Abstract
The prognostic significance of Bcl-2 protein expression and bcl-2 gene rearrangement in diffuse large cell lymphomas (DLCL) is controversial. Bcl-2 protein expression prevents apoptosis and may have an important role in clinical drug resistance. The presence of a bcl-2 gene rearrangement in de novo DLCL suggests a possible follicle center cell origin and perhaps a distinct clinical behavior more akin to low-grade non-Hodgkin's lymphoma (NHL). The purpose of this study was to determine the impact of Bcl-2 protein expression and bcl-2 gene rearrangement (mbr and mcr) on survival of a cohort of patients with DLCL who were uniformly evaluated and treated with effective chemotherapy. Patients included the original MACOP-B cohort (n = 121) and the initial 18 patients treated with the VACOP-B regimen (total = 139). All patients had advanced-stage disease, were 16 to 70 years old, and corresponded to Working Formulation categories F, G, or H. No patients had prior treatment, discordant lymphoma, or human immunodeficiency virus seropositivity. Paraffin sections from diagnostic biopsies were analyzed for bcl-2 gene rearrangement including mbr and mcr breakpoints by polymerase chain reaction and Bcl-2 protein expression by immunohistochemistry. With a median follow-up of 81 months, overall (OS), disease-free (DFS), and relapse-free survival (RFS) were measured to determine the prognostic significance of these parameters. Analyzable DNA was present in 118 of 139 (85%) cases, with 14 demonstrating a bcl-2 rearrangement (11 mbr, 3 mcr). All 14 of these bcl-2 gene rearrangement-positive cases were found in the 102 patients with a B-cell immunophenotype, but the presence of this rearrangement had no significant influence on survival. Bcl-2 protein expression was interpretable in 116 of 139 (83%) cases, with immunopositivity detected in 54 of 116 (47%). Using a cut-off of greater than 10% Bcl-2 immunopositive tumor cells for analysis, positive Bcl-2 protein expression was seen in 28 of 116 (24%) patients and the presence of this expression correlated with decreased 8-year OS (34% v 60%, P < .01), DFS (32% v 66%, P < .001), and RFS (25% v 59%, P < .001). Bcl-2 protein expression remained significant in multivariate analysis that included the clinical international prognostic index factors and immunophenotype (P < .02). In conclusion, although bcl-2 gene rearrangement status could not be shown to have an impact on outcome, Bcl-2 protein expression is a strong significant predictor of OS, DFS, and RFS in DLCLs.
DIFFUSE large cell lymphomas (DLCL) represent a diverse spectrum of lymphoid neoplasms with variable clinical, histologic, immunophenotypic, cytogenetic, and molecular genetic features.1,2 Therapy for these non-Hodgkin's lymphomas (NHLs) has greatly improved over the last two decades, with over half the patients experiencing long-term cure.3-6 Unfortunately, 40% to 50% of patients are not cured by multi-agent chemotherapy regimens, thus highlighting the need to develop models that identify potential patients better served by risk-adjusted therapies. Survival can be predicted on the basis of clinical characteristics, as recently established by the International Non-Hodgkin's Lymphoma Prognostic Factors Project.7 This model is useful for identifying at-risk patients who may benefit from more intensive therapy, but does not address the underlying biology of these heterogeneous diseases. Thus, an assessment of clinical factors is unlikely to be helpful in the design of specific therapies aimed at the molecular defects that characterize DLCLs.
The bcl-2 gene was originally discovered by virtue of its involvement in the (14; 18) (q32;q21) translocation.8-11 This cytogenetic abnormality results in deregulated expression of Bcl-2 protein and is found in the majority of follicular lymphomas (FL) and a variable number (10% to 40%) of DLCLs.12-33 Expression of Bcl-2 protein is independent of the translocation, as evidenced by its expression in a number of normal tissues, as well as a spectrum of lymphoproliferative disorders without a t(14; 18).34 A high level of Bcl-2 protein confers a survival advantage on B cells by inhibiting apoptosis and more generally may block a common cell death pathway induced by chemotherapy, conferring clinical drug resistance on cells over-expressing Bcl-2 protein.35,36 Bcl-2 protein expression has been shown to predict for poor outcome in acute myeloid leukemia, but conflicting results have been reported for acute lymphoblastic leukemia.37-39
Previous studies of bcl-2 gene rearrangement in DLCLs have been hampered by patient selection, nonuniform treatment strategies, variable molecular techniques for assessing bcl-2, and the inclusion of patients with antecedent low-grade follicular lymphoma.17-19,21-32 Recent studies have used more stringent inclusion criteria, but were analyzed using only mbr breakpoints.33 Much less is known about Bcl-2 protein expression in DLCL, but most studies have shown little impact on survival.30,31,40 Recently, several studies have suggested that Bcl-2 protein expression is an important predictor of disease-free survival (DFS), but a significant effect on overall survival was not seen.33,41 42 The purpose of this study was to determine the clinical utility of bcl-2 gene rearrangement (both mbr and mcr) and Bcl-2 protein expression for predicting overall survival (OS), DFS, and relapse-free survival (RFS) in a cohort of advanced-stage DLCL patients treated with uniform chemotherapy at a single institution.
MATERIALS AND METHODS
Patients.This study includes 145 consecutively encountered patients with diffuse aggressive lymphomas diagnosed between 1981 and June 1989 at the British Columbia Cancer Agency. This institution is the primary referral center for NHLs in the province of British Columbia, seeing the majority of patients with DLCL. Eligibility criteria were the following: age 16 to 70 years; diffuse lymphoma of large cell type (diffuse mixed, diffuse large cell, and immunoblastic lymphoma, Working Formulation categories F, G, or H); advanced disease with stage III, IV, or II with B symptoms or a mass greater than 10 cm; no prior treatment for lymphoma; and no congestive heart failure.43 Lymphomas related to acquired immune deficiency syndrome or organ transplantation were excluded. Patients with antecedent low-grade lymphoma or discordant lymphoma at diagnosis were also excluded. Eligible patients were treated with MACOP-B (n = 121)3 or VACOP-B (n = 18)4 as previously reported. This consisted of a 12-week outpatient regimen of oral and intravenous medications including prednisone, doxorubicin, and cyclophosphamide, alternating with vincristine plus either bleomycin or moderate-dose methotrexate with leucovorin rescue.3 Patients given VACOP-B received etoposide instead of methotrexate, but were otherwise treated the same. Ninety percent of the patients received more than 80% of the planned dose of chemotherapy. Details of the patients' characteristics and treatment delivery and outcome have been previously published.3 4 Of the total of 145 patients, 6 were excluded as no blocks were available for analysis (n = 139). Clinical features and histology were prospectively collected and entered into a computerized database at diagnosis and at follow-up. Bcl-2 gene rearrangement, Bcl-2 protein expression, and immunophenotype were retrospectively determined and recorded in a separate database constructed without knowledge of clinical outcome. These databases were later merged to allow this analysis of outcome.
Histology and immunohistochemistry.Tissue biopsy samples were fixed in buffered formalin or B5 fixative, routinely processed, sectioned at 3 μm, and stained with hematoxylin and eosin. All of the analyses were performed on biopsy specimens obtained before therapy. Cell lineage was assigned in each case using paraffin section immunostaining with a routine streptavidin-biotin peroxidase detection system and diaminobenzidine as chromogen. Monoclonal antibodies (MoAbs) included CD20 (L26), MB-2, CD45RO (A6, UCHL-1), CD45 (LCA), CD30 (Ber-H2), EMA, and polyclonal anti-CD3 (Dako, Carpinteria, CA). Cases were assigned a B-cell immunophenotype if positive staining was seen with CD20 and/or MB-2 but no T-cell antibodies. A T-cell immunophenotype was recorded if positive staining was seen with either CD45RO antibodies or polyclonal CD3 without B-cell staining. NHLs that failed to stain with any lineage marker were assigned a Null immunophenotype. Diagnosis of anaplastic large cell lymphoma (ALCL) was made using accepted histologic criteria and confirmed with CD30 staining.44
Bcl-2 immunostaining was performed on diagnostic biopsies using polyclonal anti–Bcl-2 antibodies (rabbit polyclonal anti–Bcl-2; Pharmingen, San Diego, CA) after microwave antigen retrieval as previously described.45 Previous studies had shown equivalent staining with MoAb clone 124 (Dako) in B5 fixed tissues, but superior staining in buffered formalin-fixed material.46 Sections were scored as negative if no large neoplastic cells stained; +1 (1% to 10% positive cells); +2 (11% to 30%); +3 (31% to 70%); and +4 (>70% positive large cells). Of the 139 total cases, 23 were excluded because Bcl-2 staining failed to stain the normal small lymphocytes that serve as positive internal controls, leaving 116 cases for analysis of Bcl-2 protein expression.
bcl-2 gene rearrangements.DNA was extracted from formalin-fixed paraffin blocks as follows: three 20-μm sections were cut from each block containing representative tissue, with the microtome blade changed between cases to avoid contamination. One section from each case was deparaffinized and DNA extracted by routine methods.12
Polymerase chain reaction (PCR) was performed with an automated thermal cycler (Perkin-Elmer Applied Biosystems Division, Foster City, CA) using a modification of published conditions as previously reported.12,47 For each PCR analysis, controls included a blank (no DNA template), a known positive control, and a 510-bp fragment of the β-globin gene used to assess for the presence of amplifiable DNA.12 Half of the amplified DNA was electrophoresed in 2% agarose and photographed under UV light after ethidium-bromide staining. A positive result was indicated by the presence of a single band of appropriate size in a lane of the gel.
Based on the intensity of the amplified β-globin fragment, the volume of template DNA in each PCR was adjusted appropriately so as to obtain optimum amplification with the primers for the mbr and mcr regions of the bcl-2 gene as follows: mbr: 5′- CCAAGTCATGTGCATTTCCACGTC- 3′; mcr: 5′-ACAGCGTGGTTAGGGTTAGGTCGTA-3′; consensus JH: 3′-ACCTGAGGAGACGGTGACC-5′.
Cases that failed to amplify the β-globin fragment when tested at three different dilutions were considered failures and were not amplified with the bcl-2–specific primers. Cases with amplifiable DNA that were negative after two attempts with the bcl-2 primers were considered bcl-2–negative. Amplifiable cases that showed a unique band with the bcl-2–specific primers were considered bcl-2–positive. The specificity of these amplified products was confirmed by transfer of the gel to a nylon membrane and probing with a radiolabeled oligonucleotide internal to the bcl-2 primers.
Statistical analysis.OS was calculated from the date of diagnosis until the patient's death or last follow-up. DFS was calculated as the interval between diagnosis and relapse, progression if the patient had less than a complete response, or death due to toxicity of treatment. RFS was calculated only for patients achieving a complete remission (CR) and was the interval between diagnosis and relapse of the disease. Survival curves were calculated by the method of Kaplan and Meier.48 Statistical comparison between curves was made by the log-rank test.49 Determination of significant differences in the distribution of clinical prognostic factors between groups was determined by the Pearson chi-squared test. Multivariate survival analysis was performed with the use of a stepwise proportional hazards model.50
RESULTS
A total of 139 patients were identified for whom there was adequate histologic material available for analysis. Their clinical characteristics are shown in Table 1. The median age was 52 years at diagnosis (range, 20 to 69 years), and 121 patients (87%) achieved a CR. After a median follow-up of 81 months (range, 1 to 183 months), the 8-year OS, DFS, and RFS were 55%, 58%, and 51%, respectively.
Clinical Characteristics of 139 Patients With DLCL
| Characteristic . | No. of Patients (%) . |
|---|---|
| Age: | |
| ≤60 yr | 99 (71) |
| >60 yr | 40 (29) |
| Sex: | |
| Male | 87 (63) |
| Female | 52 (37) |
| Site: | |
| Nodal | 96 (69) |
| Extranodal | 43 (31) |
| Stage: | |
| IIB | 50 (36) |
| III | 35 (25) |
| IV | 54 (39) |
| B symptoms | 66 (47) |
| Serum LDH: | |
| Normal | 37 (27) |
| Elevated | 102 (73) |
| Performance status (ECOG): | |
| 0,1 | 113 (81) |
| 2-4 | 26 (19) |
| Extranodal sites: | |
| 0,1 | 99 (71) |
| >1 | 40 (29) |
| BM involvement | 14 (10) |
| CR | 121 (87) |
| Characteristic . | No. of Patients (%) . |
|---|---|
| Age: | |
| ≤60 yr | 99 (71) |
| >60 yr | 40 (29) |
| Sex: | |
| Male | 87 (63) |
| Female | 52 (37) |
| Site: | |
| Nodal | 96 (69) |
| Extranodal | 43 (31) |
| Stage: | |
| IIB | 50 (36) |
| III | 35 (25) |
| IV | 54 (39) |
| B symptoms | 66 (47) |
| Serum LDH: | |
| Normal | 37 (27) |
| Elevated | 102 (73) |
| Performance status (ECOG): | |
| 0,1 | 113 (81) |
| 2-4 | 26 (19) |
| Extranodal sites: | |
| 0,1 | 99 (71) |
| >1 | 40 (29) |
| BM involvement | 14 (10) |
| CR | 121 (87) |
Abbreviations: LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group; BM, bone marrow.
Histologic subclassification showed 23 (16%) with diffuse mixed (DM), 87 (63%) diffuse large cell (DL), 19 (14%) immunoblastic, and 10 (7%) anaplastic large cell lymphomas (ALCL). Paraffin section immunophenotyping was successful in assigning lineage in 125 of 139 (90%) cases, including 115 (83%) B-cell, 10 (7%) T-cell, and 14 (10%) with a null immunophenotype.
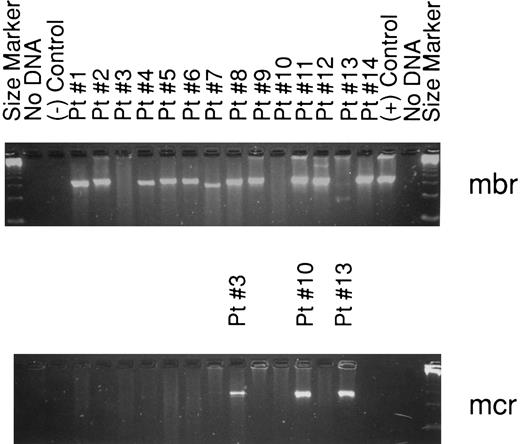
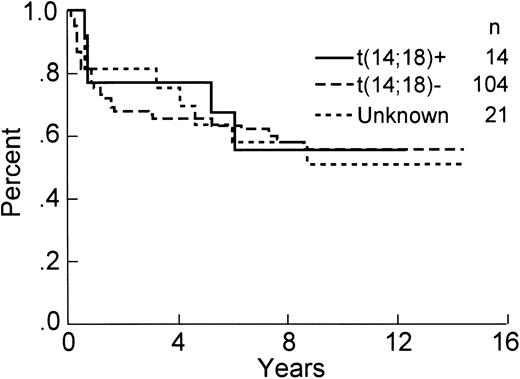
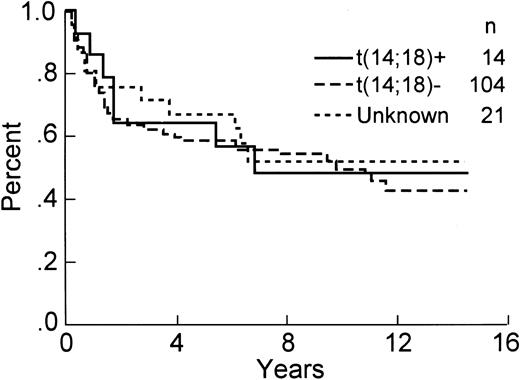
The analysis for bcl-2 gene rearrangement was performed on all 139 cases, with analyzable DNA present in 118 of 139 (85%) cases, including 14 positive samples. All cases with either an ALCL histologic subtype or T-cell immunophenotype having interpretable DNA results were negative for a bcl-2 gene rearrangement. If the PCR analysis is restricted to those cases with a B-cell immunophenotype and intact DNA, then 14 of 102 (14%) demonstrated a bcl-2 gene rearrangement, including 11 with an mbr and 3 with an mcr breakpoint (Fig 1). The relationship between clinical factors and the presence of a bcl-2 gene rearrangement is shown in Table 2. The international prognostic index factors (age > 60 years, elevated serum lactate dehydrogenase, stage III or IV, poor performance status, or >1 extranodal site) were evenly distributed between the bcl-2 positive versus negative groups, with the exception that the bcl-2–positive cases tended to be younger (P = .03). Interestingly, the bcl-2–negative cases were more frequently associated with concordant large cell lymphoma in the bone marrow as compared with the cases with a bcl-2 translocation, but this difference did not achieve statistical significance. The complete remission rate in both groups is virtually identical (see Table 2). Moreover, OS (57% v 57%, P = .85), DFS (42% v 47%, P = .73), and RFS (43% v 51%, P = .61) at 8 years were similar for bcl-2–positive and bcl-2–negative cases, respectively. The OS and DFS curves are shown in Figs 2 and 3, respectively, and include a separate curve of the 21 (15%) cases without analyzable DNA.
Agarose gel (2%) showing the 14 patients with a bcl-2 rearrangement by PCR. The top gel demonstrates all the bcl-2–positive cases. Bands are seen using mbr primers in all lanes except 3, 10, and 13. These three cases are shown in the lower gel and are positive for mcr rearrangements.
Agarose gel (2%) showing the 14 patients with a bcl-2 rearrangement by PCR. The top gel demonstrates all the bcl-2–positive cases. Bands are seen using mbr primers in all lanes except 3, 10, and 13. These three cases are shown in the lower gel and are positive for mcr rearrangements.
Clinical Characteristics of 118 Patients With DLCL Who Had DNA That Was Analyzable for t(14; 18) by PCR (bcl-2 gene rearrangement)
| Characteristic . | No. t(14; 18) Positive (%) . | No. t(14; 18) Negative (%) . | P . |
|---|---|---|---|
| Total | 14 (100) | 104 (100) | — |
| Age >60 yr | 0 | 34 (33) | .03 |
| Stage III or IV | 7 (50) | 66 (63) | NS |
| Elevated LDH | 10 (71) | 76 (73) | NS |
| Performance status (ECOG) 2-4 | 3 (21) | 17 (16) | NS |
| Extranodal sites >1 | 3 (21) | 31 (30) | NS |
| BM involvement | 0 | 10 (10) | NS |
| CR rate | 12 (86) | 88 (85) | NS |
| Characteristic . | No. t(14; 18) Positive (%) . | No. t(14; 18) Negative (%) . | P . |
|---|---|---|---|
| Total | 14 (100) | 104 (100) | — |
| Age >60 yr | 0 | 34 (33) | .03 |
| Stage III or IV | 7 (50) | 66 (63) | NS |
| Elevated LDH | 10 (71) | 76 (73) | NS |
| Performance status (ECOG) 2-4 | 3 (21) | 17 (16) | NS |
| Extranodal sites >1 | 3 (21) | 31 (30) | NS |
| BM involvement | 0 | 10 (10) | NS |
| CR rate | 12 (86) | 88 (85) | NS |
Abbreviation: NS, not significant.
OS of the 139 patients analyzed for the presence of a bcl-2 rearrangement by PCR.
OS of the 139 patients analyzed for the presence of a bcl-2 rearrangement by PCR.
DFS of the 139 patients analyzed for the presence of a bcl-2 rearrangement by PCR.
DFS of the 139 patients analyzed for the presence of a bcl-2 rearrangement by PCR.
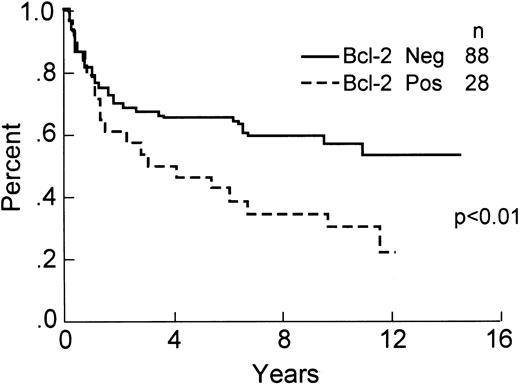
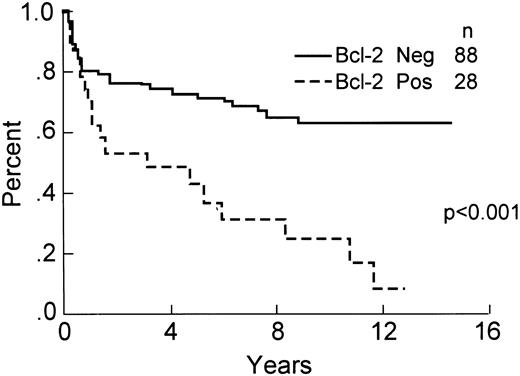
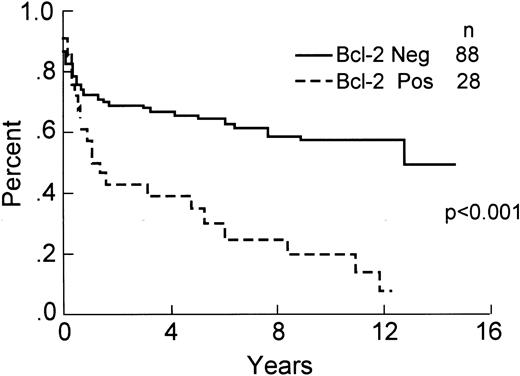
Of the 139 total cases, 116 ( 83%) had positive staining of small lymphocytes in paraffin sections and were included in the analysis of Bcl-2 protein expression. This included 63 (53%) without large cells staining (negative or 0), 26 (23%) with ≤10% large cells immunopositive (+1), 8 (7%) with between 11% and 30% cells staining (+2), 14 (12%) with 31% to 70% cells staining (+3), and 6 (5%) with >70% of the large neoplastic cells immunopositive (+4). Overall, 53 (47%) of the cases showed some expression of Bcl-2 protein. If a cut-off of >10% Bcl-2 immunopositive tumor cells is used for analysis, then 88 cases (76%) were scored as negative and 28 (24%) were positive for expression of Bcl-2 protein. The relationship between clinical characteristics, histologic subtype and Bcl-2 protein expression is shown in Table 3. The differences in 8-year OS (60% v 34%, P < .01), DFS (66% v 32%, P < .001), and RFS (59% v 25%, P < .001) are statistically significant, demonstrating a worse outcome for Bcl-2 immunopositive cases. These data are shown in Figs 4-6. The 8-year OS and DFS for the 23 cases that failed internal control staining of small lymphocytes for Bcl-2 protein was 57% and 55%, respectively. The relationship between the presence of a bcl-2 gene rearrangement and Bcl-2 protein expression is shown in Table 4. Of note, 6 cases with a bcl-2 rearrangement by PCR failed to express Bcl-2 protein, although 3 of these cases had between 1% and 10% immunopositive large cells (scored as +1).
Clinical Characteristics and Histologic Subclassification of 116 Patients With DLCL With Results Interpretable for Bcl-2 Protein Expression
| Characteristic . | No. Bcl-2 Positive (%) . | No. Bcl-2 Negative (%) . | P . |
|---|---|---|---|
| Total | 28 (100) | 88 (100) | — |
| Age >60 yr | 7 (25) | 26 (30) | NS |
| Stage III or IV | 13 (46) | 59 (67) | NS |
| Elevated LDH | 25 (89) | 64 (73) | .02 |
| Performance status (ECOG) 2-4 | 6 (21) | 15 (17) | NS |
| Extranodal sites >1 | 7 (25) | 26 (30) | NS |
| BM involvement | 2 (7) | 9 (10) | NS |
| CR rate | 25 (89) | 75 (85) | NS |
| Diffuse mixed | 7 (25) | 10 (11) | NS |
| Diffuse large cell | 20 (71) | 54 (62) | NS |
| IBL | 0 | 16 (18) | NS |
| ALCL | 1 (4) | 8 (9) | NS |
| Characteristic . | No. Bcl-2 Positive (%) . | No. Bcl-2 Negative (%) . | P . |
|---|---|---|---|
| Total | 28 (100) | 88 (100) | — |
| Age >60 yr | 7 (25) | 26 (30) | NS |
| Stage III or IV | 13 (46) | 59 (67) | NS |
| Elevated LDH | 25 (89) | 64 (73) | .02 |
| Performance status (ECOG) 2-4 | 6 (21) | 15 (17) | NS |
| Extranodal sites >1 | 7 (25) | 26 (30) | NS |
| BM involvement | 2 (7) | 9 (10) | NS |
| CR rate | 25 (89) | 75 (85) | NS |
| Diffuse mixed | 7 (25) | 10 (11) | NS |
| Diffuse large cell | 20 (71) | 54 (62) | NS |
| IBL | 0 | 16 (18) | NS |
| ALCL | 1 (4) | 8 (9) | NS |
Abbreviations: IBL, immunoblastic lymphoma; ALCL, anaplastic large cell lymphoma.
OS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
OS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
Relationship Between t(14; 18) (bcl-2 gene rearrangement) and Bcl-2 Protein Expression
| . | t(14; 18) Positive . | t(14; 18) Negative . | Unknown . |
|---|---|---|---|
| Bcl-2 positive | 7 (5%) | 19 (14%) | 2 |
| Bcl-2 negative | 6 (4%)4-150 | 66 (47%) | 14 |
| NA | 1 | 19 | 5 |
| . | t(14; 18) Positive . | t(14; 18) Negative . | Unknown . |
|---|---|---|---|
| Bcl-2 positive | 7 (5%) | 19 (14%) | 2 |
| Bcl-2 negative | 6 (4%)4-150 | 66 (47%) | 14 |
| NA | 1 | 19 | 5 |
Abbreviations: Unknown, DNA failed amplification; NA, not assessable (Bcl-2 staining uninterpretable because of failure to stain control small lymphocytes).
Three of the six cases had 1% to 10% immunopositive large cells and were scored as +1.
Multivariate analysis was performed and included the clinical variables recognized as prognostically important in the International Prognostic Factor study7 as well as immunophenotype and Bcl-2 protein expression. We had previously shown that the presence of a T-cell immunophenotype was an independent adverse prognostic factor in DLCLs.51 Bcl-2 protein expression was added to this model and was also found to be independently associated with a worse outcome in DLCL (P < .02).
DISCUSSION
In this study we sought to address two questions: (1) Does the presence of a bcl-2 gene rearrangment at the time of diagnosis predict for outcome in DLCLs? and (2) Is expression of Bcl-2 protein in DLCL an independent prognostic factor? Despite recently published work in this area, both of these questions remain controversial.30,33,40 41 To address these questions, we studied a cohort of patients who were uniformly staged and treated at a single institution with lengthy follow-up. Although many of the data are in agreement with several recent reports in the literature, this study provides some additional unique observations concerning the prognostic relevance of Bcl-2 protein expression in DLCL.
The reported frequency of t(14; 18) in DLCLs documented by cytogenetics and/or molecular genetics is highly variable in different studies, which probably reflects patient selection, use of various probes (mbr, mcr, or both), different molecular techniques (Southern blot analysis, PCR), and the inclusion of patients with either antecedent follicular lymphoma or discordant lymphoma.14-33 Not surprisingly, those studies that include patients with transformed follicular lymphomas report higher frequencies of bcl-2 gene rearrangement in DLCL.22 The majority of studies that have used PCR to evaluate bcl-2 rearrangements have analyzed only mbr breakpoints.21,22,24,25,30,33 Hill et al33 recently reviewed the literature and reported a cumulative frequency of 204 of 1,030 (19.8%), with a range of 10% to 40% for bcl-2 rearrangement in DLCL. This included their own recently published work from the British National Lymphoma Investigation (BNLI) Study in which they reported a frequency of 17% (27 of 161) using only mbr primers.33 Our data are quite similar, with 14 of 102 (14%) B-cell DLCL cases demonstrating a bcl-2 rearrangement using both mbr and mcr primer pairs. Patients with discordant lymphoma, who typically present with DLCL at a nodal or extranodal site with small cell lymphoma in the bone marrow, were excluded from our study. This latter group may be more frequently associated with a bcl-2 rearrangement, but to the best of our knowledge this has not been reported.
Although some studies have found that bcl-2 gene rearrangement positive cases have a higher rate of relapse, the majority of published series have not found a significant difference in either OS or DFS.14-33 Similarly, the BNLI report found that the presence of a bcl-2 rearrangement had no effect on RFS in DLCL. We also found that the presence of a bcl-2 gene rearrangement had no impact on either OS or DFS, and fails to predict for those patients who will ultimately relapse. We did find some patient characteristics which were associated with a bcl-2 rearrangement. They tended to be younger and had less frequent involvement of their bone marrow, although this latter difference did not reach statistical significance (see Table 2). Of note, the bcl-2–positive cases were evenly distributed between nodal and primarily extranodal presentation at diagnosis (data not shown).52
Some caution in interpreting the molecular data is required because bcl-2 PCR has a well-recognized false-negative rate. Using cytogenetics as the gold standard, both Southern blot analysis and PCR fail to detect rearrangements in approximately 15% and 25% of cases, respectively.12 The availability of paraffin material limited our analytical strategy to the use of PCR only, but the results are in keeping with those studies using cytogenetics.20 Additionally, the DNA failures were included in the analysis of outcome (see Figs 2 and 3), with similar survival as compared with either the bcl-2 gene rearrangement positive or negative patient groups.
Several studies of Bcl-2 protein expression in DLCL have been reported, but with conflicting results.30,33,40-42 Overall, the reported frequency of Bcl-2 expression has varied between 34% and 69%. Most reported series have used similar techniques and anti–Bcl-2 MoAb reagents. The original report of Ngan et al53 in 1988 used polyclonal antibody reagents and reported a frequency of 34%. In our study we used a rabbit polyclonal antisera with well-documented Bcl-2 specificity and found positive staining in 53 of 116 (47%) of cases. Previous experience with this reagent demonstrated an improved sensitivity in buffered formalin-fixed material as compared with MoAb reagents (Dako; clone 124).46 However, using a cut-off similar to other studies of greater than 10% immunopositive tumor cells, we found only 24% Bcl-2 immunopositivity in DLCLs. Importantly, a significant survival difference was shown for OS, DFS, and RFS at either threshold of Bcl-2 immunopositivity, further supporting the validity of these observations. The original reports of the prognostic significance of Bcl-2 protein expression in DLCL failed to show any impact on survival.30,40 Subsequently, three groups using similar techniques and thresholds of Bcl-2 immunopositivity have reported that Bcl-2–positive cases have a worse disease-free or cause-specific survival, although none were able to demonstrate a significant difference in OS.33,41 42 Methodological differences, uniformity of patient selection, staging and treatment, and longer follow-up may explain why we were able to demonstrate a significant difference in OS at either level of Bcl-2 immunopositivity. Moreover, we were able to show that Bcl-2 protein expression was an independent prognostic factor in DLCL, when incorporated into a model that included the clinically related International Prognostic Factor Index as well as immunophenotype. Although Bcl-2 protein expression was associated with an elevated serum LDH, it remained a statistically significant prognostic factor after multivariate analysis.
DFS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
DFS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
RFS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
RFS of the 116 patients analyzed for expression of Bcl-2 protein by immunohistochemistry.
Table 4 highlights the relationship between bcl-2 gene rearrangements and Bcl-2 protein expression. These data demonstrate that cases with a molecular rearrangement may fail to express the protein. This phenomenon has been previously described in follicular lymphoma, whereby cases with a bcl-2 translocation fail to express Bcl-2 mRNA or protein.54 Mutations in the open reading frame of the translocated bcl-2 gene may be present, leading to absent or diminished production of Bcl-2 protein.55,56 This finding has also been recently described in DLCLs that arise through transformation of follicular lymphoma, and may be one mechanism that leads to absent Bcl-2 protein expression in some cases with the translocation.57 The frequent expression of Bcl-2 protein in cases lacking t(14; 18) is well described, and suggests that mechanisms other than translocation can lead to increased Bcl-2 levels in NHLs.34 Both examples serve to point out that the presence of a bcl-2 gene rearrangement is not synonymous with overexpression of Bcl-2 protein, and vice versa.
One selective pressure against Bcl-2 expression could be related to the reported ability of Bcl-2 overexpression to inhibit cell proliferation, probably by causing a G1-phase delay or block.58-61 Thus, while high levels of Bcl-2 provide a survival advantage for malignant cells, they may also result in diminished proliferation and thereby negatively affect cell growth. A reasonable hypothesis therefore is that a t(14; 18) that activates the bcl-2 gene may create a permissive environment for the development of other genetic alterations by blocking programmed cell death, but eventually these tumors may become less dependent on Bcl-2 protein expression for their survival in vivo, and thus find it advantageous to inactivate Bcl-2 so that higher amounts of proliferation are achieved. However, the consequences of Bcl-2 protein downregulation may be deleterious to the tumor, resulting from loss of the anti-apoptotic mechanism and enhanced susceptibility to cell death from an increased tumor growth fraction killed by cell cycle active chemotheraputic agents. The balance between these opposing forces may be important for understanding the relationship between cell death and cell cycle pathways, and may provide insights into novel stategies for overcoming chemoresistance.
In summary, in this study we were unable to show that bcl-2 gene rearrangement status at diagnosis has a significant impact on clinical outcome. However, Bcl-2 protein expression is an important independent predictor of survival in patients with advanced-stage DLCL treated with chemotherapy. Consideration should therefore be given to incorporating this and perhaps other established pathologic variables into the design of treatment strategies aimed at improving the outcome of patients considered at increased risk for treatment failure.
ACKNOWLEDGMENT
The authors thank Dr P. Klimo for their help in providing clinical data for this study, Dr C. Coppin for assistance with the statistical analysis, and C. Wong for her help with data collection.
Supported by a British Columbia Health Research Foundation grant (R.D.G.) and partial support from a United States National Cancer Institute Grant to J.C.R. (CA-60421).
Address reprint requests to Randy D. Gascoyne, MD, FRCPC, Department of Pathology, B.C. Cancer Agency, 600 W 10th Ave, Vancouver, BC, V5Z 4E6 Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal