; the cytokine was released in culture supernatants from the three B-cell subsets after stimulation. These data may support the hypothesis that human TNF is involved in the paracrine and perhaps autocrine control of B-cell migration in secondary lymphoid tissues.
LEUKOCYTE MIGRATION is a nonrandom process that depends on cell-to-cell interactions through specific sets of adhesion molecules and on the binding of integrin receptors to the extracellular matrix.1,2 In response to chemotactic signals originating outside the venules, leukocytes egress from the vascular compartment by a process known as diapedesis and localize to the tissues where an inflammatory process is ongoing.1,2 Mature lymphocytes are known to recirculate continuously, moving to the tissues and returning back to the blood at least once a day.1 Discrete lymphocyte subsets, such as naive and memory B and T cells, display a differential tissue tropism which is related to the interaction of specific surface receptors, also known as “lymphocyte homing receptors,” with complementary endothelial cell adhesion molecules.1 2
In the secondary lymphoid tissues, B lymphocytes settle predominantly in three anatomical areas, namely the germinal center (GC), the follicular mantle (FM), and the marginal zone (MZ) in the spleen or its equivalent in other organs, such as, for example, the subepithelial area of tonsils.3-9 Functionally, the GC is the site of hypermutation of Ig variable (V) region genes and affinity maturation of antibody response4-8; the FM contains naive B cells that have not yet encountered specific antigen,9 and the MZ or its equivalent harbor memory B cells.9-11 GC, FM, and MZ B cells can be purified from human tonsils as IgD−, CD38+; IgD+, CD38− and IgD−, CD38− cells, respectively.7,8,11,12 Futhermore, GC, FM, and MZ B cells can be dissected by monoclonal antibodies (MoAbs) into additional subpopulations.8
Recent studies performed in mice knocked out for tumor necrosis factor (TNF )13 or lymphotoxin-α (LT-α)14,15 genes have shown that these animals display gross abnormalities in the development of secondary lymphoid tissues, with some differences in the two experimental models. In particular, LT-α deficient mice do not develop lymph nodes or Peyer's patches and show a disturbed splenic architecture, with absence of segregation of T- and B-cell zones and failure to establish a distinct marginal zone.14,15 TNF-deficient mice completely lack splenic primary B-cell follicles and cannot form organized networks of follicular dendritic cells (FDC) and GC.13
In addition, mice knocked out for the type I TNF receptor (TNFR) do not develop GC or Peyer's patches, but form lymph nodes.15 All of the above observations suggest that members of the TNF and TNFR superfamilies play a pivotal role in cell trafficking within the secondary lymphoid tissues.
Here we have addressed this issue in a human model by investigating the in vitro migration of tonsillar B lymphocytes in the presence or absence of recombinant (r) TNF. TNF has been shown previously to serve as a chemoattractant for human granulocytes,16 and monocytes,16 as well as for murine fibroblasts17 and Langerhans cells.18 No information is so far available on the effects of the cytokine on the locomotion of human B cells.
We show that rTNF stimulates the in vitro migration of human B lymphocytes through the selective engagement of the type II TNFR. The chemotactic activity of the cytokine was directed to memory and naive, but not GC, B cells. Finally, memory, naive, and GC B lymphocytes were found to express the TNF gene.
MATERIALS AND METHODS
Cell separation and culture. Mononuclear cells were isolated from surgically removed tonsils on Ficoll-Hypaque density gradients and depleted of T cells by rosetting with neuraminidase-treated sheep erythrocytes. Non T cells were subsequently deprived of monocytes and natural killer (NK) cells by incubation with CD68 and CD56 MoAbs (see below) followed by rosetting with ox erythrocytes coated with a goat antimouse Ig antiserum.19 The resulting cell preparations consistently contained more than 97% B cells, as assessed by staining with a CD19 MoAb (see below).
All of the separation procedures were performed at 4°C to prevent spontaneous apoptosis of GC B cells. Fractionation of tonsillar B lymphocytes into FM (naive), GC, and MZ (memory) cells12 was performed as follows. Naive B lymphocytes were isolated as IgD+ cells from purified B-lymphocyte suspensions by immune rosetting.12 The IgD− B-cell fractions were further separated into CD38+ (GC cells) and CD38− (memory cells) by immune rosetting.12
In some experiments, purified B-cell subsets were cultured (1 × 106/mL) for 48 hours in RPMI 1640 medium (Seromed-BiochromKG, Berlin, Germany) supplemented with L-glutamine, penicillin and streptomycin, nonessential aminoacids and 10% fetal calf serum (FCS; Seromed) in the presence or absence of the following stimuli: a CD40 MoAb and recombinant interleukin-4 (rIL-4; Genzyme, Milan, Italy; 100 U/mL)7,20 21 or phorbol 12-myristate 13-acetate (PMA) (10 ng/mL) and the A23187 calcium ionophore (0.5 μg/mL), both from Sigma Chemical Co (St Louis, MO).
MoAbs and immunofluorescence analysis. The murine MoAbs used in this study were: CD19 and CD3 from Becton Dickinson (San José, CA); CD56, anti-HLA-DR and CD10 from Coulter (Hialeah, FL); CD23, CD68 and antihuman IgM and IgG from Dako (Glostrup, Denmark); CD39 from Immunotech (Marseille Luminy, France). The CD38 MoAb used throughout this study (clone IB4) has been produced by one of us (F.M.).22 The CD40 MoAb was kindly donated by Dr Maria Grazia Roncarolo (DNAX, Palo Alto, CA). The antihuman IgD MoAb was a generous gift of Dr Maria Elisabetta Cosulich (Monoclonal Antibody Unit, CBA, Genova, Italy).
In brief, 5 × 105 cells were treated at 4°C for 30 minutes with optimal concentrations of MoAbs, washed twice in phosphate-buffered saline supplemented with 2% FCS and further incubated with a fluorescein isothiocyanate (FITC)-conjugated goat antimouse Ig (Dako) as second reagent. Stained cells were analyzed by flow cytometry with a FACScan (Becton Dickinson). Controls were untreated cells, cells treated with the FITC-second reagent alone, and cells treated with irrelevant isotype-matched MoAbs followed by the FITC-second reagent. In additional experiments, the surface expression of TNFRs by freshly isolated B-cell subsets was investigated. Cells were treated with a stripping solution23 for 2 to 3 minutes at 4°C to remove membrane-bound TNF, incubated with MoAbs to type I (htr-9), type II (utr-1) TNFR24 (kindly provided by Dr Manfred Brockaus, Hoffman-La Roche, Basel, Switzerland) or with an isotype-matched MoAb of irrelevant specificity and subsequently stained as above. Both the htr-9 and utr-1 MoAbs express the IgG1 isotype.24
Polarization assay. The purified B-cell fractions were resuspended in RPMI 1640 medium supplemented with 0.1% bovine serum albumin (Sigma) and mixed with rTNF (BioSource International, Camarillo, CA) (0, 0.1, 1, 10, 100, 1,000 ng/mL) or rIL-4 (100 U/mL). Experiments were performed using polystyrene round bottom tubes (LP Italiana, Milano, Italy). The tubes, containing 200 μL cell suspension, were incubated for 20 minutes at 37°C; thereafter, 200 μL of 10% formaldehyde solution were added. The fixed cell suspensions were examined at 400× magnification. At least 200 cells were counted in each preparation. Cells deviating from a spherical outline were scored as polarized and expressed as a percentage of the total number of cells counted.25 26
In some experiments, freshly purified B cells were incubated with or without 1 ng/mL TNF for 20 minutes at 37°C and fixed with 10% formaldehyde. Cell polarization was determined by flow cytometry as change in the profile of forward scatter (FSC).27 An aliquot of the same cell suspensions was stained with a CD19 MoAb to check their positivity.
Migration assay. Cell locomotion was studied using the leading front method.28 Tests were performed in duplicate using blind well chambers (Nucleopore, Cambridge, MA) with an 8-μm pore size cellulose ester filter (Millipore, Milano, Italy) separating the cells (4 × 105) from the chemoattractants tested at different concentrations or from medium alone. After incubation for 2 hours at 37°C, the filters were fixed in ethanol, stained with Harris haematoxylin, and cleared with xylene. Cell migration was measured as the distance between the top of the filter and the plane in which two or more nuclei of the faster cells were in focus (400 × original magnification). Five randomly chosen fields were read for each filter. The results were then pooled and the mean was determined. In some experiments, B-cell fractions were preincubated with antitype I TNFR (10 μg/mL), antitype II TNFR (10 μg/mL) or both MoAbs for 30 minutes at 4°C and washed. Thereafter, the migration assays were carried out as described above. In some experiments, the viability of the tonsillar B-cell fractions was checked by trypan blue staining.
Collagen gel invasion assay. Type I collagen solution was from Sigma (Cat. C4580, Lot. 126H4667). Gels were prepared according to Sigma protocol. Briefly, 800 μL collagen solution were mixed with 100 μL 10× Earle's buffered saline and 100 μL reconstitution buffer (2.2% sodium bicarbonate in 0.8 N sodium hydroxide) in order to restore the collagen to physiological pH and osmolarity. Thereafter, collagen solution (final gel concentration 0.88 mg/mL) was allowed to gel in 24-well plates in absence or presence of the chemoattractants (1 ng/mL rTNF or 100 U/mL rIL-4) in duplicate. After gelification, cells (8 × 105/well) were overlaid on the gel surface and incubated at 37°C for 10 hours. At the end of incubation, gels were fixed for 30 minutes with 2.5% glutaraldehyde and cell migration was measured as the distance between the top of the gel and the plane in which the two faster cells invading the gel were in focus (100 × original magnification).29
Checkerboard analysis. Assays of cell migration with different doses of rTNF (0, 0.1, 1 ng/mL) on both sides of the filter were also performed. The results of these experiments were collected in a checkerboard form by which chemokinesis (ie, change in the intensity of random locomotion) and true chemotaxis (ie, change in the directional response to the stimulus) were calculated according to Zigmond and Hirsch.25
RNA analysis by reverse transcriptase-polymerase chain reaction (RT-PCR). Total RNA was extracted from B cells according to Chomczynski and Sacchi30 and reverse transcribed into cDNA by means of a commercial kit (Clontech, Palo Alto, CA) using oligo dT as primer. Each cDNA was then diluted to a final volume of 100 μL and the efficiency of reverse transcription was evaluated by amplification of 5 μL of cDNA with the glyceraldehyde-3-phosphate-dehydrogenase(G3PDH) primers supplied with the kit. Afterwards, for each PCR analysis, 5 μL of cDNA were amplified in a 50-μL reaction by adding 25 pmol of each specific primer and 2 U of Taq polymerase (Clontech). The primers used were the following: TNF: forward GAGCACTGAAAGCATGATCCG and reverse CGAGAAGATGATCTGACTCGGT; TNFR type I: forward GCTCCTGGAGCTGTTGGTGGGAA and reverse CAGGAGAGGTGCACGGTCCCATT; TNFR type II: forward CTCAGAGAATACTATGACCAG and reverse CCTGCAAATATCCGTGGATG.
The amplification profiles were 1′ at 94°C, 1′ at 60°C, 1′ at 72°C for TNF; 1′ at 94°C and 2′ at 68°C for TNFR type I; 1′ at 94°C, 1′ at 55°C, 1′ at 72°C for TNFR type II. All the amplifications were carried out for 35 cycles. The amplified products were visualized on a 2% agarose gel stained with ethidium bromide.
TNF assay. Forty-eight–hour culture supernatants from the purified B-cell fractions were collected and frozen at −80°C until tested. TNF was tested using an enzyme-linked immunosorbent assay (ELISA) kit (Amersham, Milano, Italy) that has been calibrated to detect as low as 15 pg/mL.
Statistical analysis. Data are expressed as the mean ± 1 SE. Differences among groups were determined by the nonparametric Mann-Whitney U-test. Differences among groups in dose-response experiments were determined using Kruskal-Wallis nonparametric analysis of variance (ANOVA) followed by Dunn's test for multiple comparison.31 32
RESULTS
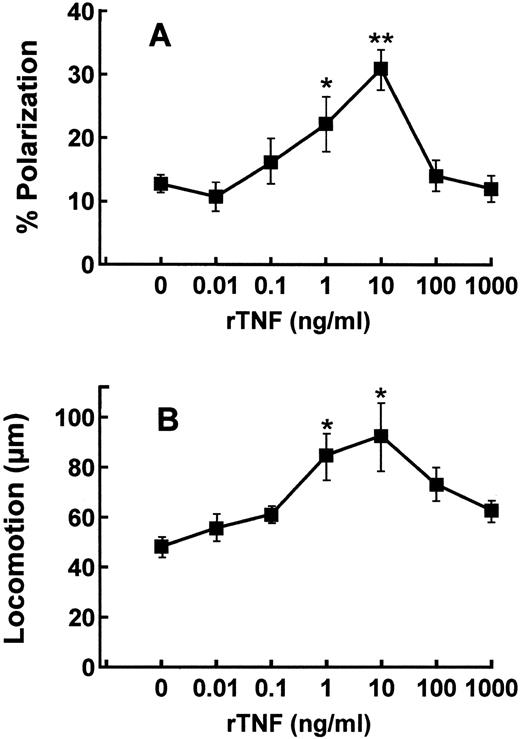
Effects of rTNF on the polarization and chemotaxis of purified tonsillar B lymphocytes. Tonsillar B cells were purified by sequential depletion of T cells, NK cells, and macrophages and subsequently tested for their ability to undergo polarization and locomotion in the presence or absence of different concentrations of rTNF (Fig 1A and B). The spontaneous polarization (Fig 1A), and locomotion (Fig 1B) of B lymphocytes were enhanced in a statistically significant manner by the exposure to 1 and 10 ng/mL rTNF (Fig 1A). At higher rTNF concentrations (100 and 1,000 ng/mL) B-cell polarization and locomotion decreased approximately to the levels observed in the absence of the cytokine (Fig 1A and B). On the basis of the above experiments, the concentration of 1 ng/mL rTNF was chosen for all the subsequent tests.
Dose-response curves of purified tonsillar B cells to rTNF in polarization and locomotion assays. B lymphocytes were purified and tested as described in Materials and Methods in polarization (A) and locomotion (B) assays. Results are means ± SE from four to six different experiments. * P < .05, ** P < .01, Kruskal-Wallis nonparametric ANOVA analysis.
Dose-response curves of purified tonsillar B cells to rTNF in polarization and locomotion assays. B lymphocytes were purified and tested as described in Materials and Methods in polarization (A) and locomotion (B) assays. Results are means ± SE from four to six different experiments. * P < .05, ** P < .01, Kruskal-Wallis nonparametric ANOVA analysis.
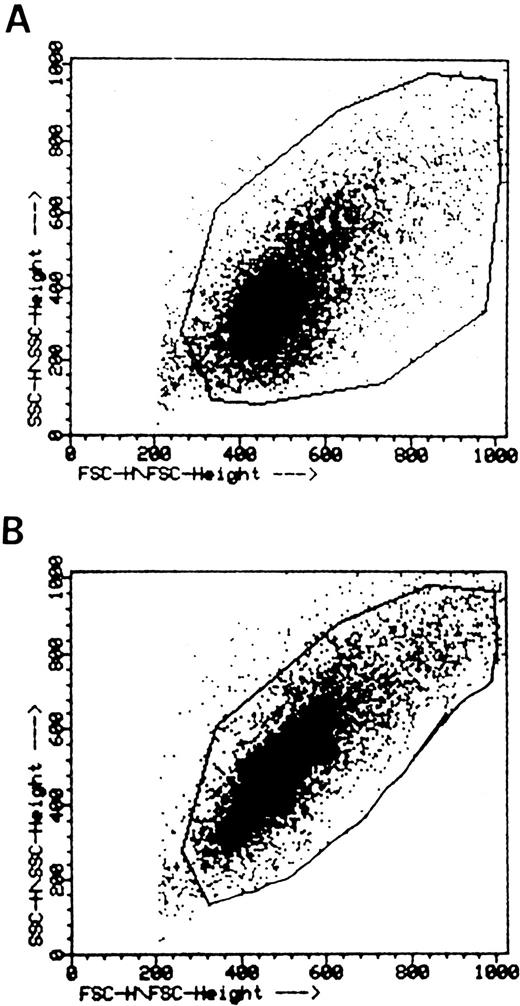
The B-cell–directed chemoattractant properties of rTNF were further documented by two additional pieces of evidence. First, when B cells were incubated with or without rTNF, fixed and analyzed by flow cytometry, a clear-cut shift of the FSC profile was observed in cells exposed to rTNF (Fig 2B) but not in control cells (Fig 2A). This finding showed objectively that rTNF induced polarization of tonsillar B lymphocytes.
Flow cytometric analysis of TNF-induced B lymphocyte polarization. B cells were incubated without (A) or with (B) 1 ng/mL rTNF as detailed in the Materials and Methods and analyzed by flow cytometry. One representative experiment out of the four carried out is shown. Staining with a CD20 MoAb showed that 98% of test cells were B lymphocytes (not shown).
Flow cytometric analysis of TNF-induced B lymphocyte polarization. B cells were incubated without (A) or with (B) 1 ng/mL rTNF as detailed in the Materials and Methods and analyzed by flow cytometry. One representative experiment out of the four carried out is shown. Staining with a CD20 MoAb showed that 98% of test cells were B lymphocytes (not shown).
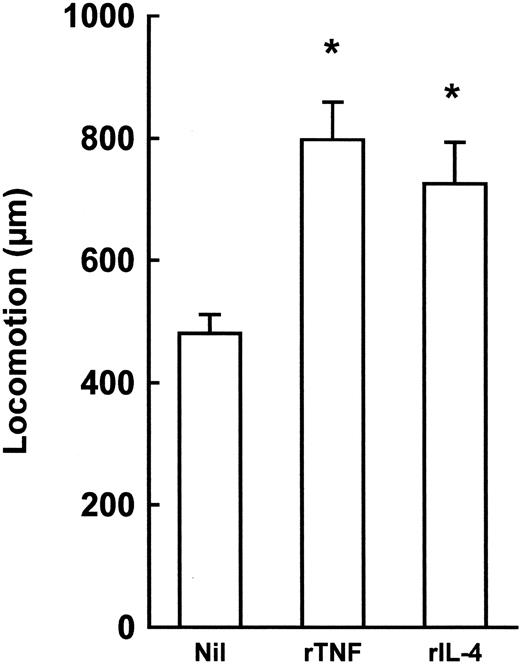
Second, experiments were performed to compare the chemoattractant properties of rTNF to those of rIL-4, an inducer of B-cell locomotion.29,33 rIL-4 caused a low-grade enhancement of B-cell responses in both polarization and locomotion assays (% polarized cells: in absence of rIL-4 = 16.5 ± 3.1, in presence of 100 U/mL rIL-4 = 20.5 ± 2.6, means ± 1 SE, n = 4, P = .4857; micrometers traveled: in absence of rIL-4 = 52.2 ± 5.3, in presence of 100 U/mL rIL-4 = 69.8 ± 8.5, means ± 1 SE, n = 4, P = .3429) as compared to rTNF (see Fig 1A and B). When tested in the collagen invasion assay that is classically used in cell migration studies,29 both rTNF and rIL-4 increased significantly the spontaneous ability of B cells to invade collagen (Fig 3).
Collagen gel invasion by purified tonsillar B cells in the presence or absence of rTNF or rIL-4. Results are expressed as micrometers travelled by the two faster cells from the top of the gels. Nil = no cytokine; rTNF = 1 ng/mL; rIL-4 = 100 U/mL. Incubation time = 10 hours. Results are means ± 1 SE from four experiments. *P < .05, nonparametric Mann-Whitney U-test.
Collagen gel invasion by purified tonsillar B cells in the presence or absence of rTNF or rIL-4. Results are expressed as micrometers travelled by the two faster cells from the top of the gels. Nil = no cytokine; rTNF = 1 ng/mL; rIL-4 = 100 U/mL. Incubation time = 10 hours. Results are means ± 1 SE from four experiments. *P < .05, nonparametric Mann-Whitney U-test.
In subsequent studies, we investigated whether the enhancement of B-cell migration induced by rTNF was due to an increased rate of locomotion and/or to a true chemotactic TNF-dependent cell response. To this end, experiments making use of different rTNF concentrations above and below the filters were performed (checkerboard analysis). As summarized in Table 1, absolute rTNF concentrations (absence of a cytokine gradient) enhanced the cell migration rate in a dose-dependent manner, suggesting chemokinetic properties of the cytokine. In particular, 1 ng/mL rTNF above and below the filter stimulated cell migration by 45.8 ± 9.9% (mean ± 1 SE, n = 3). However, cell migration was higher in the presence of positive rTNF gradients. In fact, 1 ng/mL rTNF below the filter only (positive chemotactic assay condition) enhanced the cell migration by 106.6 ± 9.9% (mean ± 1 SE, n = 3), suggesting a cytokine-dependent true chemotactic response of B cells. On the contrary, cell migration in negative gradients was lower than calculated on the basis of the expected response to absolute concentration alone. Finally, taking into account the cell migration actually detected and the migration distance expected on the basis of the chemokinesis alone, almost 50% (49.9 ± 3.7 %, mean ± 1 SE, n = 3) of the locomotory activity triggered by 1 ng/mL rTNF in the positive chemotactic assay conditions was due to a true chemotactic response. These results suggest that rTNF induces both a chemokinetic and a chemotactic B-cell response.
Checkerboard Assay of Tonsillar B Cells in Response to rTNF
| Distance Migrated ( μm in 2 h) by Leading Front of Cell | |||
| rTNF (ng/mL) Below the Filter | |||
| 0 | 0.1 | 1 | |
| rTNF (ng/mL) above the filter: | |||
| 0 | 36.9 ± 4.6 | 54.1 ± 3.8 (38.7 ± 4.6) | 76.5 ± 11.2 (41.6 ± 6.9) |
| 0.1 | 38.8 ± 4.2 (43.4 ± 3.9) | 48.1 ± 3.5 | 55.9 ± 3.6 (49.3 ± 4.4) |
| 1 | 39.4 ± 1.1 (50.5 ± 6.1) | 38.0 ± 6.1 (52.3 ± 6.2) | 53.6 ± 7.0 |
| Distance Migrated ( μm in 2 h) by Leading Front of Cell | |||
| rTNF (ng/mL) Below the Filter | |||
| 0 | 0.1 | 1 | |
| rTNF (ng/mL) above the filter: | |||
| 0 | 36.9 ± 4.6 | 54.1 ± 3.8 (38.7 ± 4.6) | 76.5 ± 11.2 (41.6 ± 6.9) |
| 0.1 | 38.8 ± 4.2 (43.4 ± 3.9) | 48.1 ± 3.5 | 55.9 ± 3.6 (49.3 ± 4.4) |
| 1 | 39.4 ± 1.1 (50.5 ± 6.1) | 38.0 ± 6.1 (52.3 ± 6.2) | 53.6 ± 7.0 |
Results are expressed as mean ± SE, n = 3. Figures in parentheses show the calculated distance migrated if cells would have not responded to the gradient of rTNF, but only to absolute concentration.
rTNF enhances the locomotion of purified tonsillar memory and naive, but not GC, B lymphocytes. As mentioned, GC, naive, and memory B lymphocytes can be isolated according to the expression of distinctive immunophenotypic markers. In particular, naive B cells express surface IgD but not CD38, the bulk of GC B cells are CD38+, IgD−, whereas memory B cells are IgD−, CD38− cells.7,8,11 12
To investigate the effects of rTNF on the locomotion of the above B-cell subsets, total tonsillar B cells were first separated into IgD+ and IgD− cells.12 IgD− cells were further fractionated into the CD38+ and CD38− subsets.12 Consistent with previous reports,11,12 most IgD+ naive B cells were CD39+, IgM+, IgG−, CD38−, CD10−, whereas approximately half of them expressed the CD23 marker (not shown). CD38+, IgD− GC B cells were CD10+, CD39−, CD23− (not shown). IgD−, CD38− memory B lymphocytes were predominantly CD39+, IgG+, CD23−, CD10− (not shown).11 12
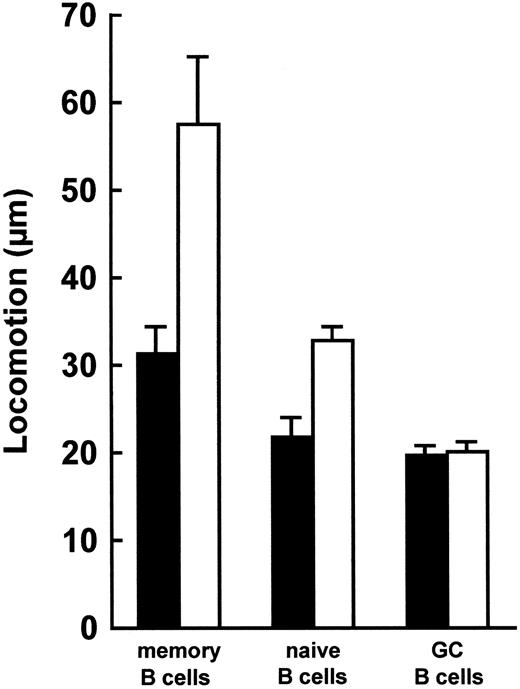
Next, memory, naive and GC B lymphocyte populations were tested for their locomotor ability in the presence or absence of rTNF (Fig 4). Memory, naive and GC B lymphocytes all migrated spontaneously (Fig 4). Upon incubation with rTNF, memory and naive, but not GC, B lymphocytes exhibited a significant (P < .05) increase in their locomotion as compared to the corresponding unstimulated B-cell fractions (Fig 4). Furthermore, in the presence of rTNF, both memory and naive B lymphocytes displayed a significantly higher degree of locomotion (P < .05) than GC B cells (Fig 4). Finally, memory B cells migrated faster (P < .05) than naive B cells in the presence of rTNF (Fig 4).
Locomotion of memory, naive, and GC B lymphocytes. Locomotory activity of IgD−CD38− memory cells, IgD+ naive cells, and IgD−CD38+ GC B cells in the presence (□) or absence (▪) of 1 ng rTNF. Data are means ± SE from four experiments. In the presence of rTNF, memory and naive, but not GC, B cells migrated faster than the corresponding unstimulated cell fractions (P < .05, nonparametric Mann-Whitney U-test). Furthermore, in the presence of rTNF, memory and naive B lymphocytes displayed a significantly higher degree of locomotion than GC B cells (P < .05) and memory B cells migrated faster than naive B cells (P < .05).
Locomotion of memory, naive, and GC B lymphocytes. Locomotory activity of IgD−CD38− memory cells, IgD+ naive cells, and IgD−CD38+ GC B cells in the presence (□) or absence (▪) of 1 ng rTNF. Data are means ± SE from four experiments. In the presence of rTNF, memory and naive, but not GC, B cells migrated faster than the corresponding unstimulated cell fractions (P < .05, nonparametric Mann-Whitney U-test). Furthermore, in the presence of rTNF, memory and naive B lymphocytes displayed a significantly higher degree of locomotion than GC B cells (P < .05) and memory B cells migrated faster than naive B cells (P < .05).
Since GC B lymphocytes are highly susceptible to spontaneous apoptosis, the possibility that their failure to migrate in response to rTNF (Fig 4) was related to the poor viability was investigated. To this end, in three different experiments, GC B cells were stained with trypan blue immediately before being subjected to locomotor assays and after a 2-hour incubation; the viability of these cell fractions was consistently higher than 90% at both time intervals (data not shown).
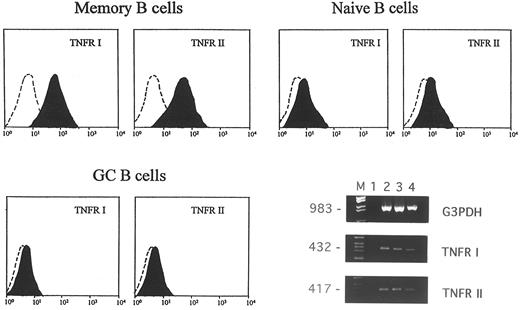
Expression of type I and type II TNFR on memory, naive, and GC tonsillar B lymphocytes. TNF binds to two different surface receptors which are known as type I or p55 and type II or p75 receptors, respectively.34-36 Therefore, in subsequent experiments, the expression of type I and type II TNFRs on the surface of memory, naive, and GC B cells was investigated by flow cytometry using the htr-9 (anti-TNFR I) and the utr-1 (anti-TNFR II) MoAbs. All of the staining procedures were performed after stripping of TNF bound in vivo to its receptors by cell treatment with an acidic solution.23 In four different experiments: (1) memory B cells expressed both type I (range 30% to 40%) and type II (range 27% to 36%) TNFRs; (2) naive B cells also expressed the two TNFRs (type I: range 5% to 15%; type II: range 8% to 16%), and (3) GC B cells did not display any expression of either receptor. Figure 5 shows the FACS profile of one representative experiment.
Expression of type I and type II TNFR in memory, naive, and GC B lymphocytes. Cells were stained with 10 μg/mL htr-9 (anti-TNFR I), 10 μg/mL utr-1 (anti-TNFR II) MoAbs or an isotype-matched irrelevant MoAb (control) and analyzed by flow cytometry. The FACS profiles are from one representative experiment of the four performed. RT-PCR analysis of TNFR gene expression in GC B cells is shown on the right (M = marker; lane 1 = negative control; lanes 2 through 4 correspond to three different GC B-cell samples). The size of the expected fragments is indicated on the left.
Expression of type I and type II TNFR in memory, naive, and GC B lymphocytes. Cells were stained with 10 μg/mL htr-9 (anti-TNFR I), 10 μg/mL utr-1 (anti-TNFR II) MoAbs or an isotype-matched irrelevant MoAb (control) and analyzed by flow cytometry. The FACS profiles are from one representative experiment of the four performed. RT-PCR analysis of TNFR gene expression in GC B cells is shown on the right (M = marker; lane 1 = negative control; lanes 2 through 4 correspond to three different GC B-cell samples). The size of the expected fragments is indicated on the left.
The finding that GC B cells did not express TNFRs on the cell surface raised the possibility that the failure of rTNF to bind to its cellular receptors was involved in the migratory unresponsiveness of GC B lymphocytes to the cytokine. Nonetheless, when type I and type II TNFR gene expression was investigated in purified GC B-lymphocyte fractions by RT-PCR, the transcripts of both genes were consistently detected (Fig 5).
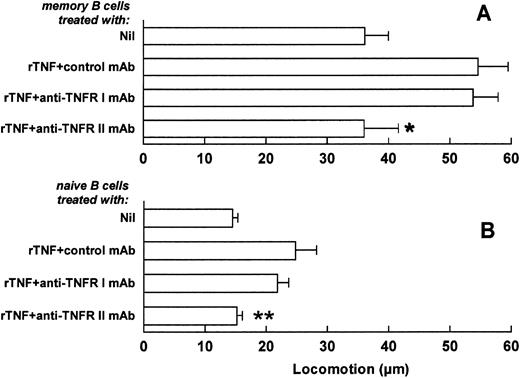
Selective involvement of type II TNFR in the TNF-mediated enhancement of memory and naive B-cell locomotion. In further experiments, the involvement of TNFRs in the TNF-mediated triggering of B-cell locomotion was investigated. Memory and naive tonsillar B cells were purified as above and incubated with MoAbs to TNFR I, TNFR II, or with an isotype-matched MoAb of irrelevant specificity (control). The two B-cell fractions were next washed and tested for locomotor ability in the presence or absence of rTNF (Fig 6A and B). The anti-TNFR II MoAb reduced the TNF-dependent locomotion of both memory and naive B lymphocytes to the levels observed in the absence of rTNF (Fig 6A and B). In contrast, no decrease in TNF-induced locomotion was observed on incubation of the two cell fractions with the anti-TNFR I MoAb (Fig 6A and B). The inhibition of TNF-induced migration by anti-TNFR MoAbs tested in combination was comparable to that observed in the experiments with the anti-TNFR II MoAb alone (not shown). The anti-TNFR MoAbs tested alone or in combination or the control antibody had no effect on the spontaneous migration of memory and naive B cells (not shown).
Involvement of TNFRs in the TNF-induced enhancement of memory and naive B-cell locomotion. Freshly purified memory (A) and naive (B) B cells were treated with anti-TNFR I, anti-TNFR II, or with an isotype-matched MoAb of irrelevant specificity (control MoAb). Cells were subsequently tested in locomotor assays in the absence (Nil) or in the presence of 1 ng/mL rTNF. Results are means ± SE from six to seven different experiments. *P < .05, **P < .005, versus isotype-matched MoAb and rTNF-treated control, nonparametric Mann-Whitney U-test.
Involvement of TNFRs in the TNF-induced enhancement of memory and naive B-cell locomotion. Freshly purified memory (A) and naive (B) B cells were treated with anti-TNFR I, anti-TNFR II, or with an isotype-matched MoAb of irrelevant specificity (control MoAb). Cells were subsequently tested in locomotor assays in the absence (Nil) or in the presence of 1 ng/mL rTNF. Results are means ± SE from six to seven different experiments. *P < .05, **P < .005, versus isotype-matched MoAb and rTNF-treated control, nonparametric Mann-Whitney U-test.
Taken together, these results show the selective involvement of the TNFR II in the TNF-mediated enhancement of memory and naive B-cell locomotion.
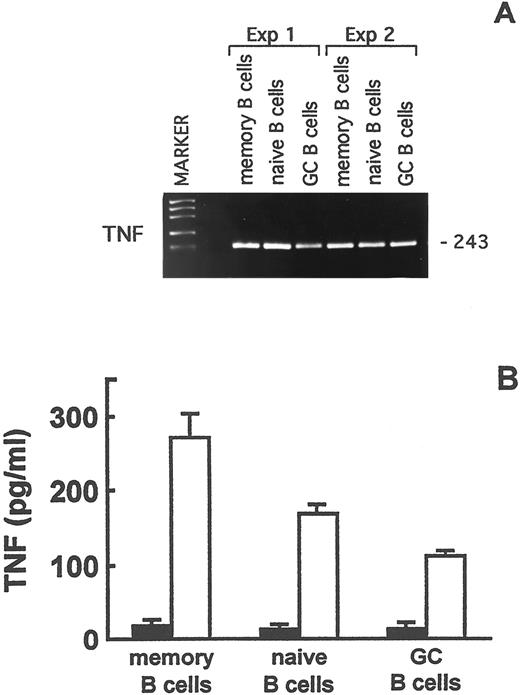
Expression of the TNF gene in naive, memory, and GC tonsillar B lymphocytes. Next, the expression of the TNF gene in naive, memory, and GC tonsillar B cells was investigated. The aim of these experiments was to assess the potential contribution of B-cell derived TNF to the TNF-dependent enhancement of memory and naive B-cell migration.
Total RNA, that had been extracted from freshly isolated naive, memory, and GC B lymphocytes, was reverse transcribed and analyzed by RT-PCR. TNF mRNA was expressed in the three B-cell subsets (Fig 7A). To determine whether TNF mRNA was translated into the corresponding protein, purified naive, memory, and GC B lymphocytes were cultured for 24 hours in the presence or absence of PMA and A23187 or of the CD40 MoAb and rIL-4. rTNF was then tested in culture supernatants by ELISA. The results of three experiments are shown in Fig 7B. No TNF was detected in the supernatants from the unstimulated cultures, whereas PMA and A23187 induced the release of immunoreactive TNF by naive, memory, and GC B lymphocytes (Fig 7B). The CD40 MoAb and IL-4 were ineffective at triggering TNF release by any of the three B-cell subsets (not shown), in accordance with that reported by Worm et al37 using total tonsillar B lymphocyte suspensions.
TNF expression in memory, naive, and GC B lymphocytes. (A) RT-PCR analysis of TNF gene expression in purified memory, naive, and GC B-cell fractions. The size of the expected fragments is indicated on the right. (B) TNF production by purified memory, naive, and GC B lymphocytes after 48-hours culture in the absence (▪) or presence (□) of PMA and A23187. TNF in culture supernatants was assayed by ELISA. Results are means ± SE from three experiments and are expressed as pg/mL.
TNF expression in memory, naive, and GC B lymphocytes. (A) RT-PCR analysis of TNF gene expression in purified memory, naive, and GC B-cell fractions. The size of the expected fragments is indicated on the right. (B) TNF production by purified memory, naive, and GC B lymphocytes after 48-hours culture in the absence (▪) or presence (□) of PMA and A23187. TNF in culture supernatants was assayed by ELISA. Results are means ± SE from three experiments and are expressed as pg/mL.
DISCUSSION
In this study we have investigated the in vitro migratory properties of human tonsillar B lymphocytes, either in the presence or absence of rTNF. The cytokine caused a significant increase in the polarization and locomotion of unfractionated B cells, as assessed by different techniques including flow cytometry, the leading front method and the collagen invasion assay. Thus, rTNF adds to the list of cytokines that influence the in vitro migration of human B lymphocytes either positively, such as IL-429,33 and IFN-γ33 or negatively, such as IL-10.38 In this respect it is noteworthy that rIL-4 and rTNF displayed a comparable chemotactic activity versus tonsillar B lymphocytes in the collagen invasion assay.
Checkerboard experiments showed that rTNF induced a true B-cell chemotaxis suggesting that rTNF itself, rather than other cytokines possibly induced by cell incubation with rTNF,39-42 was the driving force for B-lymphocyte locomotion.
Purified tonsillar B-cell subsets displayed a hierarchy in their migratory properties. Memory B lymphocytes exhibited the highest spontaneous locomotion and the strongest chemotactic response to rTNF; naive B cells also migrated under the same experimental conditions, but they were slower than memory B cells. Finally, GC B cells, although migrating spontaneously, were totally refractory to rTNF stimulation.
The expression of surface TNFRs paralleled the in vitro responsiveness of the three B-cell subsets to rTNF. Both type I and type II TNFRs were detected by flow cytometry on the surface of freshly isolated memory and naive B cells, but the percentage of positive cells was definitely higher in the memory than in the naive B-lymphocyte population. As for GC B cells, flow cytometric analyses failed consistently to detect any surface expression of TNFRs, but the same cell fractions were found to produce TNFR I and II mRNA by RT-PCR.
The following hypotheses may be envisaged to try to explain such apparent discrepancy: (1) TNFRs are indeed not expressed on the surface of GC B cells due to, for example, mRNA instability or other posttranscriptional mechanisms. This hypothesis may be supported by previous immunohistochemical studies on tonsillar tissue sections showing that, within the GC, only FDC stained for TNFR I, but not TNFR II43; (2) TNFRs are expressed on the surface of GC B cells at a density that is below the threshold of flow cytometry detection. According to this hypothesis, the few molecules of TNFRs would be insufficient to allow a migratory response to rTNF; (3) surface TNFRs cannot be detected because they are internalized or shed upon binding in vivo to their natural ligands, ie, TNF44 and LT-α. In this respect, it is of note that FDC produce TNF in vivo, thus replenishing the GC with the cytokine.43,45 46
Whatever the mechanism, it is tempting to speculate that GC B lymphocytes downregulate the surface expression of TNFRs and perhaps of other cytokine receptors to minimize the influence of exogenous stimuli in a specialized microenvironment where proliferation, antigen-driven selection and hypermutation of Ig V genes take place.4-7
The above experiments raised the question of what type(s) of TNFR was involved in the TNF-mediated enhancement of memory and naive B-lymphocyte locomotion. To address this issue, the latter cell fractions were incubated with anti-TNFR I or II MoAbs before being tested. Blocking of TNFR II, but not TNFR I, abrogated the responsiveness of both memory and naive B lymphocytes to rTNF but left the spontaneous migration of the two cell subsets unaltered.
The present results must be viewed in the general frame of the role of TNF and its receptors in the development of secondary lymphoid tissues. Studies performed in knock out mice have shown that inactivation of the TNF or of the type I TNFR15 genes impedes the formation of GC, whereas deletion of the type II TNFR gene does not influence GC development.15 These findings point to a key role of TNF and type I TNFR in cell trafficking in the secondary lymphoid tissues.
The data herein reported support this conclusion by showing that rTNF is indeed chemotactic for human B lymphocytes. However, in our study, type II rather than type I TNFR was found to be selectively involved in the migratory responses of B cells to rTNF. Notably, in this respect, Erikstein et al47 showed that SAC-activated peripheral blood B lymphocytes were induced to proliferate by rTNF through the triggering of type II TNFR. The reasons for the discrepancy between the human and mouse models are not easily explained, but may be related to differences in animal species or experimental approaches; further studies are needed to clarify this issue.
Cytokine-induced cell polarization and locomotion are strictly dependent on changes of the distribution of adhesion molecules.48 In preliminary experiments not shown here, we have addressed this issue and found that the TNF-mediated enhancement of B-cell locomotion is associated with CD54, but not CD11a, CD11b, CD11c or CD18, redistribution. Although further studies are needed, these results would point to a key role of intercellular adhesion molecule-1 in TNF-driven B-cell migration.
The ability of human Epstein-Barr virus–infected B-cell lines and unfractionated tonsillar B cells to synthesize TNF has been recognized from a decade.49 This study provides the first demonstration that memory, naive, and GC tonsillar B lymphocytes all produce TNF mRNA and protein. Since TNF mRNA was detected in the three B-cell subsets immediately after isolation, it may be hypothesized that B-cell–derived TNF synthesized in vivo upon activation contributes to the control of short-range migration of memory and naive B cells in the secondary lymphoid tissues. The finding that nonphysiologic stimuli were required to trigger TNF release in B-lymphocyte subset supernatants can be explained by the observation that most of the TNF newly synthesized by B cells upon CD40 cross-linking is retained at the surface of activated B cells as a membrane-bound molecule.37 A transmembrane form of TNF has been shown on the surface of human activated CD4+ T lymphocytes where the cytokine provides a costimulatory signal for B-cell activation.50 Therefore, it may be hypothesized that not only soluble, but also membrane-bound TNF is involved in the triggering of B-cell locomotion.
In conclusion, the identification of TNF as a novel chemotactic factor for human B lymphocytes opens up new perspectives for a better knowledge of the mechanisms governing B-cell migration in normal and pathologic conditions, eg, autoimmune and lymphoproliferative disorders. Further studies along these directions are now in progress.
ACKNOWLEDGMENT
We thank Dr Maria Grazia Roncarolo, Dr Maria Elisabetta Cosulich, Dr Manfred Brockaus for the kind supply of MoAbs; Dr Carlo E. Grossi for critically reviewing the manuscript and Elda Accettulli for secretarial assistance.
Supported by grants from Italian National Research Council (C.N.R.), Progetto Finalizzato A.C.R.O.; and Associazione Italiana Ricerca sul Cancro and Progetto Ricerca Corrente, Italian Ministry of Health.
Address reprint requests to Anna Corcione, PhD, Laboratory of Oncology, Scientific Institute G. Gaslini, Largo G. Gaslini, 5-16148 Genova, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal