Abstract
Promising results from clinical trials with unconjugated antibodies stimulated renewed interest in immune effector mechanisms of monoclonal antibodies (MoAbs). We investigated the potential of IgA as antibody isotype for cell- or complement-mediated tumor cell lysis and assessed the potential of its myeloid Fc receptor, FcαRI (CD89), as trigger molecule for bispecific antibody (BsAb)-mediated immunotherapy. Comparing hapten-directed antibodies of human IgA2 with IgG1 or IgG3 isotypes, we found all three to mediate effective killing of sensitized tumor target cells in whole blood assays. Analysis of effector mechanisms showed IgG-mediated lysis to be predominantly complement-dependent, whereas IgA-dependent killing was primarily effector cell-mediated. A comparison of effector cell populations in antibody-dependent cell-mediated cytotoxicity (ADCC) showed neutrophils to be most important for IgA-dependent tumor cell killing, involving FcαRI as shown with Fc receptor blocking antibodies. Reverse ADCC experiments against target cells sensitized with Fc receptor antibodies, or assays with FcαRI-directed bispecific antibodies confirmed FcαRI as effective trigger molecule in polymorphonuclear neutrophil (PMN)-mediated lysis. During granulocyte colony-stimulating factor (G-CSF ) therapy, (FcαRI × HER-2/neu) bispecific antibodies induced enhanced killing of HER-2/neu positive SK-BR–3 breast cancer cells in whole blood assays. This enhanced cytotoxicity was paralleled by increased PMN counts, which lead to higher effector to target cell ratios in G-CSF–primed blood. Furthermore, bispecific antibodies, directed to FcαRI and Candida albicans, enhanced neutrophils' phagocytosis of fungi. In summary, these results identify IgA as an effective antibody isotype for immunotherapy, working primarily via FcαRI on neutrophils. They suggest FcαRI-directed bispecific antibodies and G-CSF to be an attractive combination for malignant or infectious diseases.
MONOCLONAL ANTIBODIES (MoAbs) offer the potential to increase the specificity of oncologic therapy by targeting select tumor-associated antigens.1,2 Antitumor effects of MoAb may be divided into (1) direct effects on tumor cells (such as blockade of growth factors, inhibition of proliferation, or induction of apoptosis), or (2) indirect effects. These latter involve recruitment of immune effector mechanisms such as complement- or cell-mediated lysis of target cells and require interaction of therapeutic antibodies with complement proteins or with immunoglobulin receptors on effector cells. An improved interaction was shown with chimeric or humanized antibodies relative to their murine counterparts.3,4 Recent clinical trials with MoAbs in oncology focused largely on human IgG1 as therapeutic isotype.5,6 Human IgG1 effectively activates human complement and interacts well with human IgG Fc receptors, especially FcγRIIIa (CD16) involved in natural killer (NK) cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC). Data in this report support a role for human IgA as a promising therapeutic antibody isotype because it effectively recruits neutrophils for ADCC. Neutrophils have tumor-cytolytic potential against a broad spectrum of tumor cells7-10 and represent the most populous Fc receptor-expressing leukocytes. In addition, their number can be expanded several-fold by therapeutic application of granulocyte colony-stimulating factor (G-CSF ).
Cell-mediated effects of MoAbs require binding of their Fc portions to activating Fc receptors on immune effector cells. Most Fc receptors belong to the family of multichain immune recognition receptors (MIRR), consisting of distinct ligand binding α-chains, which noncovalently associate with shared signaling components. These promiscuous signaling molecules include FcR γ-, β-, or ζ- chains.11,12 For the family of leukocyte IgG receptors, eight α-chain genes were identified, all located on chromosome 1. They encode a total of 12 transmembrane or soluble receptor isoforms, which are grouped based on genetic, biochemical, and functional characteristics, into three Fcγ receptor classes: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). For IgA, in comparison, one α gene (FcαRI), located on chromosome 19, has been characterized that encodes several alternatively spliced transmembrane isoforms of 55 to 110 kD.13-15 FcαRI (CD89) is constitutively expressed on monocytes/macrophages, eosinophilic and neutrophilic granulocytes, but importantly, not on noneffector cell populations. FcαRI has medium affinity (≈5 × 107 mol/L−1) for both IgA1 and IgA2,16,17 which is increased on exposure to G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF ).18 For signaling, the α-chain of the IgA receptor requires association with the common FcRγ-chain, which results in the phosphorylation of an ITAM (immunoreceptor tyrosine-based activation motif ) within the γ-chain, initiating further downstream signaling events.19 Functions described for FcαRI include ADCC, phagocytosis, induction of a respiratory burst, and inflammatory mediator or cytokine release.16 17
In vivo, therapeutic antibodies compete with high concentrations of endogenous immunoglobulins for binding to Fc receptors. In addition, many Fc receptor-expressing cells are not cytolytic for tumor cells (eg, B cells, platelets), and some Fc receptor isoforms (eg, FcγRIIb, FcγRIIIb) bind antibodies, but do not trigger cytolytic cascades. However, select cytotoxic Fc receptors on effector cells can be triggered by antibodies reacting with Fc receptors via their variable regions,20 a process called reverse ADCC. This mechanism is used in the therapeutic concept of bispecific antibodies, where antibodies directed to cytotoxic trigger molecules, such as Fc receptors, are genetically or chemically linked to tumor-directed antibodies.21 Bispecific antibodies engaging FcγRI or FcγRIII are currently being tested with promising results in clinical trials and may help to overcome some of the above-mentioned limitations of conventional antibodies.22
Besides their potential as effector cells in antibody-based tumor therapy, neutrophils are the main effector cell population in the host defense against fungi.23 24 Therefore, we investigated the role of bispecific antibodies directed to FcαRI and Candida albicans. These constructs were found to mediate very effective phagocytosis of Candida by neutrophils. Thus, we identify FcαRI as a promising trigger molecule for bispecific antibody (BsAb)-based therapy and show synergistic effects in combination with G-CSF.
MATERIALS AND METHODS
Blood donors. Experiments reported here were approved by the Ethical Committee of the University of Erlangen, Nürnberg, in accordance with the Declaration of Helsinki. After informed consent, 10 to 20 mL of citrate anticoagulated peripheral blood was drawn from healthy volunteers or from patients receiving G-CSF therapy. Patients were treated with rh-met-G-CSF (Neupogen, 3 to 5 μg/kg of body weight) from Hoffmann La-Roche (Basel, Switzerland), based on clinical indications. As the kinetics of neutrophil recovery during G-CSF treatment vary, the following criteria were defined for analysis: (1) at least 3 days of G-CSF treatment, and (2) absolute neutrophil count > 2,500/μL. Total leukocyte counts were determined on a Coulter cytometer (Coulter, Hialeah, FL), and absolute polymorphonuclear neutrophil (PMN) counts were calculated from total leukocyte counts and differentials of Wright-stained blood smears.
Cell lines. The human breast carcinoma cell line SK-BR-3 and the hybridoma cell line HB58 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). All cells were kept in RF10 + medium consisting of RPMI 1640 medium (Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 U/mL streptomycin, and 4 mmol/L L-glutamine (all Life Technologies).
Monoclonal and bispecific antibodies. Human/mouse chimeric antibodies against the chemical hapten 5-iodo-4-hydroxyl-3-nitrophenacetyl (NIP) consisting of mouse VH , VL , and CL chains of λ isotype, and CH chains of human γ1, or γ3 isotypes were produced from Chinese hamster ovary (CHO)-K1 transfectomas and were purified on antigen columns as described.25 Anti-NIP IgA2 was used as supernatant from the original transfectoma,3 which was obtained from the European Collection of Animal Cell Cultures (Porton Down, UK).
Antibody 520C9 (mIgG1) against the proto-oncogene product HER-2/neu26 and Fc receptor antibodies A77 (mIgG1) and My43 (mIgM) both to FcαRI,27,28 22 (mIgG1) and 197 (mIgG2a) both to FcγRI,20 whole antibody and Fab fragments of IV.3 (mIgG2b) to FcγRII,29 and whole antibody and F(ab′)2 fragments of 3G8 (mIgG1) to FcγRIII30 were from Medarex (Annandale, NJ). Antibody MMA (mIgM) to CD15 and negative control antibody 3.6.2 (mIgG2a) were produced from hybridomas obtained from the ATCC. For detection of human/mouse chimeric NIP antibodies or murine Fc receptor antibodies, fluorescein isothiocyanate (FITC)-labeled anti-mouse λ light chain antibodies or FITC-labeled F(ab′)2 fragments of goat anti-mouse were used as staining reagents, respectively (both from Cappel, Cochranville, PA).
BsAb (FcαRI × HER-2/neu) was produced as described31 by chemically cross-linking F(ab′) fragments of MoAb A77 (FcαRI; CD89) with F(ab′) fragments of MoAb 520C9 (HER-2/neu). Briefly, F(ab′)2 fragments were produced by limited digestion with pepsin and were then reduced with mercaptoethanol to provide F(ab′) with free hinge-region sulfur hydroxyl (SH) groups. The SH groups on one of the F(ab′) (SH) partners were then fully derivatized with excess o-phenylenedimaleimide (o-PDM) to provide free maleimide groups. Finally, the F(ab′)-o–PDM and F(ab′)-SH were combined at a ratio of 1:1 to generate heterodimeric F(ab′)-o-PDM–F(ab′) constructs. After purification by size exclusion chromatography and characterization by high-performance liquid chromatography (HPLC), samples were sterilized by filtration. For production of (FcαRI × Candida) BsAb, F(ab′)2 fragments of a polyclonal anti-Candida IgG isolated from immunized rabbits (Biodesign, Kennebunk, MA) were treated with sulfo-SMCC, which adds maleimide groups to free amino groups. These F(ab′)2 fragments were then reacted with an equimolar concentration of A77 F(ab′), and the conjugate was purified from uncoupled molecules by chromatography on Superdex 200 (Pharmacia, Picsataway, NJ). Both BsAb were stored at 4°C and showed binding to both effectors and targets consistent with the specificity patterns of the parental antibodies.
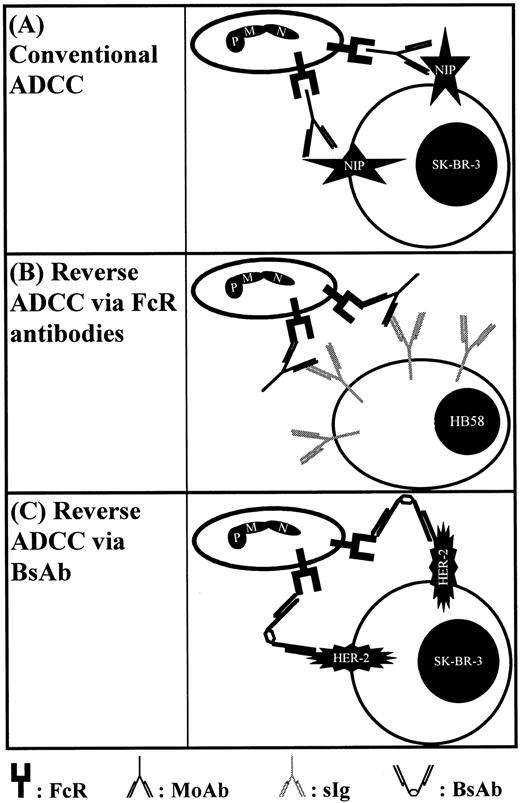
Schematic representation of different types of cytotoxicity assays employed in this study. (A) Shows conventional ADCC against haptenized SK-BR–3 breast cancer cells via NIP-directed antibodies. FcR blocking antibodies served to identify trigger molecules involved in IgA-mediated ADCC. In (B), a novel reverse ADCC assay is shown using hybridoma target cells, which were selected for high membrane expression of their surface Ig directed to mouse κ light chains. In the presence of Fc receptor antibodies, these hybridoma cells are sensitized for reverse ADCC. (C) Shows recruitment of Fc receptor positive effector cells via BsAbs.
Schematic representation of different types of cytotoxicity assays employed in this study. (A) Shows conventional ADCC against haptenized SK-BR–3 breast cancer cells via NIP-directed antibodies. FcR blocking antibodies served to identify trigger molecules involved in IgA-mediated ADCC. In (B), a novel reverse ADCC assay is shown using hybridoma target cells, which were selected for high membrane expression of their surface Ig directed to mouse κ light chains. In the presence of Fc receptor antibodies, these hybridoma cells are sensitized for reverse ADCC. (C) Shows recruitment of Fc receptor positive effector cells via BsAbs.
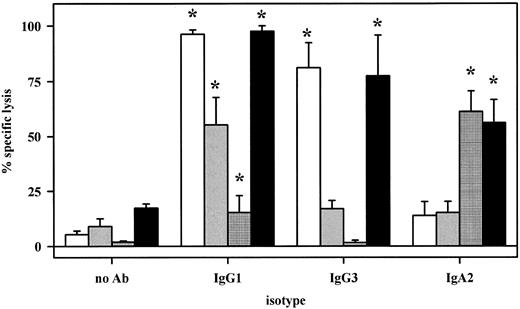
Antibody-mediated cytotoxicity against NIP-haptenized SK-BR–3 breast cancer cells was analyzed using NIP antibodies of human IgG1, IgG3, or IgA2 isotypes. As effector source, whole blood (▪), fresh human plasma (□), isolated MNC (▨), or PMN (▦) were compared. All three isotypes induced significant lysis with whole blood. Interestingly, IgA-dependent lysis was predominantly mediated by PMN, whereas IgG1- or IgG3- dependent killing resided mainly in the plasma fraction. Results from five experiments are presented as mean ± SEM of % specific lysis. Significant (P < .05) antibody-mediated lysis is marked by an asterisk (*).
Antibody-mediated cytotoxicity against NIP-haptenized SK-BR–3 breast cancer cells was analyzed using NIP antibodies of human IgG1, IgG3, or IgA2 isotypes. As effector source, whole blood (▪), fresh human plasma (□), isolated MNC (▨), or PMN (▦) were compared. All three isotypes induced significant lysis with whole blood. Interestingly, IgA-dependent lysis was predominantly mediated by PMN, whereas IgG1- or IgG3- dependent killing resided mainly in the plasma fraction. Results from five experiments are presented as mean ± SEM of % specific lysis. Significant (P < .05) antibody-mediated lysis is marked by an asterisk (*).
Isolation of mononuclear and neutrophil effector cells. Isolated neutrophils were obtained by a method described by Elsässer et al.9 Briefly, citrate anticoagulated blood was layered over a discontinuous percoll (Seromed, Berlin, Germany) gradient. After centrifugation, neutrophils were collected at the interphase between the two percoll layers and mononuclear cells from the percoll/plasma interphase. Remaining erythrocytes were removed by hypotonic lysis. Purity of neutrophils was determined by cytospin preparations and exceeded 95%, with few contaminating eosinophils, and less than 1% mononuclear cells. Viability of cells tested by Trypan blue exclusion was always greater than 95%.
Immunofluorescence analysis. Isolated neutrophils, mononuclear cells (MNC) or SK-BR–3 breast cancer cells were incubated with MoAb. During incubation of effector cells with MoAb, polyclonal human IgG (4 mg/mL) was added to inhibit nonspecific binding to FcγRI. After staining with FITC-labeled reagents, fluorescence was analyzed on an EPICS Profile flow cytometer (Coulter). For each cell population, relative fluorescence intensity (RFI) was calculated as the ratio of mean linear fluorescence intensity of relevant to irrelevant, isotype-matched antibodies.
Antibody-dependent cellular cytotoxicity assays. ADCC assays were performed as described.10 Briefly, target cells were labeled with 200 μCi 51Cr for 2 hours. For assays with NIP antibodies, SK-BR–3 cells were then haptenized by adding 0.5 mg of 3-nitro-4-hydroxy-5-iodophenacetylacetic acid (succinimide ester of NIP-CAP-OSU, Genosys Biotechnologies, Cambridge, UK) dissolved in 7.5 μL of dimethyl sulfoxide (DMSO) to a 5-mL suspension of SK-BR–3 cells in borate buffer (pH 8.3). After 5 minutes at room temperature, the reaction was stopped by washing with RF10+. Cells were then adjusted to 105/mL. Whole blood, plasma, or isolated effector cells (50 μL), sensitizing antibodies (10 μg/mL for anti-NIP IgG antibodies, 50 μL supernant for anti-NIP IgA, or 0.4 μg/mL for BsAb, unless specified otherwise) and RF10+ were added into round-bottom microtiter plates. In some experiments, Fc receptor blocking antibodies were used at a final concentration of 10 μg/mL. Assays were started by adding target cell suspensions (50 μL), giving a final volume of 200 μL, and an effector to target cell ratio of 80:1 with isolated cells. After 3 hours at 37°C, assays were stopped by centrifugation, and 51Cr release from triplicates was measured. Percentage of cellular cytotoxicity was calculated using the formula:
with maximal 51Cr release determined by adding perchloric acid (3% final concentration) to target cells, and basal release measured in the presence of sensitizing antibodies without effector cells. For analysis of effects induced by Fc receptor antibodies, “% inhibition” was calculated:
Negative values determined by this formula are reported as “% stimulation” in the presence of Fc receptor antibody.
Candida phagocytosis.C albicans (strain ATCC 448585) was cultured overnight at 37°C in Sabouraud Maltose Broth (DIFCO, Detroit, MI), harvested by centrifugation, washed three times with phosphate-buffered saline (PBS), and labeled by incubation with FITC (Sigma, St Louis, MO) at a concentration of 0.1 mg/mL in 0.1 mol/L NaH2PO4 /Na2HPO4 buffer (pH 9.6) for 30 minutes at 4°C. After three washes with PBS, 5 × 105 yeast particles were incubated for 30 minutes at 37°C with 2 × 105 isolated PMN without or with 10 μg/mL (A77 × αCandida) BsAb. C albicans phagocytosis was quantitated by measuring FITC-fluorescence intensities of cells on a flow cytometer using phycoerythrin (PE)-conjugated CD11b MoAb (Becton Dickinson, San Jose, CA) as a gate marker for PMN. Alternatively, phagocytosis was determined in cytospin preparations evaluated by microscopy.
Statistical analysis. Group data are reported as mean ± standard error of the mean (SEM). Differences between groups were analyzed by unpaired (or, when appropriate, paired) Student's t-test. Levels of significance are indicated.
RESULTS AND DISCUSSION
To investigate the role of IgA and its myeloid receptor FcαRI (CD89) for antibody-based tumor therapy, different cytotoxicity assays were established, which are schematically represented in Fig 1. First, SK-BR–3 breast cancer cells were covalently coated with the hapten NIP and sensitized with NIP-specific chimeric antibodies of human IgA2, IgG1, or IgG3 isotypes (Fig 1A). All three antibodies gave high levels of target cell sensitization (RFI = 130, 276, or 238, respectively) and did not induce direct target cell killing. In the presence of whole blood, all three mediated effective target cell lysis. For further analysis of effector mechanisms, whole blood was fractionated into plasma, isolated PMN, or MNC effector cells, which were then compared for their capacity to mediate lysis via NIP antibodies (Fig 2). Remarkably, IgA2-induced killing was predominantly mediated by PMN, and to a smaller extent, by plasma or MNC effector cells. In contrast, IgG1- or IgG3-mediated killing resided predominantly in the plasma fraction, with significant cell-mediated lysis only observed with IgG1. Antibody-mediated lysis in the presence of plasma was completely abrogated when plasma was heat-inactivated at 56°C for 30 minutes (n = 3), indicating complement-mediated lysis to be the underlying effector mechanism. In accordance with previous reports,17 IgA was found to be less effective than IgG1 in complement-dependent target cell killing. However, uncontrolled complement activation induced by therapeutic antibodies in vivo may cause serious side effects in patients.32 On the other hand, IgA proved considerably more effective in recruiting PMN for ADCC than IgG1. To our knowledge, no therapeutic human IgA MoAb has yet been given to patients. Together with our previous observation that neutrophil-mediated ADCC against malignant B cells was strongly target antigen restricted,9 results reported here suggest IgA antibodies to be particularly promising when ADCC by neutrophils is a major effector mechanism for MoAbs. Further studies are in progress to investigate whether neutrophils' target antigen restriction can be overcome by using therapeutic antibodies of IgA isotype.
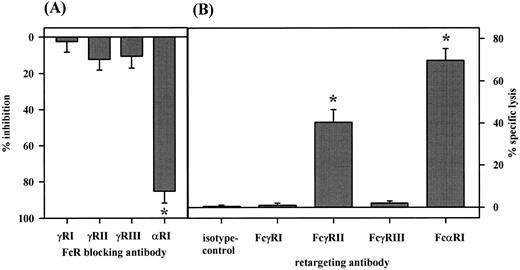
The role of FcαRI (CD89) in PMN-mediated ADCC was investigated by using Fc receptor-blocking antibodies in NIP-IgA2–mediated ADCC against SK-BR–3 cells (A) and in reverse ADCC against HB58 target cells (B). Significant inhibition of ADCC was observed with antibody My43 to FcαRI, but not with antibodies 197, IV.3 or 3G8 to FcγRI, FcγRII, or FcγRIII, respectively. Data from four experiments are presented as mean ± SEM of % specific inhibition. For reverse ADCC assays (B), HB58 hybridoma cells were selected for high membrane expression of their surface immunoglobulin, which is directed against mouse κ light chain, leading to sensitization of targets in the presence of murine antibodies against potential cytotoxic trigger molecules on PMN. In these experiments, PMN were effective with FcαRI or FcγRII-directed antibodies, but not with antibodies to FcγRI or FcγRIII. Results from five experiments are presented as mean ± SEM of % specific lysis. Significant (P < .05) inhibition (A) or reverse ADCC (B) is marked by an asterisk (*).
The role of FcαRI (CD89) in PMN-mediated ADCC was investigated by using Fc receptor-blocking antibodies in NIP-IgA2–mediated ADCC against SK-BR–3 cells (A) and in reverse ADCC against HB58 target cells (B). Significant inhibition of ADCC was observed with antibody My43 to FcαRI, but not with antibodies 197, IV.3 or 3G8 to FcγRI, FcγRII, or FcγRIII, respectively. Data from four experiments are presented as mean ± SEM of % specific inhibition. For reverse ADCC assays (B), HB58 hybridoma cells were selected for high membrane expression of their surface immunoglobulin, which is directed against mouse κ light chain, leading to sensitization of targets in the presence of murine antibodies against potential cytotoxic trigger molecules on PMN. In these experiments, PMN were effective with FcαRI or FcγRII-directed antibodies, but not with antibodies to FcγRI or FcγRIII. Results from five experiments are presented as mean ± SEM of % specific lysis. Significant (P < .05) inhibition (A) or reverse ADCC (B) is marked by an asterisk (*).
Increasing concentrations of (A77 × 520C9) BsAb directed to FcαRI and HER-2/neu were analyzed in ADCC against SK-BR–3 breast cancer cells. As effector cell source, unfractionated whole blood (A, •) was compared with human plasma (▵), isolated MNC (○), or PMN effector cells (▪) (B) at an E:T ratio of 80 to 1. Significant ADCC (P < .05 indicated by an asterisk [*]) was observed with BsAb concentrations higher than 0.08 μg/mL in whole blood and with PMN, but not with plasma or MNC. Results from three experiments are presented as mean ± SEM of % specific lysis.
Increasing concentrations of (A77 × 520C9) BsAb directed to FcαRI and HER-2/neu were analyzed in ADCC against SK-BR–3 breast cancer cells. As effector cell source, unfractionated whole blood (A, •) was compared with human plasma (▵), isolated MNC (○), or PMN effector cells (▪) (B) at an E:T ratio of 80 to 1. Significant ADCC (P < .05 indicated by an asterisk [*]) was observed with BsAb concentrations higher than 0.08 μg/mL in whole blood and with PMN, but not with plasma or MNC. Results from three experiments are presented as mean ± SEM of % specific lysis.
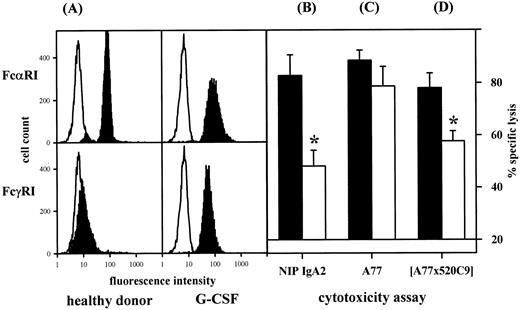
Isolated neutrophils from healthy donors or from G-CSF–treated patients were compared for expression of FcαRI (A), conventional ADCC via IgA2 (B), or reverse ADCC using either A77 sensitized HB58 target cells (C), or FcαRI-directed BsAb (D). Both effector cell populations expressed similar levels of FcαRI. At constant effector to target cell ratios, G-CSF–primed PMN were less effective than healthy donor PMN, with differences being significant for ADCC against SK-BR–3, either haptenized using anti-NIP IgA or HER-2/neu-directed BsAb (P = .01, or .03, respectively). Results from at least eight experiments are expressed as mean ± SEM. (A) (▪), Relevant antibody; (□), irrelevant antibody. (B through D) (▪), Healthy donor; (□), G-CSF–treated patient.
Isolated neutrophils from healthy donors or from G-CSF–treated patients were compared for expression of FcαRI (A), conventional ADCC via IgA2 (B), or reverse ADCC using either A77 sensitized HB58 target cells (C), or FcαRI-directed BsAb (D). Both effector cell populations expressed similar levels of FcαRI. At constant effector to target cell ratios, G-CSF–primed PMN were less effective than healthy donor PMN, with differences being significant for ADCC against SK-BR–3, either haptenized using anti-NIP IgA or HER-2/neu-directed BsAb (P = .01, or .03, respectively). Results from at least eight experiments are expressed as mean ± SEM. (A) (▪), Relevant antibody; (□), irrelevant antibody. (B through D) (▪), Healthy donor; (□), G-CSF–treated patient.
The influence of increased neutrophil counts during G-CSF therapy on ADCC against SK-BR–3 breast cancer cells was investigated in the presence of 0.4 μg/mL of BsAb (A77 × 520C9) directed to HER-2/neu and FcαRI. As effector cell source, 50 μL whole blood from patients before G-CSF (before), after 3 and 7 days of G-CSF (3 days and 7 days, respectively), or after G-CSF treatment (after) were compared. During G-CSF therapy, significant enhancement of BsAb-mediated ADCC was paralleled by significant increases in neutrophil numbers (P < .05, indicated by an asterisk [*]). Results from three experiments are presented as mean ± SEM of % specific lysis. (○), No antibody; (▴), (A77 × 520C9); (•), PMN count.
The influence of increased neutrophil counts during G-CSF therapy on ADCC against SK-BR–3 breast cancer cells was investigated in the presence of 0.4 μg/mL of BsAb (A77 × 520C9) directed to HER-2/neu and FcαRI. As effector cell source, 50 μL whole blood from patients before G-CSF (before), after 3 and 7 days of G-CSF (3 days and 7 days, respectively), or after G-CSF treatment (after) were compared. During G-CSF therapy, significant enhancement of BsAb-mediated ADCC was paralleled by significant increases in neutrophil numbers (P < .05, indicated by an asterisk [*]). Results from three experiments are presented as mean ± SEM of % specific lysis. (○), No antibody; (▴), (A77 × 520C9); (•), PMN count.
To identify cellular receptors involved in IgA-mediated ADCC, we analyzed PMNs' expression of FcαRI (CD89), FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16) by indirect immunofluorescence with MoAb A77, 22, IV.3, or 3G8, respectively. Thus, PMN expressed FcαRI, FcγRII, and FcγRIII (RFI 11.0 ± 1.1, 16.5 ± 2.2, and 99.1 ± 20.5, respectively, n = 8), but very low levels of FcγRI (RFI = 1.6 ± 0.2, n = 8). Blocking antibodies to FcγRI (197, whole antibody), FcγRII (IV.3, F(ab) fragments), FcγRIII (3G8, F(ab′)2 fragments), or FcαRI (My43, whole antibody) served to identify trigger molecules on PMN involved in IgA-mediated killing. IgA-mediated ADCC by PMN was significantly (P < .05) inhibited by FcαRI antibody My43, but not by isotype control antibodies or by Fcγ receptor antibodies, which were documented to block IgG-mediated ADCC11 12 (Fig 3A).
Reverse ADCC, induced by antibodies directed against surface molecules on effector cells, represents a sensitive and specific approach to identify tumor-cytolytic trigger molecules. To compare different Fc receptors for their capacity to mediate reverse cytotoxicity, we set-up a novel assay using rat HB58 hybridoma cells as targets in ADCC (Fig 1B). These cells were selected for high membrane expression of their surface immunoglobulin, a rat antibody directed to mouse κ light chains. In the presence of murine effector cell-directed antibodies, these target cells are sensitized for reverse ADCC. Importantly, healthy donor PMN very effectively lysed HB58 hybridoma cells in the presence of FcαRI antibody A77 (Fig 3B). Furthermore, they were effective with FcγRII antibody IV.3, but not with FcγRI or FcγRIII antibodies (22, and 3G8, respectively). G-CSF primed PMN were additionally effective with FcγRI antibody 22 (not shown). Data with this broadly applicable assay establish FcαRI on PMN as novel cytotoxic trigger molecule. For Fcγ receptors, they are in agreement with results using Fc receptor-specific hybridoma targets in reverse ADCC assays, which were selected for high surface expression of their immunoglobulins.9 33
The mechanism of reverse ADCC is exploited in the therapeutic concept of bispecific antibodies (Fig 1C). We, therefore, tested a BsAb constructed by chemically cross-linking F(ab′) fragments of antibodies A77 and 520C9, specific for FcαRI (CD89), or the proto-oncogene protein HER-2/neu, respectively. This BsAb showed the expected binding characteristics and was tested in ADCC against HER-2/neu positive SK-BR–3 breast cancer cells (Fig 4) using either whole blood (Fig 4A), or fractions consisting of human plasma, isolated MNC or PMN effector cells (Fig 4B). As shown, (A77 × 520C9) BsAb induced significant target cell lysis at concentrations higher than 80 pg/mL, with optimal killing at 2.0 μg/mL, by whole blood or PMN. Isolated MNC or human plasma had no activity with this BsAb, indicating that reverse ADCC by PMN represents the predominant effector mechanism in these whole blood assays.
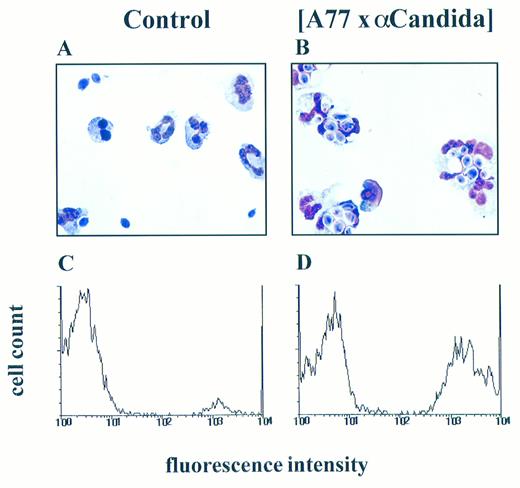
Role of FcαRI (CD89) in PMN-mediated phagocytosis of fungi. PMN were cultured with C albicans in medium alone (A and C) or in the presence of (A77 × αCandida) BsAb (B and D). Phagocytosis was analyzed microscopically with (A and B) showing representative photographs or by flow cytometry (C and D), plotting FITC-fluorescence (arbitrary units on a logarithmic scale) against the relative cell number. These experiments were repeated three times yielding essentially identical results.
Role of FcαRI (CD89) in PMN-mediated phagocytosis of fungi. PMN were cultured with C albicans in medium alone (A and C) or in the presence of (A77 × αCandida) BsAb (B and D). Phagocytosis was analyzed microscopically with (A and B) showing representative photographs or by flow cytometry (C and D), plotting FITC-fluorescence (arbitrary units on a logarithmic scale) against the relative cell number. These experiments were repeated three times yielding essentially identical results.
G-CSF is known to stimulate IgG-dependent ADCC by PMN via induction of FcγRI expression34 and to increase the affinity of IgA binding.18 Therefore, we compared isolated PMN from healthy donors and from patients during G-CSF therapy (Fig 5) for FcαRI expression (Fig 5A), IgA-mediated ADCC against haptenized targets (Fig 5B), reverse ADCC against A77-sensitized HB58 hybridoma cells (Fig 5C), and (A77 × 520C9) BsAb mediated lysis of SK-BR-3 cells (Fig 5D). As reported before,34,35 PMN isolated from patients during G-CSF therapy expressed higher levels of FcγRI compared with healthy donor PMN (RFI 8.1 ± 1.3 v 1.6 ± 0.2, n = 5, P < .01). However, both effector cell populations expressed similar levels of FcαRI (RFI 11.0 ± 1.1 v 16.2 ± 3.1, n = 12, P = .09). In ADCC assays (at constant effector to target cell ratios), G-CSF–primed PMN were less effective than healthy donor PMN, with differences being significant for ADCC against SK-BR–3, either haptenized using anti-NIP IgA or HER-2/neu directed BsAb (P = .01, or .03, respectively). This may be explained by competition for FcR γ-chain association between FcαRI and upregulated FcγRI on G-CSF–primed PMN, as has been shown for other Fc receptors.36,37 An alternative explanation is regulated expression of different FcαR isoforms, leading to expression of isoforms with reduced affinity for IgA13 or diminished signaling capacity.14 As ADCC is critically dependent on effector to target cell ratios,10 we performed whole blood ADCC assays before, on days 3 and 7 during, and after G-CSF therapy (Fig 6). Consistent with a critical role for PMN in FcαRI-mediated ADCC, increases in neutrophil counts were closely paralleled by significantly enhanced lysis of SK-BR–3 breast cancer cells in the presence of (FcαRI × HER-2/neu) BsAb.
Neutrophils are the most important effector cell population against pathogenic fungi such as candida and aspergillus species.23 24 Therefore, we investigated the role of FcαRI in mediating Candida phagocytosis by neutrophils. As shown in Fig 7, very effective phagocytosis of Candida was observed in the presence of (FcαRI × αCandida) BsAb. Comparing G-CSF–primed and healthy donor PMN, 59.4% ± 7.1% versus 62.7% ± 8.1% of neutrophils had phagocytosed Candida in the presence of BsAb (n = 3, difference not significant), whereas in its absence, only 3.9% ± 1.9% versus 7.5% ± 2.4% of PMN had ingested yeast particles.
In summary, these results suggest IgA antibodies or FcαRI-directed BsAbs to be effective reagents for immunotherapy, working primarily by recruiting PMN for ADCC or phagocytosis. The safety of G-CSF to increase neutrophil numbers in combination with (FcγRI × HER-2/neu) BsAb is currently evaluated in phase I/II studies.38,39 Preliminary results from these trials show biological responses, such as release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and an acceptable toxicity profile.38 39 Results reported here suggest combinations of FcαRI-directed BsAb and G-CSF to be promising for clinical evaluation.
ACKNOWLEDGMENT
We acknowledge the excellent technical assistance by S. Gehr and B. Bock, and H. Vile and K. Sundarpandian for preparation of bispecific antibodies. We thank Dr R.J. Owens for his support generating chimeric anti-NIP IgG1 and IgG3 transfectomas and Dr P. Guyre for valuable comments on the manuscript.
Supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (Va 124/1-3).
Address reprint requests to Thomas Valerius, MD, Division of Hematology/Oncology, Department of Medicine III, University Erlangen, Nürnberg, Krankenhausstraβe 12, 91054 Erlangen, Germany.

![Fig. 4. Increasing concentrations of (A77 × 520C9) BsAb directed to FcαRI and HER-2/neu were analyzed in ADCC against SK-BR–3 breast cancer cells. As effector cell source, unfractionated whole blood (A, •) was compared with human plasma (▵), isolated MNC (○), or PMN effector cells (▪) (B) at an E:T ratio of 80 to 1. Significant ADCC (P < .05 indicated by an asterisk [*]) was observed with BsAb concentrations higher than 0.08 μg/mL in whole blood and with PMN, but not with plasma or MNC. Results from three experiments are presented as mean ± SEM of % specific lysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4485/3/m_bl_0031f4.jpeg?Expires=1765933039&Signature=aE-ctiodH7gyumb0SHZJftkAMuB1M3rwkrsyB950rHAhNDxZLqx6js-yN0XSA0QhLm8kgQsSzSpF3AtvzrU2ynx6HpADNArdCvqRotVeIKjphFgTRlBPCvP8SuUTowZjuGYZPFWR~Zf-8n8lOwKWbJRGNUCvaZpjN6l967PL~ka2~a0HiuJDshd8~i0NAwZA7jet6GCpbc0iIIxSU0rIg3UKwMavr0ZGXMxaJNICU0uW5GiWboxhKsOlg8j6rnTee-BKDjZu89yLpS3IjTc3HnxYl5kuIexY7kxEqYTM1flEt0nhdGUZOqrgjxrtJuDD8HbdUHBJDk~SeaIT3RkHAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The influence of increased neutrophil counts during G-CSF therapy on ADCC against SK-BR–3 breast cancer cells was investigated in the presence of 0.4 μg/mL of BsAb (A77 × 520C9) directed to HER-2/neu and FcαRI. As effector cell source, 50 μL whole blood from patients before G-CSF (before), after 3 and 7 days of G-CSF (3 days and 7 days, respectively), or after G-CSF treatment (after) were compared. During G-CSF therapy, significant enhancement of BsAb-mediated ADCC was paralleled by significant increases in neutrophil numbers (P < .05, indicated by an asterisk [*]). Results from three experiments are presented as mean ± SEM of % specific lysis. (○), No antibody; (▴), (A77 × 520C9); (•), PMN count.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4485/3/m_bl_0031f6.jpeg?Expires=1765933039&Signature=M4HO9Sm3mpFUPrh1i1Db5Y2wCKsyftHYu1WplpcjrSj2d9Yjb~H3xkkUkKq0yg3pXLvTC4s1mQvTUv8BAwLlsfYk7H-4w-4VNwwfecoyQ31F8i5Og~ZD0jrjzRttEDGslkf8tvtBIimRB9vEnTarUML2jU-K7u1oaVKxVkpqIdo5AntSThGZs5hGx4GqkuiSipJrTeggypWmYr3swwcLPJjiciGvm4sCBS8giE3rimLcfI8YnOULbPNWTeso7k~M7X2dPX26C9MBrSSqj~sjsiD4Fs4mB91uhxCE6KIkPruk89qEag~OWoNet5YyQLfZ92DiYSjW2IX4Alq2JF73Tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal