Abstract
Cooperation between in vitro exogenous prolactin (PRL), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-3 (IL-3) at an early step of in vitro erythroid differentiation has been shown in a previous study. To gain more insight into the role of PRL in in vivo hematopoiesis, we have now addressed the involvement of endogenous PRL in the growth of hematopoietic progenitors in a bone marrow (BM) stroma environment. The possible modulation of local PRL production by the inflammatory mediator platelet-activating factor (PAF), which is known to be produced by BM cells and to regulate pituitary PRL release, has also been evaluated. Development of burst-forming unit-erythroid (BFU-E) colonies from CD34+ hematopoietic progenitors cultured on a BM stroma cells (BMSC) layer was slightly, but significantly, reduced in the presence of an antihuman PRL antibody. Pretreatment of BMSC with PAF increased the BFU-E colony efficiency of cocultured CD34+ cells, and this effect was completely abrogated by the antiserum. PAF-modulated release of PRL by BMSC was confirmed by an enzyme-linked-immunospot (Elispot) technique. In addition, immunoprecipitation and Western blotting experiments showed two immunoreactive products in the BMSC culture medium. These corresponded to the nonglycosylated (23 kD) and glycosylated (25.5 kD) forms of pituitary PRL that are also expressed by the B-lymphoblastoid cell line IM9-P3. Specific increase of the nonglycosylated form and decrease of the glycosylated form was observed after PAF treatment. Polymerase chain reaction (PCR) amplification of reverse transcribed RNA using PRL-specific primers showed the presence of PRL message in BMSC and IM9-P3 cells. In situ hybridization experiments with a rat PRL cDNA probe cross-reacting with human PRL mRNA confirmed its presence in a small fraction of unstimulated BMSC and in the majority of PAF-stimulated BMSC. The enhancing effect of PAF on PRL-mediated colony formation, PRL release, and mRNA activation was counteracted by pretreating BMSC with the PAF-receptor (R) antagonist WEB 2170. Lastly, responsiveness of BMSC to PAF was substantiated by the presence of the PAF-R mRNA on these cells.
BLOOD CELL development in bone marrow (BM) occurs in close association with nonhematopoietic cells that form the marrow stromal microenvironment.1 The BM stroma consists of an organized and not completely defined array of cell types, including fibroblasts, endothelial cells, adipocytes, epithelial cells, macrophages, and dendritic cells,2 which provide nutritive and adhesive support for stem cell development through cell-cell interaction and production of short-range acting hematopoietic growth factors (HGF).3-5 Production of these factors is hardly detectable under resting conditions and increases in response to inflammatory mediators.6 7
The hormone prolactin (PRL) has long been suggested to participate in regulation of hematopoiesis.8,9 Its receptors have been included in the superfamily of hematopoietin/cytokine receptors, based on their structural similarities10 and the use of common or related signal transduction molecules, such as cytoplasmic kinases and signal transduction activators of transcription (STATs).11 The importance of PRL in the hematopoietic system has been reinforced by the identification of specific membrane binding sites in a subset of human CD34+ hematopoietic progenitor cells, and by the observation of a functional interplay between PRL and HGF, leading to stimulation of the erythroid maturation pathway.12 Although most of the biologic actions of PRL are believed to be sustained by pituitary release, recent evidence points to its paracrine/autocrine role during the immune response.13-15
The question of whether local production of PRL is involved in normal hematopoiesis is addressed in this report through a study of the participation of endogenous PRL in the growth of hematopoietic progenitors in an in vitro BM stroma environment. The possible modulation of local PRL production by the inflammatory mediator platelet-activating factor (PAF),16 known to regulate pituitary PRL release,17 has also been evaluated. Both basal and PAF-induced colony formation were specifically inhibited by an antihuman PRL antibody. The participation of endogenous PRL was substantiated by the demonstration of PRL mRNA expression and PRL release by the BM stroma cells (BMSC).
MATERIALS AND METHODS
Reagents and polymerase chain reaction (PCR) oligonucleotide primers.Human recombinant interleukin-3 (IL-3) granulocyte-macrophage colony-stimulating factor (GM-CSF) (a gift from Dr S. Clark, Genetics Institute, Cambridge, MA), and erythropoietin (Epo) (Eritrogen, Boehringer Mannheim, Germany) were used at concentrations of 50, 20, and 2 U/mL respectively. Synthetic C16 PAF (1-hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine), from Bachem Feinchemikalien (Bubendorf, Switzerland), was used at the physiological dose of 10−12 mol/L (final concentration). WEB 2170 (Boehringer, Ingelheim Kg, Germany) was used as PAF receptor (R) antagonist (final concentration 10−11 mol/L).18
Rabbit antisera to human PRL were: IC-5 (kindly provided by Dr A.F. Parlow, Harbor-UCLA Medical Center, Torrance, CA) and neutralizing AB960 (Chemicon, Temicula, CA). Monoclonal antibodies (MoAbs) were: clones B010 and B021 (directed against distinct epitopes of human PRL) (Serotec Ltd, Oxford, UK); B36.1 (anti-CD5), B67.1 (anti-CD2), B59.2 (anti-CD41), B13.4 (anti-granulocytes and monocytes), B52.1 (anti-CD14), B159.5 (anti-CD56), generously provided by Dr G. Trinchieri (Wistar Institute, Philadelphia, PA); OKT3 (anti-CD3), BC1 (anti-CD20), IV.3 (anti-CDw32), 3G8 (anti-CD16), J5 (anti-CD10), and Gly NN3.1 (anti-glycophorin A), produced from cells obtained from the American Tissue Culture Collection (Rockville, MD). The nucleotide sequences of the oligonucleotide primers were as follows: PRL primers: 5′ primer 5′ CCCGAAGACAAGGAGCAAGCC 3′; 3′ primer 5′ GTCGATTTTATGTGAATCCCTGCG 3′; PAF-R19 primers: 5′ primer 5′ ATGGACTCTGAGTTCCGATACACT 3′; primer 3′ 5′ ATGGACTCTGAGTTCCGATACACT 3′; β-actin primers: 5′ primer 5′ ACACTGTGTGCCCATCTACGAGGGG; primer 3′ 5′ ATGATGGAGTTGAAGGTAGTTTCGTGGAT 3′. The expected sizes of the PCR fragments for PRL, PAF-R, and β-actin were 354, 587, and 386 bp, respectively.
Cell preparations.BM cells were collected into preservative-free heparin according to institutional guide lines from allogeneic BM transplant donors at the time of marrow harvest. BM mononuclear cells (BMMC) were isolated on Ficoll-Hypaque density gradient, depleted of adherent cells by 1 hour plastic adherence in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) (Hyclone Laboratories, Inc, Logan UT) and cryopreserved until use. BMSC established from plastic adherent BMMC were grown at 37°C in 5% CO2 in Iscove's modified Dulbecco's medium (IMDM) (GIBCO) supplemented with 10% FCS. Highly enriched populations of BM hematopoietic progenitor cells (BMHPC) were obtained by negative selection with the immunorosetting technique from thawed light-density BMMC treated with a mixture of anti-CD3, -CD2, -CD5, -CD20, -CD14, -CD56, -CD32, -CD16, -CD41, and -CD10 MoAbs, as described in detail.20
Cell lines.Lymphoblastoid cell line IM9 clone P3 (IM9-P3),21 hepatoma cell line Hep-G2, and monocytic cell line U937 were grown in RPMI 1640 supplemented with 10% FCS.
Stimulation of cells.Confluent BMSC at the fourth passage, grown in 24- or 6-well tissue plates (Nunc Inc, Naperville, IL), were extensively washed with phosphate-buffered saline (PBS; pH 7.3), incubated for 30 minutes in the presence or absence of WEB 2170 and then cultured with or without PAF for 5 and 20 hours in serum-free IMDM containing 0.25% heat shock, fatty-acid-free BSA Fraction V (Boehringer Mannheim, GmbH Biochemica, Mannheim, Germany).
Analysis of support of hematopoiesis by BMSC.BMSC cultured in 24-well plates were pretreated or not with WEB 2170 and incubated for 20 hours in serum-free conditions in the absence or presence of PAF. They were then extensively washed in PBS and γ-irradiated (3,000 rad). Five × 102 autologous BMHPC were plated onto a BMSC monolayer in 0.5 mL vol of IMDM containing 0.9% methylcellulose, 30% FCS, 10% BSA, 10−4 mol/L 2-mercaptoethanol (2-ME), IL-3, GM-CSF, and Epo in the presence or absence of neutralizing anti-PRL polyclonal antibody or control antiserum. After 14 days of culture, colony-forming unit (CFU)-GM and burst-forming unit-erythroid (BFU-E) colonies were enumerated in situ with an inverted microscope.
Elispot assay.To evaluate the production of PRL at the single cell level an enzyme-linked immunospot (Elispot) assay was performed as described by Czerkisky et al.22 BMSC were cultured for 24 hours at 37°C in a humidified atmosphere with 5% CO2 in serum-free conditions in the presence or absence of WEB 2170 and PAF, alone or in combination, in nitrocellulose-bottomed 96-well Multiscreen plates (Millipore, Bedford, MA) precoated with anti-PRL polyclonal antibody IC-5. After washing with PBS containing 0.01% Tween 20 (PBS-T), biotinylated anti-PRL MoAbs B010 and B021 were added to the wells. Horseradish peroxidase (HRP)-conjugated streptavidin (Dako, Copenhagen, Denmark) was used as the secondary layer of reagents. HRP reaction was developed with 3-amino-9-ethyl carbazole (AEC) (Sigma Chemical Co, St Louis, MO). Each spot was referred to a PRL-producing cell.
Immunoprecipitation.Supernatants from serum-free cultures of BMSC (5 × 104 cells/mL) were collected, concentrated 10×, and diluted with an equal volume of 2× immunoprecipitation buffer (IP) (1% PBS, 0.1% NP40, 0.1% sodium dodecyl sulfate [SDS], 2 mol/L Na2EDTA, 2 mmol/L phenylmethylsulfonyl fluoride [PMSF]). Supernatants from serum-free cultures of IM9-P3 and Hep-G2 cell (5 × 105 cells/mL) were used as positive and negative control, respectively. The samples were precleared for 1 hour with Agarose-protein A coated with nonimmune rabbit antiserum (Sigma), reacted overnight at 4°C with IC-5, and then incubated 1 hour at 4°C with Agarose-protein A before centrifugation at 6,000g for 2 minutes. The pellets were washed five times with IP, boiled 5 minutes in sample buffer with 5% 2-ME, then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% bovine serum albumin (BSA) in PBS with 0.05% Tween 20, immunoblotted with the anti-PRL MoAbs B010 and B021, and incubated in Protein A peroxidase conjugate (Biorad, Hercules, CA). The reactivity was visualized by chemiluminescence with the Renaissance kit (NEN DuPont, Boston, MA).
Reverse transcriptase (RT)-PCR.Resting and treated BMSC, IM9-P3, Hep-G2, and U937 cells were pelleted and total cellular RNA was isolated using the single-step RNAzol method (Cinna/Biotecx, Houston, TX). The first cDNA strand was synthesized at 37°C for 1 hour with oligo-dT primer in a final volume of 20 μL containing 20 U of Moloney murine leukemia virus (MMLV) reverse transcriptase, 1× reverse transcriptase buffer, 24 U of RNAsin, and 0.5 mmol/L of dNTP mix. Ten microliters of first-strand cDNA was added to 20 μL of a PCR mix containing 100 ng each of upstream and downstream primers and 1 U Taq DNA polymerase. All reagents were purchased from GIBCO-BRL Life Technologies (Paisley, UK). The PCR amplification reactions were: for PRL, 94°C/3 min for denaturation step, 62°C/20 s for annealing, and 72°C/35 s for extension, followed by 33 cycles of denaturation at 94°C for 15 seconds, annealing at 62°C for 20 seconds, and extension at 72°C for 35 seconds; for PAF-R, 94°C/4 min for denaturation step, 67°C/20 s for annealing, and 72°C/1 min for extension, followed by 34 cycles of denaturation at 94°C for 15 seconds, annealing at 67°C for 20 seconds, and extension at 72°C for 1 minute; for β-actin, 94°C/1 min for denaturation step, 55°C/1 min for annealing, and 72°C/1 min for extension, followed by 29 cycles of denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute.
In situ hybridization (ISH).ISH was performed as described previously19 with the rat PRL cDNA probe (pPRL-1) (681 bp) (a gift from Dr R.A. Maurer, Health Sciences University, Oregon) cross-reacting with human mRNA for PRL. The cDNA was nonradioactively labeled with digoxigenin-labeled dUDP for 20 hours at 37°C using the Klenow fragment of DNA polymerase I (DIG DNA Labelling kit; Boehringer). Resting and stimulated BMSC, cultured in Lab-Tek Chamber Slides (Nunc), IM9-P3, and Hep-G2 cells were fixed in 4% (vol/vol) paraformaldehyde in PBS and dehydrated in increasing concentration of ethanol. The cells were predigested with Proteinase K (0.01 mg/mL) (Boehringer) for 10 minutes at 37°C and incubated for 2 hours at 37°C with a prehybridization solution containing 10% (wt/vol) dextran sulfate, 50% (vol/vol) formamide, 5× SSC, 5× Denhardt's solution, 0.02% (wt/vol) SDS, 0.02 mmol EDTA/L, 10 mmol sodium phosphate buffer/L (pH 6.5), 50 mg tRNA/L (Sigma), and 100 mg salmon testes DNA/L (Sigma). Cells were then incubated with a hybridization solution containing 250 ng/mL of heat-denaturated probe. After 18 hours at 42°C, the cells were washed with increasingly stringent solutions as follows: 2× SSC for 1 hour at room temperature, 1× SSC for 1 hour at 37°C, and 0.5× SSC for 1 hour at 42°C. The hybrids were detected by an enzyme-linked immunoassay using an antidigoxigenin MoAb conjugated to alkaline phosphatase (Boehringer), and nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate as chromogen. Cells were counterstained with methyl green. Negative controls were performed by treating the cells with RNAse A (100 μg/mL) (Boehringer) for 30 minutes at 37°C or no probe-hybridization. As positive controls, BMSC, IM9-P3, and Hep-G2 cells were hybridized with oligo-dT instead of probe.
Statistical analysis.The Wilcoxon test was used for statistical analysis. Significance was defined as P < .05.
RESULTS
Contribution of BMSC-derived PRL to colony formation from cocultured CD34+ hematopoietic progenitors.BMSC monolayers derived from selective growth of BMMC in 10% FCS-IMDM were irradiated and used as feeders for colony formation from autologous CD34+ hematopoietic progenitors in the presence of exogenous GM-CSF, IL-3, and Epo. Colonies were scored on day 14 (Tables 1 and 2). Anti-PRL antiserum at a concentration previously reported to neutralize the effect of extractive pituitary human PRL on hematopoietic growth in semisolid medium21 induced a slight (28 ± 8 v 32 ± 10, mean ± SD of triplicates from four different experiments) though significant (P = .004) inhibition of BFU-E colonies (Table 1). By contrast, CFU-GM were not significantly affected by the antibody (P = .6) (Table 2). When BMSC were pretreated with physiologic concentrations of PAF (10−12 mol/L) before being used as feeder, a significant increase of both BFU-E and CFU-GM colonies (54 ± 13 v 32 ± 10, P = .002 for BFU-E and 32 ± 16 v 20 ± 12, P = .007 for CFU-GM) was seen. The effect was specific because it was not observed when PAF pretreatment was preceded by exposure to the PAF-R antagonist WEB 2170. PAF-induced enhancement of BFU-E colony promoting activity was significantly decreased (40 ± 12 v 54 ± 13, P = .004) by the anti-PRL antiserum, thus demonstrating its dependence on the rise of endogenous PRL. By contrast, the reduction of PAF-stimulated CFU-GM induced by the antibody did not reach statistical significance (P = .4).
Effect of PAF Treatment on the Ability of BMSC to Support BFU-E Colony Formation From Autologous CD34+ Cells
| Culture Conditions . | Exp 1 . | Exp 2 . | Exp 3 . | Exp 4 . |
|---|---|---|---|---|
| Control | 29 ± 2* | 45 ± 8 | 34 ± 8 | 24 ± 3 |
| PAF | 55 ± 5 | 72 ± 7 | 50 ± 7 | 39 ± 4 |
| WEB2170 | 31 ± 3 | 49 ± 5 | 37 ± 7 | 24 ± 1 |
| PAF + WEB2170 | 28 ± 3 | 43 ± 6 | 48 ± 3 | 30 ± 3 |
| Control + anti-PRL | 25 ± 4 | 39 ± 5 | 31 ± 7 | 20 ± 1 |
| PAF + anti-PRL | 33 ± 3 | 56 ± 4 | 46 ± 3 | 27 ± 6 |
| WEB2170 + anti-PRL | 26 ± 2 | 40 ± 5 | 36 ± 11 | 24 ± 5 |
| PAF + WEB2170 + anti-PRL | 27 ± 5 | 46 ± 7 | 35 ± 10 | 23 ± 4 |
| Culture Conditions . | Exp 1 . | Exp 2 . | Exp 3 . | Exp 4 . |
|---|---|---|---|---|
| Control | 29 ± 2* | 45 ± 8 | 34 ± 8 | 24 ± 3 |
| PAF | 55 ± 5 | 72 ± 7 | 50 ± 7 | 39 ± 4 |
| WEB2170 | 31 ± 3 | 49 ± 5 | 37 ± 7 | 24 ± 1 |
| PAF + WEB2170 | 28 ± 3 | 43 ± 6 | 48 ± 3 | 30 ± 3 |
| Control + anti-PRL | 25 ± 4 | 39 ± 5 | 31 ± 7 | 20 ± 1 |
| PAF + anti-PRL | 33 ± 3 | 56 ± 4 | 46 ± 3 | 27 ± 6 |
| WEB2170 + anti-PRL | 26 ± 2 | 40 ± 5 | 36 ± 11 | 24 ± 5 |
| PAF + WEB2170 + anti-PRL | 27 ± 5 | 46 ± 7 | 35 ± 10 | 23 ± 4 |
Abbreviations: anti-PRL, rabbit antiserum to human prolactin; WEB2170, PAF receptor antagonist.
Data represent the mean ± SD number of BFU-E colonies per well in triplicate cultures each seeded with 5 × 102 cells. Cultures were performed in the presence of IL-3, GM-CSF, and Epo. No nonspecific inhibition was observed when using a nonimmune rabbit antiserum (not shown).
Effect of PAF Treatment on the Ability of BMSC to Support CFU-GM Colony Formation From Autologous CD34+ Cells
| Culture Conditions . | Exp 1 . | Exp 2 . | Exp 3 . | Exp 4 . |
|---|---|---|---|---|
| Control | 15 ± 1* | 39 ± 6 | 19 ± 1 | 17 ± 7 |
| PAF | 39 ± 5 | 52 ± 9 | 14 ± 6 | 22 ± 2 |
| WEB2170 | 16 ± 3 | 41 ± 4 | 11 ± 4 | 21 ± 4 |
| PAF + WEB2170 | 16 ± 2 | 45 ± 2 | 10 ± 4 | 21 ± 2 |
| Control + anti-PRL | 17 ± 3 | 33 ± 4 | 10 ± 3 | 18 ± 5 |
| PAF + anti-PRL | 24 ± 4 | 49 ± 7 | 19 ± 2 | 24 ± 4 |
| WEB2170 + anti-PRL | 18 ± 2 | 38 ± 6 | 11 ± 2 | 19 ± 3 |
| PAF + WEB2170 + anti-PRL | 26 ± 3 | 40 ± 4 | 11 ± 7 | 20 ± 2 |
| Culture Conditions . | Exp 1 . | Exp 2 . | Exp 3 . | Exp 4 . |
|---|---|---|---|---|
| Control | 15 ± 1* | 39 ± 6 | 19 ± 1 | 17 ± 7 |
| PAF | 39 ± 5 | 52 ± 9 | 14 ± 6 | 22 ± 2 |
| WEB2170 | 16 ± 3 | 41 ± 4 | 11 ± 4 | 21 ± 4 |
| PAF + WEB2170 | 16 ± 2 | 45 ± 2 | 10 ± 4 | 21 ± 2 |
| Control + anti-PRL | 17 ± 3 | 33 ± 4 | 10 ± 3 | 18 ± 5 |
| PAF + anti-PRL | 24 ± 4 | 49 ± 7 | 19 ± 2 | 24 ± 4 |
| WEB2170 + anti-PRL | 18 ± 2 | 38 ± 6 | 11 ± 2 | 19 ± 3 |
| PAF + WEB2170 + anti-PRL | 26 ± 3 | 40 ± 4 | 11 ± 7 | 20 ± 2 |
Abbreviations: anti-PRL, rabbit antiserum to human prolactin; WEB2170, PAF receptor antagonist.
Data represent the mean ± SD number of CFU-GM colonies per well in triplicate cultures each seeded with 5 × 102 cells. Cultures were performed in the presence of IL-3, GM-CSF, and Epo. No nonspecific inhibition was observed when using a nonimmune rabbit antiserum (not shown).
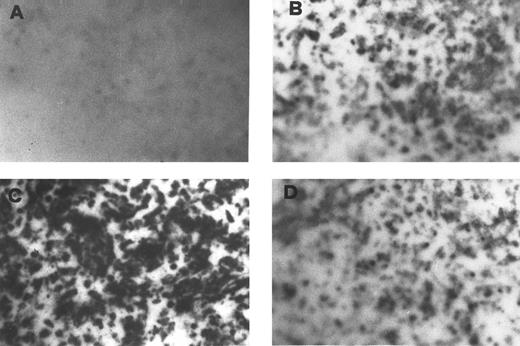
Secretion of a PRL-immunoreactive molecule by BMSC.This was assessed by Elispot, which detects PRL secretion by peripheral blood MC.22 The number of spots indicates low basal secretion by BMSC (Fig 1B). This was markedly increased after activation with PAF (Fig 1C). Specificity of the effect was established by pretreating BMSC with WEB 2170 before PAF exposure (Fig 1D).
Detection of a BMSC-secreted PRL-immunoreactive molecule by Elispot assay. Specific PRL spots in unstimulated BMSC (B) are increased after PAF treatment (C) and the effect is abrogated by the PAF-R antagonist WEB 2170 (D). (A) Elispot of PAF-stimulated BMSC in which the anti-PRL antibody has been omitted.
Detection of a BMSC-secreted PRL-immunoreactive molecule by Elispot assay. Specific PRL spots in unstimulated BMSC (B) are increased after PAF treatment (C) and the effect is abrogated by the PAF-R antagonist WEB 2170 (D). (A) Elispot of PAF-stimulated BMSC in which the anti-PRL antibody has been omitted.
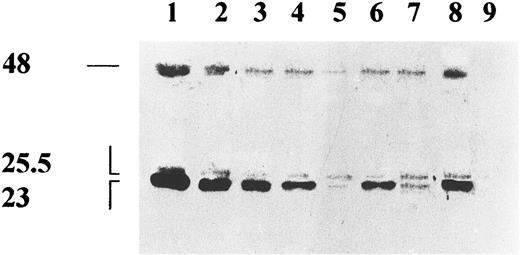
Characteristics of the PRL-like molecule released by BMSC.BMSC were cultured in the presence (Fig 2, lane 6) or absence (Fig 2, lane 5) of PAF, or with PAF plus WEB 2170 (Fig 2, lane 7) and the supernatants were collected after 32 hours, 10× concentrated, and assayed for the presence of PRL by immunoprecipitation followed by Western blotting. Resting BMSC supernatant contained both the glycosylated (G) (25.5 kD) and the nonglycosylated (NG) (23 kD) PRL forms (Fig 2, lane 5), which are also present in standard pituitary human PRL (Fig 2, lanes 1 through 4) and in the IM9-P3 conditioned medium (Fig 2, lane 8). However, equal amounts of G-PRL and NG-PRL were detected in BMSC. Upmodulation of the NG-PRL and downmodulation of G-PRL were observed in PAF-treated BMSC (Fig 2, lane 6). These effects were not observed when BMSC were pretreated with WEB 2170 (Fig 2, lane 7)
Biochemical characterization of PRL-immunoreactive peptide in the BMSC supernatants. Supernatants of resting (lane 5), PAF-stimulated (lane 6), WEB 2170 plus PAF stimulated (lane 7) BMSC; positive control IM9-P3 (lane 8) and negative control Hep-G2 (lane 9) cell lines were immunoprecipitated with rabbit-antihuman PRL antiserum (IC-5) and run on SDS-PAGE. Decreasing amounts (100, 50, 25, 12.5 ng) of standard human PRL (NIDDK) (lanes 1 through 4) were also run. Filters were blotted with two anti-PRL MoAbs direct against distinct epitopes and the reactivity revealed by peroxidase conjugated Protein A and chemiluminescence. No signal was observed when human PRL (100 ng) and supernatant of IM9-P3 were immunoprecipitated with control serum (not shown).
Biochemical characterization of PRL-immunoreactive peptide in the BMSC supernatants. Supernatants of resting (lane 5), PAF-stimulated (lane 6), WEB 2170 plus PAF stimulated (lane 7) BMSC; positive control IM9-P3 (lane 8) and negative control Hep-G2 (lane 9) cell lines were immunoprecipitated with rabbit-antihuman PRL antiserum (IC-5) and run on SDS-PAGE. Decreasing amounts (100, 50, 25, 12.5 ng) of standard human PRL (NIDDK) (lanes 1 through 4) were also run. Filters were blotted with two anti-PRL MoAbs direct against distinct epitopes and the reactivity revealed by peroxidase conjugated Protein A and chemiluminescence. No signal was observed when human PRL (100 ng) and supernatant of IM9-P3 were immunoprecipitated with control serum (not shown).
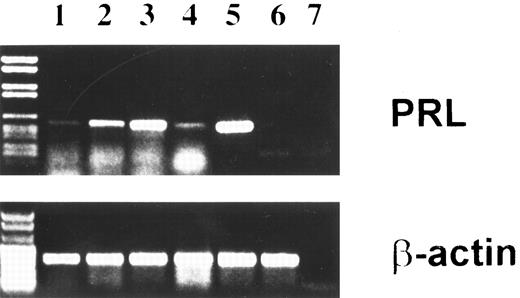
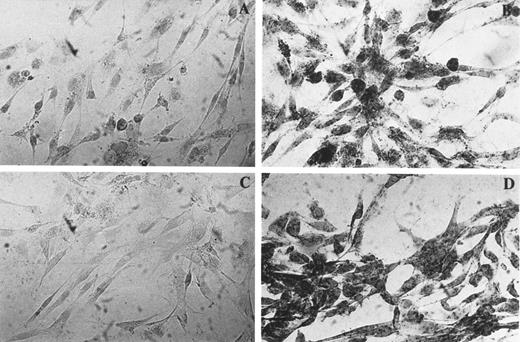
Evidence for PRL mRNA in BMSC.To verify that the basal and PAF-modulated, antiserum-inhibitable effect of BMSC was caused by synthesis of PRL by the BMSC, expression of PRL mRNA was assessed by amplification of cDNA reverse transcribed from total RNA and ISH. As shown in Fig 3, a 354-bp PCR product was found in the IM9-P3 cell line (lane 5) used as positive control for extra-pituitary PRL, as well as in BMSC (lanes 2 through 4). The faint signal observed in unstimulated BMSC (lane 1) was increased after exposure to PAF for 5 hours (lane 2) and 20 hours (lane 3), the maximal enhancement being observed toward the end of the incubation. Reversal of the PAF effect was observed in the presence of WEB 2170 (lane 4). PCR data were confirmed by ISH experiments in which BMSC microcultured on slide chambers were hybridized with a rat cDNA complementary for PRL mRNA labeled with digoxigenin. Figure 4 shows moderate (A) and marked reactivity (B) by resting (A) and PAF-stimulated (B) BMSC. Again, the reactivity was not seen after WEB pretreatment of PAF-stimulated cells (C). Specificity of the probe was confirmed by a strong message on the PRL-producing cell line IM9-P3 and negativity on the Hep-G2 hepatoma cell line23 (data not shown).
PRL transcript in BMSC identified by RT-PCR. Passaged stromal layers were cultured in the absence (lane 1) or presence of PAF for 5 (lane 2) and 20 (lane 3) hours, or PAF plus the PAF-R antagonist WEB 2170 (20 hours) (lane 4). Total cell RNA was reverse transcribed and cDNA was amplified using PRL-specific primers and various amplification cycles as described in Materials and Methods. Lane 7 is the no-template control. The base-pair length was determinated with marker DNA fragments (left). Positive and negative controls for PRL mRNA were IM9-P3 (lane 5) and Hep-G2 (lane 6). β-Actin gene expression was also examined as an internal control to ensure RNA integrity and proper amplification. Data are representative of three experiments.
PRL transcript in BMSC identified by RT-PCR. Passaged stromal layers were cultured in the absence (lane 1) or presence of PAF for 5 (lane 2) and 20 (lane 3) hours, or PAF plus the PAF-R antagonist WEB 2170 (20 hours) (lane 4). Total cell RNA was reverse transcribed and cDNA was amplified using PRL-specific primers and various amplification cycles as described in Materials and Methods. Lane 7 is the no-template control. The base-pair length was determinated with marker DNA fragments (left). Positive and negative controls for PRL mRNA were IM9-P3 (lane 5) and Hep-G2 (lane 6). β-Actin gene expression was also examined as an internal control to ensure RNA integrity and proper amplification. Data are representative of three experiments.
Identification of mRNA in BMSC by ISH using digoxigenin-labeled probe. BMSC were cultured in the absence (A) or in the presence (B, C, D) of PAF for 20 hours and hybridized with PRL cDNA probe (A, B, and C) or with poly dT probe (D). The specificity of the reaction was assessed by pretreating PAF-stimulated cells with RNAse before hybridization (C). These findings were confirmed in other two experiments.
Identification of mRNA in BMSC by ISH using digoxigenin-labeled probe. BMSC were cultured in the absence (A) or in the presence (B, C, D) of PAF for 20 hours and hybridized with PRL cDNA probe (A, B, and C) or with poly dT probe (D). The specificity of the reaction was assessed by pretreating PAF-stimulated cells with RNAse before hybridization (C). These findings were confirmed in other two experiments.
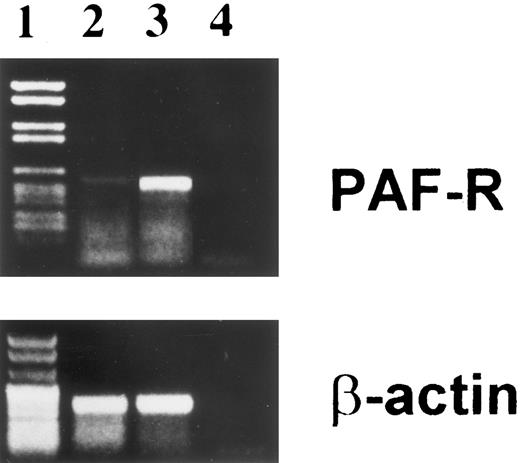
Presence of PAF-R on BMSC.Because of the strong enhancing effect of PAF on the colony formation and PRL-synthetic activity by BMSC, we studied the expression of PAF-R mRNA on these cells. Figure 5 shows the presence of PAF-R PCR products of the appropriate size in the reverse transcribed mRNA of BMSC (lane 2) and in the U937 cell line (lane 3) used as positive control.
Expression of PAF-R mRNA by BMSC. PAF-R was detected on BMSC (lane 2) and monocytic cell line U937 as positive control (lane 3), using specific primers and various amplification cycles as described in Materials and Methods. The base-pair length was determined with marker DNA fragments (lane 1). Lane 4 is the no-template control. β-Actin gene expression was also examined as an internal control to ensure RNA integrity and proper amplification.
Expression of PAF-R mRNA by BMSC. PAF-R was detected on BMSC (lane 2) and monocytic cell line U937 as positive control (lane 3), using specific primers and various amplification cycles as described in Materials and Methods. The base-pair length was determined with marker DNA fragments (lane 1). Lane 4 is the no-template control. β-Actin gene expression was also examined as an internal control to ensure RNA integrity and proper amplification.
DISCUSSION
Several lines of evidence suggest that PRL behaves as a cytokine-like molecule in that it is produced by lymphocytes and is required for their activation.13,15 In addition, there are data indicating its involvement in hematopoiesis.8,9 We have recently shown cooperation between exogenous PRL, GM-CSF, and IL-3 at an early step of in vitro erythroid differentiation.12 This report confirms and extends those data by showing that differentiation of erythroid progenitors in a BMSC microenvironment is dependent on locally produced PRL.
Formation of BFU-E colonies from GM-CSF–, IL-3–, and Epo–stimulated CD34+ progenitors seeded on BMSC feeder layers was slightly, but significantly, inhibited by an anti-PRL antibody, whereas CFU-GM were unaffected. Indeed, release of a PRL-immunoreactive protein from BMSC was detected by Elispot assay and two forms of PRL were found in the serum-free culture medium of BMSC immunoprecipitated with an anti-PRL antibody. Interestingly, compared with standard pituitary human PRL (Fig 2, lanes 1 through 4), human serum PRL,24 and lymphocyte PRL (Fig 2, lane 8), PRL detected in the BMSC medium (Fig 2, lane 5) was predominantly represented by the glycosylated form. Active synthesis of PRL by BMSC was suggested by the presence of PRL mRNA, as assessed by both PCR and ISH. Participation of extra-pituitary PRL in normal hematopoiesis has already been demonstrated by Nagy and Berczi,25 though these authors did not investigate the direct participation of BM PRL to the anti-PRL antiserum-inhibitable hematopoietic activity of hypophysectomized animals.
Hematopoiesis is regulated by external stimuli, which act primarily by modifying the secretory activity of BMSC. Constitutive production of HGFs such as GM-CSF, G-CSF, stem cell factor, and IL-6 is increased by inflammatory molecules (lipopolysaccharides, IL-1, and tumor necrosis factor).6,7 PAF is also secreted during inflammation16 and stimulates 3H-thymidine uptake in freshly isolated human BM cells,26 an observation reinforced by our present finding of PAF-R mRNA expression by BMSC. In addition, PAF increases the release of PRL from the pituitary.17 Here we show that the colony-forming activity of BMSC was strongly enhanced by PAF, an effect that was neutralized by a PAF-R antagonist and by the anti-PRL antibody. Increased production of PRL protein and mRNA was also demonstrated in BMSC stimulated by PAF and was also inhibited by the PAF-R antagonist. Interestingly, NG-PRL (23 kD) was strongly upmodulated by PAF, whereas G-PRL (25.5 kD) was downmodulated. It is worth noting that most of the biologic activity of PRL has been associated with NG-PRL27 and that, in line with our findings, NG-PRL is increased more markedly than G-PRL after pharmacologic stimulation by the PRL releasing hormone TRH or metoclopropramide.28 In addition, the suggestion of Brue et al29 that glycosylation selectively downregulates PRL action at individual target tissues may explain why the PAF-induced activation of PRL-mediated colony-promoting activity observed here is accompanied by synthesis of the NG-PRL form. In keeping with this line of reasoning, the abnormally high proportion of G-PRL found in unstimulated BMSC may serve to maintain the action of PRL at subliminal levels (or restrain it) during basal hematopoiesis.
In conclusion, the present study shows that PRL is constitutively produced by BMSC and participates, although to a small extent, in basal erythroid colony formation. These findings, together with our previous observation of a synergistic effect of exogenous PRL on GM-CSF–, IL-3–, and Epo–driven BFU-E colony formation, suggest an interactive role for PRL and other HGF in vivo at the sites of hematopoiesis. In addition, the present data reinforce the modulatory role of PAF in hematopoiesis and indicate that PRL may be one of the mediators of its effect.
Extension of these studies to factors known to affect HGF release by the BMSC or PRL release by the pituitary and identification of the BMSC type(s) responsible for PRL production are needed to precisely delineate the role of PRL in the BM hematopoietic cytokine network.
ACKNOWLEDGMENT
We are indebted to Drs F. Bussolino and G. Camussi for critically reading the paper.
Supported by research grants from the National Research Council (CNR) (no. 95.02124.CT04) to G.B. and the Ministry of Education (40% Neuroimmunology) to L.M., Rome, Italy.
Address reprint requests to Lina Matera, PhD, Department of Internal Medicine, Corso A.M. Dogliotti 14, 10126 Turin, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal