Abstract
The Fas (Apo-1/CD95) ligand (FasL) plays a central role in the elimination of target cells by effector T lymphocytes and in the suppression of cellular immune responses against nonmalignant and malignant cells. We show the expression of FasL on the surface of neoplastic plasma cells. We provide evidence that the FasL is functionally active because five of five neoplastic plasma cell lines tested killed CEM-C7H2 T-acute lymphoblastic leukemia (T-ALL) cells. The effect was mediated via the Fas (Apo-1/CD95) receptor molecule because blocking of Fas on the target cells or the FasL on the tumor cells by receptor- and ligand-specific monoclonal antibodies (MoAbs), respectively, protected T cells from being killed by myeloma cells. In addition, overexpression of the cowpox virus protein CrmA, a molecule with inhibitory potential on caspase-1 and caspase-8, specifically involved in Fas-induced signaling, protected T cells from being destroyed by the neoplastic cells or the agonistic anti-Fas MoAb. The potential of the malignant plasma cells to extinguish target T cells was independent of their own sensitivity to the agonistic anti-Fas MoAb, and FasL-positive (FasL+) CEM-C7H2 T cells were incapable of killing myeloma cells. Our results suggest that tumor cell–induced suppression of the immune system may be exerted via the FasL active on malignant plasma cells. Furthermore, loss of Fas expression or insensitivity to the agonistic anti-Fas MoAb do not seem to be prerequisites for myeloma cells to defeat T cells via Fas/FasL interaction.
THE FAS LIGAND (FasL) recognizes and cross-links the Fas (Apo-1/CD95) receptor, a member of the tumor necrosis factor/nerve growth factor (TNF/NGF) receptor supergene family, which is expressed in a wide variety of normal and neoplastic tissues.1-3 The extracellular portion of the FasL protein has been shown to share significant homology with other members of the TNF-family like TNF-α, CD30 ligand, CD40 ligand, or lymphotoxin.4,5 The functional FasL molecule induces programmed cell death in Fas+, FasL-sensitive target cells.1,4 The molecule usually acts in a membrane-bound manner,4 but after being shed by the cell membrane, also in its soluble form.6 7
The recognized biologic roles of the FasL lie in its participation in the process of the acquisition of self tolerance by clonal deletion of thymocytes,8 as well as in T-cell–mediated target cell killing as a part of the host-defense against virally infected or transformed cells.9 Until recently, T cells were thought to actively control the direction of Fas-induced target cell killing and to be the major source of active FasL molecules10,11 — a supposition that is in good agreement with the functions mentioned above as well as with the rather broad expression profile of the Fas/Apo-1 receptor. However, this picture was incomplete because, in addition to T cells, cells of the murine testis and retina also were shown to express FasL mRNA and protein.4,12,13 Further studies in the murine system revealed the role of FasL-induced apoptosis for the establishment of immune privileged sites, because retina cells were shown to be capable of suppressing cellular immune responses after inoculation of the anterior chamber of the eye with viral antigen. Thereby, tolerance was acquired via FasL-mediated killing of infiltrating T cells.12,14 More recently, T-cell–derived neoplastic cells such as natural killer cell lymphomas, large granular lymphocytic leukemias,15 as well as a T-cell acute lymphocytic leukemia (T-ALL) cell line16 also were shown to constitutively express FasL. These observations raise the question of whether the FasL-induced active suppression of tumor-specific, Fas+ T cells might be one of the mechanisms by which neoplastic cells escape from immune surveillance. In contrast to other mechanisms by which tumor cells escape immune responses, such as aberrant regulation of processing and presentation of antigen,17,18 changes in the immunologic profile of the cell surface,19 or the release of cytotoxic substances, aminosugars, or gangliosides,20-22 FasL expression would be an active immunosuppressive strategy mediated by cell-cell interaction which defeats T cells using their own weapons. As a caveat against this hypothesis it must be pointed out that the expression of FasL on malignant T cells might be caused by their lineage-specific derivation. Remarkably enough, however, the expression of the FasL molecule was not found to be restricted to T cells or hematologic neoplasms derived from the T-cell lineage, but was also detected on human macrophages23 and neutrophil granulocytes,24 thus pointing to a more ubiquitous distribution of the FasL within the hematopoetic system. Moreover, the expression of functionally active FasL was also observed on phorbol 12-myristate 13-acetate (PMA)-activated murine B cells.25 These findings prompted us to investigate the potential expression and functional relevance of the FasL molecule on human malignant cells of the B-cell lineage. Multiple myeloma, plasma cell leukemia, and plasmacytoma cell lines were chosen as a model for peripheral mature B-cell neoplasias26 because these cell lines were previously shown to express the Fas antigen while displaying a varying sensitivity to treatment with the agonistic anti-Fas monoclonal antibody (MoAb).27 Furthermore, the expression and function of the Fas receptor on neoplastic plasma cells was under the control of immunologically active cytokines,27 thus underlining its role as a target of the natural and the therapeutically activated immune surveillance.
The present study proves the expression of functional FasL on neoplastic plasma cells and their ability to kill target T cells regardless of the myeloma cells expression level of the Fas receptor and their own sensitivity to Fas-mediated signaling. This not only points to a possible mechanism by which myeloma cells escape the natural or therapeutically activated immune system, but may also open opportunities for novel therapeutic strategies. Thus, downregulation of the FasL by means of antisense or other immunomodulatory strategies could prove helpful in the treatment of this disease by disarming myeloma cells and, thereby, disabeling them from deleting activated T-cell clones.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
The myeloma cell lines NCI-H929, RPMI-8226, and U-266, the plasma cell leukemia lines ARH-77 and LP-1, the plasmacytoma cell line MC/CAR, and the IM-9 myeloma/lymphoblastoid cell line were used in this investigation. The LP-1, IM-9, and U-266 cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), while the NCI-H929, MC/CAR, and ARH-77 cell lines were purchased from the American Type Culture Collection (ATCC; Rockville, MD). The RPMI-8226 was kindly provided by Dr Takeshi Otani (Fujisaki Cell Center, Okayama, Japan). The human T-ALL cell line CEM-C7H2, a steroid sensitive subclone of the CCRF-CEM T-ALL cell line,28 was used as a Fas+/FasL+ control and target cell line. All cells were cultured in RPMI 1640 media (Seromed, Berlin, Germany) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS, Biological Inc, Beth Haemek, Israel), 2 mmol L-glutamine (Seromed, Berlin, Germany), and 100 μg/mL gentamicin (GIBCO, Grand Island, NY) at 37°C in a humidified atmosphere containing 5% CO2 .
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was isolated using RNAzol (BIOTECX LAB Inc, Houston, TX) according to the manufacturer's recommendation. Total RNA (1 μg) was subjected to cDNA synthesis using avian myoblastosis virus (AMV) reverse transcriptase (Promega, Madison, WI) in the supplied buffer system and 100 pmol hexanucleotide random primer (Boehringer Mannheim, Mannheim, Germany). Synthesis was performed at 42°C for 60 minutes and followed by denaturation of the cDNA strands at 95°C for 10 minutes. Samples were cooled rapidly to 4°C and 2 to 4 μL was used for PCR. PCR (50 cycles: 1 min/94°C, 1 min/60°C, 2 min/72°C) was performed using Roche-Ampli-Taq-Gold DNA Polymerase (Cetus, Vienna, Austria) in the supplied buffer system with 0.2 mmol/L dNTP mixture (Boehringer Mannheim) and 50 pmol of the following relevant 5′/3′ primers (Microsynth, Balgach, Switzerland). Primers were chosen using the OLIGO 4.0 computer program: β-Actin primers: (upper 5′ ATCTGGCACCACACCTTCTACAATGAGCTGCG 3′; lower 5′ CGTCATACTCCTGCTTGCTGATCCACATCTGC 3′), product size 838 bp. FasL primers: (upper 5′ TTCTTCCCTGTCCAACCTCTGTGC 3′, lower 5′ TCATCTTCCCCTCCATCATCACCA 3′), product size 627 bp.
Northern Analysis
Ten micrograms of total RNA was analyzed by electrophoresis using 1% agarose-formaldehyde gels, followed by capillary transfer overnight to a positively charged nitrocellulose membrane (Boehringer Mannheim). After transfer, the membrane was dried, UV cross-linked, and blocked for 2 hours in 25 mL (pre)-hybridization solution (5 × sodium/sodium citrate [SSC], 50% deionized formamide, 0.1% sodium-lauryl-sarcosine, 0.02% sodium dodecyl sulfate [SDS] 10 × Denhardt's reagent) at 55°C. The membrane was hybridized with 2 × 106 cpm/mL 32[P]-labeled FasL DNA probe overnight at the same temperature. For posthybridization washes, 2 × SSC/0.1% SDS (2 × 10 minutes, room temperature) as well as 0.1 × SSC/0.1% SDS (1 × 5 minutes room temperature, 1 × 5 minutes, 55°C) were used. The membrane was stripped and reprobed at 50°C using a human GAPDH probe. The FasL-specific probe was prepared as follows: CEM-C7H2 cDNA was amplified using FasL-specific primers as detailed in section “RT-PCR.” After gel electrophoresis, the FasL PCR product was excised out of the gel, purified using a Bio-101 Gen-clean kit (San Francisco, CA) and labeled with 20 μCi α32[P]-CTP using a random primer labeling kit (Boehringer Mannheim) to a specific activity of ∼5 × 106 cpm/μg.
Immunoblotting
Immunoblotting of cellular proteins was performed as previously described.26 Cells (2 × 106) were resuspended in 200 μL lysis buffer (50 mmol/L Tris, 150 mmol/L NaCl, 2 mmol/L EDTA, 1 mmol/L EGTA pH 7.5, supplemented with 25 μg/mL aprotinin, 25 μg/mL leupeptin, and 1% Triton X-100 [Sigma, St Louis, MO]). The samples were cleared by centrifugation (16,000g, 30 minutes, 4°C) and corrected for protein concentrations. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (14%) was performed under reducing conditions on Tris/glycine-buffered gels (Novex, San Diego, CA). Proteins were transferred onto a polyvinyl-difluoridon (PVDF) membrane (Millipore, Bedford, MA) by tank-blotting (220 mA, 80 minutes, 4°C). The anti-FasL MoAb (Transduction Laboratories, Lexington, KY) was diluted 1:2,000 in Tris-buffered saline (TBS) containing 0.05% Tween-20 and 5% nonfat dry milk for incubation. Rabbit-antimouse peroxidase-conjugated antibodies (Dako, Copenhagen, Denmark) served as secondary antibodies (1:1,000). Incubations were performed for 1 hour each at room temperature. Diaminobenzidine diluted in TBS served as substrate solution.
Immunofluorescence
FasL staining.Cells (0.2 × 106) were washed twice in phosphate-buffered saline (PBS), resuspended, and fixed in 1 mL paraformaldehyde (4%) and incubated for 15 minutes at 4°C. After an additional wash with PBS the pellet was resuspended in chilled methanol (100%) and incubated for 60 minutes on ice. Cells were washed twice in PBS, incubated with 1 μg anti-FasL MoAb NOK-1 (Pharmingen, San Diego, CA) for 30 minutes at 4°C. Again, cells were washed twice with PBS and incubated with 5 μL of fluorescein isothiocyanate (FITC)-labeled goat-antimouse antibody F0479 (DAKO, Copenhagen, Denmark) for 30 minutes at 4°C. The pellets were resuspended in 200 μL PBS/1% bovine serum albumin (BSA) and analyzed immediately by FACScan (5,000 cells per sample). Negative controls were carried out simultaneously using a mouse-antihuman IgG1 MoAb (no. X0931; DAKO) instead of the FasL MoAb.
CD95 staining.Cells (0.2 × 106) were incubated with 10 μL FITC-conjugated anti-Fas MoAb MC 064 (Kamiya, Thousand Oaks, CA) for 30 minutes at room temperature and gently shaken. Cells were washed twice with PBS, resuspended in 150 μL PBS/2% FCS, and analyzed immediately by flow cytometry (5,000 cells per sample). Negative controls were performed simultaneously using an FITC-conjugated mouse-antihuman IgG1 MoAb (no. X927; DAKO) instead of the anti-Fas MoAb. The mean specific fluorescence intensities (MSFIs) were calculated as the ratio of mean fluorescence channels specific MoAb/isotype-matched negative control MoAb.
Determination of FasL Expression on Native Neoplastic Plasma Cells
Bone marrow (BM) samples of six patients with multiple myeloma and one patient with breast cancer were analyzed. Samples were collected during routinely scheduled examinations after informed consent had been obtained. The patients characteristics are given in Table 2 (Results section). BM samples were pressed through a 0.2 × 20-mm syringe and diluted in RPMI-1640 medium 1:1 (vol/vol). The solution was applied onto a FICOLL density gradient (Lymphoprep; NYCOMED, Oslo, Norway) [2:1 (vol/vol)] and centrifuged with 500g for 30 minutes at room temperature. The mononuclear cell fraction was carefully aspirated, washed twice with PBS, and BM cells (0.5 × 106) were forwarded to FasL or isotype staining, respectively (see “FasL Staining”). After FasL staining, the cells were washed three times in PBS and thereafter subjected to CD38 immunostaining (30 minutes on ice) using 20 μL of a phycoerythrin (PE)-conjugated mouse-antihuman CD38 MoAb (Pharmingen). The plasma cell population was gated according to their unique position in the correlation of forward light scattering, orthogonal light scattering, and immunofluorescent-labeled CD38.29 Plasma cells stained with the FasL MoAb were compared with samples stained with the relevant FasL isotype control to determine FasL expression on the plasma cells.
Patients' Characteristics and Determination of FasL Expression in Native Malignant Plasma Cells
| Patient No. . | Age (yr) . | Treatment . | Paraprotein . | BM Plasma Cells (%) . | FasL Expression (MSFI)* . |
|---|---|---|---|---|---|
| 1 | 55 | No | IgGλ | 19 | 1.06 |
| 2 | 52 | Yes | IgGλ | 2 | 1.25 |
| 3 | 76 | No | Nonsecretory | 50 | 1.46 |
| 4 | 70 | No | IgG-κ | 40 | 1.30 |
| 5 | 69 | Yes | IgG-λ | 5 | 1.13 |
| 6 | 65 | No | κ-Light chain | 35 | 1.26 |
| Patient No. . | Age (yr) . | Treatment . | Paraprotein . | BM Plasma Cells (%) . | FasL Expression (MSFI)* . |
|---|---|---|---|---|---|
| 1 | 55 | No | IgGλ | 19 | 1.06 |
| 2 | 52 | Yes | IgGλ | 2 | 1.25 |
| 3 | 76 | No | Nonsecretory | 50 | 1.46 |
| 4 | 70 | No | IgG-κ | 40 | 1.30 |
| 5 | 69 | Yes | IgG-λ | 5 | 1.13 |
| 6 | 65 | No | κ-Light chain | 35 | 1.26 |
MSFI was determined by immunofluorescence staining and FACS analysis and calculated as the ratio of the MSFI achieved with anti-FasL MoAb/isotype-matched control MoAb. Mean of two experiments is given. A ratio >1.2 was considered as positive.
Assay for Fas/FasL-Induced Cell Death
Target cell death of CEM-C7H2 T-ALL cells resulting from their cocultivation with “effector” myeloma cells was quantified by measuring target cell DNA fragmentation using the JAM-Test.30 31 For this purpose, T-ALL cells were incubated with 10 μCi/mL 3[H]-thymidine (Amersham, Buckinghamshire, UK) for 5 hours, washed three times with PBS, resuspended in regular culture medium, and 100 μL of the suspension (2 × 104/mL) was cocultivated in 96-well plates with or without 100 μL of the suspension of the relevant myeloma cell lines (2 × 105/mL). The myeloma cells were incubated with 2.5 μg/mL anti-FasL MoAb (NOK-2) or a relevant negative control IgG2a MoAb (no. X0943; DAKO), respectively, 30 minutes before cocultivation with the labeled CEM-C7H2 cells. Alternatively, the labeled T cells were coincubated with 0.25 μg/mL of the Fas receptor blocking ZB4 MoAb (Kamiya, Thousand Oaks, CA) or a relevant negative control IgM MoAb (no. X0942; DAKO), respectively, 30 minutes before cocultivation with the myeloma cells. Treatment of labeled T cells with 0.25 μg/mL of the death-inducing anti-Fas MoAb (clone CH11; Kamiya) served as a positive control for Fas-mediated apoptosis. To determine the effect of CEM-C7H2 T cells on myeloma cells, myeloma cells were endogenously labeled with 3[H]-thymidine and exposed to unlabeled CEM-C7H2 cells using adequately adapted effector cell/target cell (E/T) ratios. Cocultivation of cells was performed for 72 hours at 37°C. Cells were obtained automatically, transferred onto filter papers, and washed six times. Incorporated radioactivity from undegraded chromosomal DNA was measured with a β-scintillation counter. The reduction in incorporated radioactivity was used to calculate the percentage of specific target cell killing [(cpm Untreated Cells − cpm Cocultured Cells)/cpm Untreated Cells × 100].
Assay for Anti-Fas MoAb-Induced Apoptosis
Cells were taken out of continuous culture, washed, and seeded at a density of 0.2 × 106/mL in 10% RPMI 1640 medium. For induction of apoptosis, 0.25 μg/mL of the IgM anti-Fas MoAb no. 1504, clone CH11 (Kamiya), was used. After 8 hours cells were procured, washed, and analyzed for morphologic changes characteristic for apoptosis32 as well as for the diminution of DNA-content by the propidium iodide (PI) assay. Cells were procured, washed by centrifugation in PBS, and incubated with 300 μL/well PI solution (50 μg/mL PI, 0.1% sodium citrate, and 0.1% Triton X-100) for permeabilization and DNA-staining. Analysis of cell size and fluorescence intensity was performed in the forward/side scatter program of a FACScan (Becton Dickinson, Vienna, Austria) as recently described.27 After exclusion of necrotic debris, apoptotic cells in PI assays were defined by a decrease in DNA content as compared to G1 phase cells of myeloma cell lines and normal T lymphocytes.
Detection of FasL mRNA in malignant plasma cell lines. (a) After treatment of cells with PMA (50 nmol/L) and ionomycin (1 μg/mL) for the indicated period of time, RNA was extracted from the CEM-C7H2 T-ALL and MC/CAR plasmacytoma cells, reverse-transcribed into cDNA, amplified by PCR using FasL-specific primers, and separated on 2% agarose gels. The PCR product size was confirmed by image analysis and the used size marker (bp). Determination of β-actin expression levels served as a control. (b) Ten micrograms of total RNA was separated from the indicated neoplastic plasma cells and subjected to Northern analysis, which showed basal expression of FasL mRNA in all cell lines tested. Total RNA extracted from CEM-C7H2 T cells was used as a FasL+ control. GAPDH mRNA expression served as an internal standard for loading of the gel and, together with the location of 18s rRNA on the gel, for the evaluation of the size of the FasL signal (∼2 kb).
Detection of FasL mRNA in malignant plasma cell lines. (a) After treatment of cells with PMA (50 nmol/L) and ionomycin (1 μg/mL) for the indicated period of time, RNA was extracted from the CEM-C7H2 T-ALL and MC/CAR plasmacytoma cells, reverse-transcribed into cDNA, amplified by PCR using FasL-specific primers, and separated on 2% agarose gels. The PCR product size was confirmed by image analysis and the used size marker (bp). Determination of β-actin expression levels served as a control. (b) Ten micrograms of total RNA was separated from the indicated neoplastic plasma cells and subjected to Northern analysis, which showed basal expression of FasL mRNA in all cell lines tested. Total RNA extracted from CEM-C7H2 T cells was used as a FasL+ control. GAPDH mRNA expression served as an internal standard for loading of the gel and, together with the location of 18s rRNA on the gel, for the evaluation of the size of the FasL signal (∼2 kb).
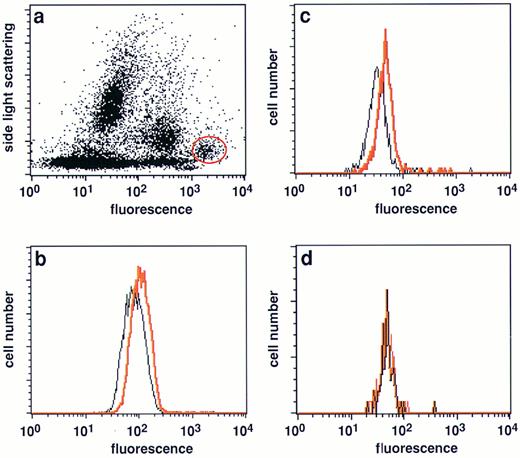
Expression of FasL in native malignant plasma cells from myeloma patients. (a) Typical side light scattering profile of a BM sample from a myeloma patient stained with a PE-conjugated CD38 MoAb. The plasma cell population was defined by its unique position at a very low side light scattering profile and highest CD38 expression levels29 and gated (red circle) for further analysis. (b and c) Analysis of FasL expression in the gated plasma cell population of two patients with multiple myeloma (nos. 4 and 6, Table 2). A significant shift in fluorescence intensitiy in samples stained with the NOK-1 FasL antibody (red line) was observed when compared with samples treated with the isotype-matched control MoAbs only (black line). (d) FasL negative plasma cell sample (no. 1, Table 2).
Expression of FasL in native malignant plasma cells from myeloma patients. (a) Typical side light scattering profile of a BM sample from a myeloma patient stained with a PE-conjugated CD38 MoAb. The plasma cell population was defined by its unique position at a very low side light scattering profile and highest CD38 expression levels29 and gated (red circle) for further analysis. (b and c) Analysis of FasL expression in the gated plasma cell population of two patients with multiple myeloma (nos. 4 and 6, Table 2). A significant shift in fluorescence intensitiy in samples stained with the NOK-1 FasL antibody (red line) was observed when compared with samples treated with the isotype-matched control MoAbs only (black line). (d) FasL negative plasma cell sample (no. 1, Table 2).
Determination of FasL protein expression in malignant plasma cell lines. (a) Cellular protein was extracted from the indicated cell lines. Protein extract from endothelial cells was provided by the manufacturer of the FasL MoAb and served as a positive control. Equal amounts of protein were separated on 14% Tris-glycine gels by PAGE and transferred onto a nylon membrane. Immunostaining showed a specific signal in all myeloma extracts at the predicted molecular weight of the FasL protein (ie, 37 kD) matching the signal obtained with control and T-cell extract. (b) LP-1 plasma cell leukemia cells were stained with the FasL-specific MoAb and characterized for staining by means of flow cytometry. A shift in fluorescence intensity in samples stained with the NOK-1 FasL antibody (red line) was observed when compared with samples treated with the isotype-matched control MoAbs only (black line).
Determination of FasL protein expression in malignant plasma cell lines. (a) Cellular protein was extracted from the indicated cell lines. Protein extract from endothelial cells was provided by the manufacturer of the FasL MoAb and served as a positive control. Equal amounts of protein were separated on 14% Tris-glycine gels by PAGE and transferred onto a nylon membrane. Immunostaining showed a specific signal in all myeloma extracts at the predicted molecular weight of the FasL protein (ie, 37 kD) matching the signal obtained with control and T-cell extract. (b) LP-1 plasma cell leukemia cells were stained with the FasL-specific MoAb and characterized for staining by means of flow cytometry. A shift in fluorescence intensity in samples stained with the NOK-1 FasL antibody (red line) was observed when compared with samples treated with the isotype-matched control MoAbs only (black line).
Transfection of CEM-C7H2 T-ALL Cells
Logarithmically growing CEM-C7H2 cells were washed in PBS, pelleted at 300g, and resuspended at a density of 1 × 107 cells/400 mL PBS. Cells were mixed with 20 μg of plasmid pHD1.2, a CrmA expression vector driven by the chicken β-actin promoter, kindly provided by V. Gagliardini,33 incubated for 10 minutes on ice, and permeabilized with the eletroporator (Biorad Lab, Vienna, Austria) set at 960 mF and 300 V. After electroporation, cells were again placed on ice for 10 minutes diluted in 20 mL growth medium, and seeded on 96-well flat-bottom plates. Selection of stably transfected cells was initiated 48 hours after electroporation using 1 mg/mL G418 (bioactivity 70%). CrmA expression was monitored by RNA dot-blot analysis28 using the CrmA insert as a probe. The CrmA expressing subclone 2E8 was used for analysis. IC3 is a CEM-C7H2 subclone stably transfected with the “empty” control vector.
RESULTS
The FasL mRNA and Protein Are Expressed in Malignant Plasma Cells
Since it has been shown in murine B lymphocytes that treatment with PMA induces the expression of FasL mRNA which was undetectable before such induction,25 we analyzed expression levels of FasL mRNA in myeloma cells in response to treatment with PMA/ionomycin. CEM-C7H2 T-ALL cells treated for 4 hours with PMA/ionomycin served as a positive control for FasL mRNA expression. When MC/CAR plasmacytoma cells were treated with PMA/ionomycin for up to 8 hours and the extracted total RNA was subjected to RT-PCR analysis, an FasL-specific PCR product could be detected (Fig 1a). The PCR product appeared exactly at the product size predicted for the chosen FasL primer set used and at the same product size as the T-cell–derived PCR product. The FasL mRNA was already detected in unstimulated MC/CAR plasmacytoma cells and slightly increased in response to PMA/ionomycin when a ratio between the relative expression levels of FasL and β-actin mRNA was determined by image analysis. Similar results were observed in IM-9 cells and the RPMI-8226 myeloma cell line, too. However, basal FasL expression was not detected in the epithelial carcinoma cell lines HELA and KB (data not shown). We subsequently analyzed FasL mRNA expression by Northern analysis. In all neoplastic plasma cell lines tested, the RNA analysis showed a solitary signal (Fig 1b) at the size expected for the mRNA transcript specific for FasL (∼2 kb, Fig 1b), as determined by comparison with the GAPDH signal.
To verify FasL expression at the protein level, we performed immunologic analysis using immunoblotting and immunofluorescence staining. Subsequent quantitation of fluorescence intensity by flow cytometry. The Western blotting experiments showed a single band at the predicted size of ∼37 kD typical of the FasL molecule. The protein stained with the anti–FasL-specific MoAb appeared at the identical level of molecular weight as the one obtained from endothelial cell extracts provided as a positive control by the manufacturer (Fig 2a). In addition, immunofluorescence staining yielded consistently positive results in the malignant plasma cell lines tested (Fig 2b, Table 1). The MSFI, calculated as the quotient mean fluorescence channels of anti-FasL MoAb/isotype-matched negative control MoAbs, ranged between 2.1 and 5.7 in all cell lines investigated (Table 1). To get an insight into the in vivo situation, we further analyzed native malignant plasma cells derived from the BM of myeloma patients. Flow cytometric analysis of native plasma cells showed FasL expression in four of six samples investigated (Fig 3, Table 2), with expression levels below those observed in the malignant plasma cell lines. However, in BM plasma cells from two patients with myeloma and one patient with breast cancer (not shown) clearly negative results were obtained.
Expression of FasL and Fas/Apo-1 Receptor (CD95) in Malignant Plasma Cell Lines and Their Sensitivity to the Agonistic Anti-Fas MoAb
| Cell Line . | FasL Expression (MSFI)* . | Fas/Apo-1 (CD95) Expression (MSFI)* . | % Anti-Fas MoAb-Induced Apoptosis† . | Survival of Myeloma Cells Coincubated With FasL+ CEM-C7H2 Cells (% control)‡ . |
|---|---|---|---|---|
| ARH-77 | 3.79 | 3.91 | 37 | 116 |
| IM-9 | 2.10 | 11.85 | 62 | 108 |
| LP-1 | 2.40 | 9.83 | 31 | 94 |
| MC/CAR | 3.06 | 7.96 | 44 | 117 |
| NCI-H929 | 5.15 | 1.05 | neg | 98 |
| RPMI-8226 | 5.70 | 4.10 | 51 | 113 |
| U-266 | 5.06 | 1.13 | neg | 99 |
| CEM-C7H2-wt/IC3ρ | 2.40 | 3.60 | 50 | — |
| CEM-C7H2-CrmA/2E8 | 2.10 | 4.30 | 4 | — |
| Cell Line . | FasL Expression (MSFI)* . | Fas/Apo-1 (CD95) Expression (MSFI)* . | % Anti-Fas MoAb-Induced Apoptosis† . | Survival of Myeloma Cells Coincubated With FasL+ CEM-C7H2 Cells (% control)‡ . |
|---|---|---|---|---|
| ARH-77 | 3.79 | 3.91 | 37 | 116 |
| IM-9 | 2.10 | 11.85 | 62 | 108 |
| LP-1 | 2.40 | 9.83 | 31 | 94 |
| MC/CAR | 3.06 | 7.96 | 44 | 117 |
| NCI-H929 | 5.15 | 1.05 | neg | 98 |
| RPMI-8226 | 5.70 | 4.10 | 51 | 113 |
| U-266 | 5.06 | 1.13 | neg | 99 |
| CEM-C7H2-wt/IC3ρ | 2.40 | 3.60 | 50 | — |
| CEM-C7H2-CrmA/2E8 | 2.10 | 4.30 | 4 | — |
MSFI was determined by immunofluorescence staining and FACS analysis and calculated as the ratio of the MSFI achieved with anti-FasL MoAb/isotype-matched control MoAb. Mean values of three independent experiments are given.
Induction of apoptosis in myeloma cells was determined after 8 hours of incubation with anti-Fas MoAb (0.25 μg/mL) using the PI assay.27 Mean values of three independent experiments are given. The standard error was <10% in all cell lines.
Using myeloma cells as targets for FasL+ CEM-C7H2 T cells, cells of the relevant lines were endogeneously labeled with 3[H]-thymidine and incubated with an excess of effector T cells (E/T ratio 10:1) for 72 hours (JAM test30 ). Mean values (n = 8) were used to calculate the percentage of myeloma cell survival.
ρ CEM-C7H2 T-ALL cells served as a FasL positive control.
FasL Expressed on Malignant Plasma Cells Is Functionally Active and Able to Induce Cell Death in CEM-C7H2 T Cells
To define the physiologic activity of the FasL protein detected, we tried to determine tumor cell–mediated killing of CEM-C7H2 T-ALL target cells using the JAM-Test.30 T-ALL cells such as Jurkat and CEM cells are known to display several features of activated T cells, like expression of Fas8,16 and a functionally active FasL,16 thus allowing their use as a surrogate of activated T cells in the JAM test.30 The T cells were pulse-labeled with 3[H]-thymidine for 5 hours. Cocultivation for 72 hours of endogenously labeled T cells with unlabeled ARH-77, IM-9, MC/CAR, and U-266 cells, respectively, induced a significant decrease in T-cell–specific 3[H]-thymidine content when compared with untreated controls, indicating the induction of cell death in target T cells (Fig 4). The effector cell to target cell ratio (E/T) was tested over a broad range (1:1 to 20:1) and at an E/T ratio of 10:1 T-cell killing was most effective. Preincubation of target T cells with the Fas blocking ZB4 MoAb (0.25 μg/mL), or of malignant plasma cells with the FasL neutralizing NOK-2 MoAb (2.5 μg/mL), respectively, significantly protected T-ALL cells from being killed by the neoplastic plasma cells (Fig 4a and b), whereas isotype-matched control MoAbs used in the same concentrations had no protective effect (<2%; data not shown). Treatment of CEM-C7H2 target cells with the agonistic anti-Fas MoAb served as a positive control for the assay. Preincubation of T-ALL cells with the Fas blocking MoAb ZB4 partially protected the cells from being destroyed by the agonistic anti-Fas MoAb. As with plasma cell–mediated cytotoxicity, the antibody was unable to rescue all T cells from anti-Fas MoAb-induced cell death even when higher concentrations of the ZB4 MoAb were applied (<1.5 μg/mL, data not shown). Analysis of the Fas expression status and reactivity to anti-Fas MoAb treatment of the plasma cell lines used showed that the killing of CEM-C7H2 T cells by myeloma cells was independent of their own sensitivity to the agonistic anti-Fas MoAb (Table 1). When the direction of the FasL-induced apoptosis was checked by inverting target and effector cell population of the assay and by exposing endogenously labeled myeloma target cells to FasL-positive CEM-C7H2 T-ALL cells (E/T = 10:1), no decrease in the plasma cell–specific 3[H]-thymidine content could be observed (Table 1).
CEM-C7H2 T-ALL cells are eliminated by myeloma cells via the Fas/FasL pathway. CEM-C7H2 T-ALL cells were labeled with 3[H]-thymidine for 5 hours and cocultivated with the indicated neoplastic plasma cell lines, respectively, for 72 hours. An efficient, predetermined E/T ratio of 10:1 was applied. The decrease in DNA-incorporated radioactivity was used to calculate the degree of cell death induced in target T cells by their cocultivation with malignant plasma cells (▪). Treatment of T cells with 0.25 μg/mL of the agonistic anti-Fas MoAb served as an internal control of the assay. Blockage of the Fas receptor with the antagonistic ZB4 anti-Fas MoAb (0.25 μg/mL, a) or neutralization of FasL molecules using the NOK-2 FasL MoAb (2.5 μg/mL, b) was performed to prove the dependence of the observed cytotoxicity on active Fas signaling (□). Mean values ± SEM (n = 8) are depicted.
CEM-C7H2 T-ALL cells are eliminated by myeloma cells via the Fas/FasL pathway. CEM-C7H2 T-ALL cells were labeled with 3[H]-thymidine for 5 hours and cocultivated with the indicated neoplastic plasma cell lines, respectively, for 72 hours. An efficient, predetermined E/T ratio of 10:1 was applied. The decrease in DNA-incorporated radioactivity was used to calculate the degree of cell death induced in target T cells by their cocultivation with malignant plasma cells (▪). Treatment of T cells with 0.25 μg/mL of the agonistic anti-Fas MoAb served as an internal control of the assay. Blockage of the Fas receptor with the antagonistic ZB4 anti-Fas MoAb (0.25 μg/mL, a) or neutralization of FasL molecules using the NOK-2 FasL MoAb (2.5 μg/mL, b) was performed to prove the dependence of the observed cytotoxicity on active Fas signaling (□). Mean values ± SEM (n = 8) are depicted.
Transfection of a CrmA Expression Plasmid Protects CEM-C7H2 T Cells From Myeloma Cell-Induced Cytotoxicity
To further investigate at the molecular level whether the observed decrease in 3[H]-thymidine content was caused by FasL-mediated cell death induced by malignant plasma cells, we generated CEM-C7H2 subclones that were transfected with a cowpox-virus CrmA expression plasmid. Three clones were analyzed for sufficient levels of CrmA expression by monitoring their resistance to apoptosis induced by the agonistic anti-Fas MoAb, as determined by PI assays. After 24 hours of incubation with the anti-Fas MoAb, the mean percentage (n = 3) of Fas-induced cell death was 4%, 6%, and 10%, respectively, whereas the untransfected cell line or the subclone bearing the relevant “empty” vector control (termed IC3) remained highly sensitive to the anti-Fas MoAb (mean of MoAb-induced cell death: 50%, n = 3). The CrmA expressing subclone with the highest resistance to the anti-Fas MoAb (termed 2E8) was chosen for further experiments. Coculture experiments using CrmA-expressing T cells showed a high resistance of these cells to plasma cell–mediated cytotoxicity (Fig 5), thus supporting the assumption that the myeloma cell–induced cytotoxicity was mediated via CrmA-sensitive caspases, as described for Fas-mediated apoptosis.34 Increasing the E/T ratio up to 20:1 or prolonging the incubation period to 96 hours did not alter the results obtained (data not shown).
CrmA expression protects CEM-C7H2 T cells from being killed by malignant plasma cells. CrmA-transfected T cells (clone 2E8, □) and T cells carrying the relevant vector control (clone IC3, ▪) were labeled with 3[H]-thymidine for 5 hours and cocultivated with the unlabeled neoplastic plasma cell lines for 72 hours using the predetermined E/T ratio of 10:1. Treatment of T cells with 0.25 μg/mL anti-Fas MoAb served as an internal control for the assay. Mean values ± SEM (n = 8) are given. The decrease in DNA-incorporated radioactivity was used to calculate the percentage of myeloma cell–mediated target cell death. Mean values ± SEM (n = 8) are shown.
CrmA expression protects CEM-C7H2 T cells from being killed by malignant plasma cells. CrmA-transfected T cells (clone 2E8, □) and T cells carrying the relevant vector control (clone IC3, ▪) were labeled with 3[H]-thymidine for 5 hours and cocultivated with the unlabeled neoplastic plasma cell lines for 72 hours using the predetermined E/T ratio of 10:1. Treatment of T cells with 0.25 μg/mL anti-Fas MoAb served as an internal control for the assay. Mean values ± SEM (n = 8) are given. The decrease in DNA-incorporated radioactivity was used to calculate the percentage of myeloma cell–mediated target cell death. Mean values ± SEM (n = 8) are shown.
DISCUSSION
Our experiments indicate the constitutive expression of FasL in seven of seven neoplastic plasma cell lines as shown at the RNA level by means of RT-PCR and Northern blot analysis (Fig 1a and b). These FasL-specific transcripts are translated into detectable amounts of protein as shown by immunoblotting experiments as well as by single cell analysis using flow cytometry (Figs 2a and b, Table 1). Furthermore, FasL protein could also be detected in the plasma cell fraction of BM samples from four of six patients with multiple myeloma (Fig 3, Table 2). The FasL expressed on the malignant plasma cell lines was functionally active, because five of five cell lines tested in the JAM assay30,31 were able to kill CEM-C7H2 T-ALL target cells (Figs 4 and 5). This cytotoxic effect of the neoplastic cells could specifically be inhibited by an MoAb neutralizing the FasL on myeloma cells6 as well as by the MoAb ZB4, which prevents the binding of the ligand to its receptor (Figs 4a and b). To confirm the role of the Fas/FasL system in the achievement of the cytotoxic effect on the level of intracellular signal transduction, CEM-C7H2 target cells were replaced by the subclone 2E8 transfected with a vector overexpressing the cowpox virus CrmA protein known to inhibit particulary ICE/caspase-135 and FLICE/caspase-8.36 Both caspases have been implicated in the effector phase of apoptosis triggered by antibodies to the Fas/Apo-1 membrane protein.34,37 Furthermore, the central role of caspase1 in the mediation of Fas/Apo-1-induced apoptosis was supported by experiments using ICE-deficient mice.38 Our results obtained in cocultivation experiments with CrmA-expressing CEM-C7H2 cells confirmed the data obtained in the antibody inhibition experiments, because CrmA-expressing cells were essentialy resistant to cell death caused by cocultivated myeloma cells or by treatment with anti-Fas MoAb (Fig 5). The degree of cytotoxicity blocked by transfection of CEM-C7H2 T-ALL cells with the vector allowing overexpression of the CrmA protein was superior to the degree of inhibition obtained with MoAbs against the FasL or its receptor. This phenomenon might be explained by submaximal inhibitory capacity of the antibodies used in this investigation, since in all cocultivation experiments protection of target cells from undergoing anti-Fas MoAb-induced apoptosis was achieved more efficiently by CrmA-transfection than by blocking of the Fas/FasL system with the inhibitory MoAbs (Figs 4 and 5). As an alternative explanation, other molecules like cytotoxic cytokines might in part be involved in the tumor cell–induced target cell killing. Signaling along the TNF-α receptor, which has recently been shown to cause programmed cell death via activation of ICE proteases,39 also induces apoptosis in CCRF-CEM T-ALL cells.40 Therefore, we tried to rule out that TNF-α secreted by myeloma cells may be involved in the killing of CEM-C7H2 target cells by analyzing culture supernatants using a TNF-α–specific enzyme-linked immunosorbent assay (ELISA). In our hands, TNF-α could not be detected in the supernatants of the culture media as determined after 72 hours of cultivation, but the presence of TNF-α in concentrations below the detection limit of the assay (4 pg/mL) cannot be ruled out (results not shown). However, the results are in line with previous investigations which failed to detect TNF-α–mediated bioactivity in 11 of 12 culture supernatants derived from purified native malignant plasma cells and the RPMI-8226 cell line.41 These observations suggest that myeloma cell–mediated cytotoxicity might be exerted independently from TNF-α in the test system used. Taken together, our findings suggest a new concept by which malignant B cells could fight the host immune system via FasL-mediated T-cell killing. This concept is further supported by recent studies demonstrating the presence of the FasL on cells of a human colon carcinoma cell line,31 murine melanoma,42 and on native human hepatocarcinoma cells,43 thus indicating a more ubiquitous role of FasL expression in the expansion of an immune-privileged neoplastic clone.
The fact that five of seven neoplastic plasma cell lines investigated simultaneously expressed the Fas receptor as well as the death-inducing FasL (Table 1) raises the question of why the tumor cells do not commit suicide or fratricide via autologous cell/cell contact or via soluble FasL. Because the Fas+/FasL+ phenotype is shared by the CEM-C7H2 T cells (Table 1), a further question arises as to whether the T cells are capable of using the same effector system for killing myeloma cells in reverse. However, the malignant plasma cells were protected from death induced by FasL+ myeloma or T cells as demonstrated by two findings: (1) Spontaneous rates of apoptosis in continuous cultures are low in these cell lines (<10%) and cannot be reduced any further by the addition of MoAbs blocking the Fas receptor or the FasL over an observation period of 96 hours (results not shown). (2) By endogenously labeling the neoplastic plasma cell lines, inverting target and effector cell population of the assay and by changing E/T ratios accordingly, we observed that all malignant plasma cells remained unaffected by the FasL expressed on CEM-C7H2 cells (Table 1). These data suggest a highly active intrinsic control mechanism preventing autocrine suicide of myeloma cells and governing the direction of FasL-induced apoptosis, a requirement for survival of tumor cells which was also postulated to be active in FasL-expressing malignant T cells.16,44 This intrinsic control could theoretically be exerted by the following divergent mechanisms of action: (1) Some of the tumor cells, like the U-266 and the NCI-H929 myeloma cell lines, might become resistant to the FasL on both myeloma cells and T cells by their lack of the Fas molecule (Table 1). This is in line with the observation that cirrhotic livers expressed Fas/Apo-1, while loss of the receptor molecule accompanied the acquisition of the FasL in hepatocellular carcinoma cells.43 In contrast to a loss of the Fas molecule, intrinsic deficiencies in the signaling pathway could also explain insensitivity of tumor cells toward FasL. This mechanism was suggested for the SW620 colon carcinoma cell line which still expressed the Fas molecule but was insensitive to treatment with the agonistic anti-Fas MoAb.31 (2) However, five of seven neoplastic plasma cell lines investigated were sensitive to the anti-Fas MoAb but all were resistant to FasL exposed on T-ALL cells, thus refuting the suggestion of the selection of a Fas−/FasL+ phenotype as a prerequisite for the utilization of FasL pathway for the expansion of the neoplastic clone. Additional signals delivered during homologous cell/cell or myeloma/T-cell contact but not during cross-linking of the Fas receptor by binding of the agonistic MoAb might protect myeloma cells from being killed by fratricidal action or by the effectors of the immune system. This explanation is supported by the observation that PMA/ionomycin increased the cytotoxic potential of murine prestimulated B lymphoblasts against B lymphoma cells, which was interpreted as indicative for a coregulatory role of PMA-induced adhesion molecules in the process of FasL-induced apoptosis.25 (3) Alternatively, immunomodulatory cytokines released by the myeloma cells or the microenvironment might also contribute to the suppression of plasma cell suicide. We recently reported that interferon-α (IFN-α) protected malignant plasma cells from Fas-induced cell death via induction of a protein kinase C pathway27 and we have also observed that myeloma cell lines as well as native plasma cells derived from the BM of myeloma patients widely expressed IFN-α mRNA (Villunger A., Egle A., Greil R., unpublished observations, January 1996). Furthermore, B cells are protected from Fas-induced apoptosis by interleukin-4; thus, constitutive activation or mimicry of this signaling pathway could also protect myeloma cells from FasL-mediated cell death.45
Taken together, the data presented shed new light on the mechanisms by which neoplastic plasma cells and possibly tumor cells in general escape the immune surveillance and thus enable the expansion of the neoplastic clone. They also point to the possibility of developing new therapeutic strategies targeting FasL expression in malignant cells to prevent the suppression of specific immune responses of the host.
Supported by a grant of the Province of Tyrol (R.G.) and the Austrian FWF grants (P11946-Med and Project F-204).
Address reprint requests to Richard Greil, MD, Department of Internal Medicine, University of Innsbruck, Anichstrasse 35, A-6020 Innsbruck, Austria.

![Fig. 4. CEM-C7H2 T-ALL cells are eliminated by myeloma cells via the Fas/FasL pathway. CEM-C7H2 T-ALL cells were labeled with 3[H]-thymidine for 5 hours and cocultivated with the indicated neoplastic plasma cell lines, respectively, for 72 hours. An efficient, predetermined E/T ratio of 10:1 was applied. The decrease in DNA-incorporated radioactivity was used to calculate the degree of cell death induced in target T cells by their cocultivation with malignant plasma cells (▪). Treatment of T cells with 0.25 μg/mL of the agonistic anti-Fas MoAb served as an internal control of the assay. Blockage of the Fas receptor with the antagonistic ZB4 anti-Fas MoAb (0.25 μg/mL, a) or neutralization of FasL molecules using the NOK-2 FasL MoAb (2.5 μg/mL, b) was performed to prove the dependence of the observed cytotoxicity on active Fas signaling (□). Mean values ± SEM (n = 8) are depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.12/5/m_bl_0055f4.jpeg?Expires=1769091786&Signature=Fl04Jimeq~Fxr1yiHFzD~6UEl5eFzJI2-cO7~4I-ArUT2e5g5o3~dLvVxDFDq6GrO9cm~4ds9kmoY2VbBQ~C3QG94sbEzY2oLV0lu45YErsGOPRAQV6hzAyI27DzhN94m5IfMJUp7I-StLiCrkvYRiUc4nAKKdEDQswoWNS4fyacjwgPa8SIPhu3c7sQoxhb0~HdcAuuHqHhR4~RToKBLZYkoa-f9yVBIXQUowizcLRzYiYtrsaaIiKGqWTbPuaPx5NUhwG7n-rhthucRM0gDJlVYDrLZFQRzVVDW~6XXiY9ISLBQp41k19D4-1DoEEJENthwmQvVjfwPVU6UVmJsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. CrmA expression protects CEM-C7H2 T cells from being killed by malignant plasma cells. CrmA-transfected T cells (clone 2E8, □) and T cells carrying the relevant vector control (clone IC3, ▪) were labeled with 3[H]-thymidine for 5 hours and cocultivated with the unlabeled neoplastic plasma cell lines for 72 hours using the predetermined E/T ratio of 10:1. Treatment of T cells with 0.25 μg/mL anti-Fas MoAb served as an internal control for the assay. Mean values ± SEM (n = 8) are given. The decrease in DNA-incorporated radioactivity was used to calculate the percentage of myeloma cell–mediated target cell death. Mean values ± SEM (n = 8) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.12/5/m_bl_0055f5.jpeg?Expires=1769091786&Signature=0zqL41YLNTROq~ZFobvwL-6SYDVfXCUFxnjWkyNF-uAXENo5d-SIj0U0i6Ai7gH3PkYXVEIO1Go76i6uKUXoKWWPh1~l-tF07FVPYCpROKZYP07K0D~RpEL7AxdfCBGojJNOM0ZZG~QyvLYpaII82yxBfy-ye6U2SPrKxf2Hs3H2mIEEPxf7VBV~HwQdxmn4ftBuiy1DetiT2QRuzQAa3cMetdzsZ1B2J2rp1Udr~6LGd-QkiCpoxZ0IvMsYT7edo4171d6AuA-FAaDpmFkoJdJCrJPSlBOOC7JcM1txqodO2c11ozhwYeiby6RmkqYUyI6-H~RDNHzeRs3mq9sS2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal