Abstract
The appropriate timing of bone marrow transplantation (BMT) for adults with acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) is controversial. Although allogeneic transplantation results in a lower risk of disease recurrence than intensive chemotherapy alone, overall outcome following BMT may not be improved due to the higher incidence of therapy-related fatal complications, frequently as a result of the development of graft-versus-host disease (GVHD). Selective T-cell depletion of donor marrow can reduce the incidence of GVHD and thereby limit transplant-related toxicity. Herein we report the risk of GVHD, incidence of transplant related mortality (TRM), likelihood of disease relapse, and overall survival in adult patients undergoing BMT with CD6 depleted allogeneic marrow for acute leukemia in first remission. Forty-one consecutive allogeneic transplants were performed on patients with acute leukemia and high-risk features (28 AML, 13 ALL) using T12 monoclonal antibody and complement to remove CD6+ T cells from donor marrow. No pre- or posttransplant immune suppressive medications for GVHD prophylaxis were administered. The actuarial estimated risk of grade 2 to 4 acute GVHD was 15% in patients receiving HLA identical grafts. Chronic GVHD developed in five patients. The estimated risk of TRM for patients in first complete remission was 5% at Day +100 and 16% at 2 years. Fatalities attributable to infection with cytomegalovirus or Epstein-Barr virus occurred in only three patients. Estimated probabilities of relapse, overall survival, and event-free survival at 4 years were 25%, 71%, and 63%, respectively. No significant differences in GVHD, TRM, relapse rate, or survival was observed for patients with AML compared with those with ALL. Allogeneic transplantation with CD6 depleted bone marrow is effective in consolidating remissions of high-risk patients with acute leukemia in first remission without excessive toxicity.
OVER THE PAST 20 YEARS, bone marrow transplantation (BMT) has been used with increasing frequency in the treatment of patients with acute leukemia.1,2 Reports from many centers indicate that adults undergoing allogeneic BMT for acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL) have a lower risk of relapse than individuals treated with intensive chemotherapy alone.3-6 However, since the toxicities associated with BMT are significant and represent the primary cause of treatment failure for patients with leukemia transplanted in first remission, there is considerable controversy over the appropriate timing of transplantation.7
Allogeneic BMT would be a far more attractive treatment modality for patients with acute leukemia in first remission if the risks associated with the procedure could be limited. Transplant-related mortality often comes as a direct or indirect result of the development of graft-versus-host disease (GVHD).8-10 GVHD is initiated, in large part, by T lymphocytes in the donor marrow.11 Decreasing the number of donor marrow T cells by a variety of purging methods will decrease the incidence of GVHD.12-15 However, the overall utility of T-cell depletion has been questioned because of reports of higher rates of relapse, graft failure, and Epstein-Barr virus (EBV) related lymphoproliferative disorders post-BMT.16-20 Cellular populations lost in many T-cell depletion procedures appear to play critical roles in promoting donor marrow engraftment, suppressing outgrowth of EBV-transformed lymphocytes, and eliminating residual disease post-BMT.
In vitro purging methods are not uniform, and the precise extent of T cells removed by different techniques vary.21,22 Some depletion approaches eliminate other potentially important cell types as well, such as natural killer (NK) cells, B lymphocytes, monocytes, and myeloid progenitors. We have used T12, an IgM monoclonal antibody (MoAb) that recognizes the CD6 antigen on T cells, to purge donor marrow. T12 reacts with 90% to 95% of mature T cells, but does not affect immature thymocytes, NK cells, monocytes, or other hematopoietic elements.23 Three cycles of incubation with T12 plus rabbit complement removes approximately 1.5 to 2.0 logs of T lymphocytes from normal bone marrow.24 We have previously demonstrated that depletion of CD6+ T cells from donor marrow with T12 and complement can, without the addition of any immune suppressive agents, significantly reduce the incidence of grades 2 to 4 acute GVHD in patients transplanted for hematologic malignancy.25 We now report results of CD6 depletion in BMTs performed for acute leukemia in first remission at our institution through April 1995.
MATERIALS AND METHODS
Patient population.Forty-one consecutive adults with a diagnosis of AML or ALL who underwent BMT at our institution between March 1984 and April 1995 are included in this analysis. Results are analyzed as of April 1, 1996. Since allogeneic transplantation had not been included in the routine treatment of patients entered onto cooperative group or institutional studies at our institution, these patients represent a minority of the individuals presenting to Dana-Farber since we initiated our transplant program. These patients were selected for transplantation because they were felt to be at high risk for relapse following intensive chemotherapy based on their clinical characteristics including adverse karyotypic anomalies, FAB classification, antecedent myelodysplastic syndrome, and slow response to induction chemotherapy. Inpatients were cared for at the Brigham and Women's Hospital from 1985 to 1987 (n = 6) and at Dana-Farber Cancer Institute from 1987 to 1995 (n = 35). Treatment protocols were approved by the Human Subjects Protection Committees of DFCI and BWH. Written informed consent was obtained in all cases.
Treatment protocol.Forty of the 41 patients received cyclophosphamide 60 mg/kg intravenously (IV) × 2 on 2 consecutive days followed by total body irradiation (TBI) as ablation. The dose of TBI was sequentially escalated from 1,300 cGy in 1985 to 1,560 cGy in 1995 (Table 1). One patient who had previously received TBI as part of a conditioning regimen for an autologous transplant for non-Hodgkin's lymphoma received busulfan (1 mg/kg × 16 doses) instead of TBI. In all patients who received TBI, the total dose was administered in six to eight equal fractions administered twice daily, 5 to 6 hours apart. All patients were treated at 10 cGy/min on a dedicated linear accelerator. Patients who received marrow from HLA mismatched donors (n = 7) also received total lymphoid irradiation (TLI) at 750 to 1,050 cGy 1 week before admission as previously described to prevent graft rejection.26
Patient Characteristics
| Variable . | N . |
|---|---|
| Diagnosis | |
| AML | 28 |
| ALL | 13 |
| Patient/donor sex | |
| Male/male | 9 |
| Male/female | 13 |
| Female/female | 7 |
| Female/male | 12 |
| Age | |
| Patient | 34 (22-54) |
| Donor | 34 (16-51) |
| HLA disparity | |
| Matched | 34 |
| Mismatched | 7 |
| 1 antigen | 6 |
| 2 antigens | 1 |
| Ablative regimen | |
| Cyclophosphamide/TBI (1,300 cGy) | 4 |
| Cyclophosphamide/TBI (1,400 cGy) | 26 |
| Cyclophosphamide/TBI (1,480 cGy) | 7 |
| Cyclophosphamide/TBI (1,560 cGy) | 2 |
| Cyclophosphamide/Busulfan | 1 |
| Cell dose | |
| Median BM cells infused | 5.6 × 107 cells/kg |
| Median CD3+ BM cells infused | 1.5 × 106 cells/kg |
| Variable . | N . |
|---|---|
| Diagnosis | |
| AML | 28 |
| ALL | 13 |
| Patient/donor sex | |
| Male/male | 9 |
| Male/female | 13 |
| Female/female | 7 |
| Female/male | 12 |
| Age | |
| Patient | 34 (22-54) |
| Donor | 34 (16-51) |
| HLA disparity | |
| Matched | 34 |
| Mismatched | 7 |
| 1 antigen | 6 |
| 2 antigens | 1 |
| Ablative regimen | |
| Cyclophosphamide/TBI (1,300 cGy) | 4 |
| Cyclophosphamide/TBI (1,400 cGy) | 26 |
| Cyclophosphamide/TBI (1,480 cGy) | 7 |
| Cyclophosphamide/TBI (1,560 cGy) | 2 |
| Cyclophosphamide/Busulfan | 1 |
| Cell dose | |
| Median BM cells infused | 5.6 × 107 cells/kg |
| Median CD3+ BM cells infused | 1.5 × 106 cells/kg |
Donor bone marrow was harvested on the last day of TBI administration. CD6+ T cells were removed from donor marrow by complement-mediated antibody lysis using anti-T12 MoAb as previously described.25 Marrow was infused into the patient through an indwelling central venous catheter the same day it was harvested. No patients received prophylactic immunosuppressive therapy of any type, including corticosteroids, methotrexate, or cyclosporine. Since January 1994, six patients received granulocyte colony stimulating factor (G-CSF ) beginning at day + 1 and continuing until the absolute neutrophil count exceeded 1.0 × 109 cells/L.
All patients were treated in HEPA-filtered rooms using standard reverse isolation procedures. Oral prophylactic antibiotics, either ciprofloxacin or trimethoprim-sulfamethoxazole, were administered to all patients. These were discontinued when patients developed fevers that required initiation of broad spectrum IV antibiotics. Since 1987, all patients have received prophylactic acyclovir. All patients received single donor platelets from cytomegalovirus sero-negative individuals and frozen deglycerolized red blood cells. All blood components were irradiated to prevent transfusion-related GVHD. After BMT, three of the 41 patients participated on trials in which they received low-dose interleukin-2 (IL-2; Hoffmann-LaRoche [Nutley, NJ] or Amgen, Inc) administered either IV by continuous infusion or by subcutaneous bolus at doses of 2 to 6 × 105 U/m2/d for up to 90 days.27
Immunofluorescence assays.Bone marrow and peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Hypaque density centrifugation and were analyzed by direct or indirect immunofluorescence for reactivity with a series of MoAbs using standard techniques. Antibodies used included T3 (CD3), T4 (CD4), T8 (CD8), T11 (CD2), T12 (CD6), NKH1 (CD56), B1 (CD20), and My4 (CD14) (Coulter Immunology, Hialeah, FL). Ten thousand cells were analyzed in each sample using automated flow cytometry (EPICS-C; Coulter Electronics, Hialeah, FL). Bone marrow cells were analyzed before and after in vitro treatment with anti-T12 plus complement. PBMC were analyzed at regular intervals post-BMT.
Statistical analysis.Descriptive statistics are reported as proportions, medians, and means. Fisher's exact test was used to compare proportions.28 The duration of survival, event-free survival, time to relapse, GVHD, and transplant-related mortality were computed from the date of marrow infusion. GVHD was graded by standard criteria.29 Patients were considered censored at the time of death for “time to relapse” and censored at the time of relapse for “time to death without relapse” (transplant-related mortality). Survival, event-free survival, time to relapse (risk), and transplant-related mortality curves were constructed using the Kaplan-Meier product limit method.30 The logrank test of survival analysis was used to compare the various subgroups with respect to their survival, event-free survival, time to relapse, and time to death without relapse (transplant mortality) distributions.31 Fisher's exact test and Wilcoxon rank sum tests were used to identify possible predictors of GVHD. Predictors of treatment-related mortality, relapse, and event-free survival and overall survival were examined using the logrank test.
RESULTS
Patient characteristics.Twenty-eight patients had a diagnosis of AML and 13 patients had ALL (Table 1). The median age of patients was 34 years (range, 22 to 54 years). There were 22 males and 19 females. The majority of patients received 1,400 cGy TBI in seven fractions over 4 days. Thirty-four received marrow from sibling donors who were genotypically matched while seven received marrow from HLA-mismatched related donors. The average age of donors was 34 years (16 to 51 years). Patients were sex matched with their donors in 16 cases and mismatched in 25 cases. Of the 20 females donors, 12 were multiparous, 5 nulliparous, and 3 unknown. The median time from diagnosis to transplant for patients grafted in first remission was 4.5 months (range, 2 to 12 months).
The majority of patients reported possessed adverse clinical features that prompted the decision to have them undergo BMT. Cytogenetics conveying a poor prognosis for patients treated with chemotherapy alone were present in 19 patients (Table 2). Normal karyotypes were documented in 11 patients, and two patients had favorable cytogenetics. In nine patients, no cytogenetic data were available. Six patients transplanted for AML had a history of antecedent myelodysplasia. An additional three patients (1 AML, 2 ALL) had been treated with chemotherapy for a prior malignancy. Five of the 28 patients with AML had histologic features of M5, M6, or M7 leukemia at diagnosis. Five patients with AML had initially failed induction with two courses of anthracycline and cytosine arabinoside and had achieved complete remission only after treatment with high dose ara-C. Four patients with ALL required at least 8 weeks to achieve a complete remission and five ALL patients presented with a WBC count in excess of 50,000 × 106/L.
Cytogenetics at Diagnosis (CR1)
| Disease . | Karyotype . | N . |
|---|---|---|
| AML | Unfavorable | 11 |
| Trisomy 8 | 4 | |
| Multiple abnormalities | 2 | |
| Monosomy 7 | 2 | |
| t(9; 11) | 2 | |
| t(9; 22) | 1 | |
| Normal | 8 | |
| Favorable (inv 16) | 2 | |
| Unknown | 7 | |
| ALL | Unfavorable | 8 |
| t(9; 22) | 4 | |
| Multiple abnormalities | 2 | |
| t(4; 11) | 1 | |
| t(8; 14) | 1 | |
| Normal | 3 | |
| Unknown | 2 |
| Disease . | Karyotype . | N . |
|---|---|---|
| AML | Unfavorable | 11 |
| Trisomy 8 | 4 | |
| Multiple abnormalities | 2 | |
| Monosomy 7 | 2 | |
| t(9; 11) | 2 | |
| t(9; 22) | 1 | |
| Normal | 8 | |
| Favorable (inv 16) | 2 | |
| Unknown | 7 | |
| ALL | Unfavorable | 8 |
| t(9; 22) | 4 | |
| Multiple abnormalities | 2 | |
| t(4; 11) | 1 | |
| t(8; 14) | 1 | |
| Normal | 3 | |
| Unknown | 2 |
Marrow infusion.The median number of nucleated marrow cells infused into patients was 4.0 × 109 (1.4 to 12.7 × 109) which was the equivalent of 5.6 × 107 cells/kg (2.0 to 25.4 × 107). Immunophenotypic analysis was performed on infused marrow following CD6 depletion. The median number of CD3 cells infused was 1.5 × 106 cells/kg. In the majority of patients, no cells expressing CD6 could be detected in the infused marrow by immunophenotypic analysis.
Engraftment.Forty of 41 patients demonstrated successful initial engraftment (>1.0 × 109 WBC/L) within 30 days of marrow infusion. The patient who failed to engraft had received a one-antigen HLA mismatched marrow. This patient was successfully engrafted with previously cryopreserved autologous bone marrow. The median time to achieve a neutrophil count of 0.5 × 109 cells/L was 19 days (11 to 30 d). Multivariable analysis of factors influencing neutrophil engraftment (including patient age, donor age, sex mismatch, number of cells infused/kg, number of CD3+ cells infused/kg, TBI dose, use of G-CSF ) revealed that only use of G-CSF correlated with speed of engraftment (logrank, P = .003). Platelet counts of 50,000 × 106/L were achieved at a median of 25 days (range, 14 to 55 days) post-BMT.
Late graft failure, defined as a drop in absolute neutrophil count to a level <0.1 × 109 cells/L beyond day +30, was noted in two patients who had no evidence of GVHD. It was recognized 54 to 76 days after initial marrow infusion. An active cellular rejection process could not be demonstrated. Both patients were successfully engrafted following reconditioning with cyclophosphamide (30 mg/kg × 4 days), antithymocyte globulin (30 mg/kg for 3 days), and infusion of unmanipulated marrow from the original donor. One patient developed GVHD despite prophylaxis with cyclosporine and corticosteroids.
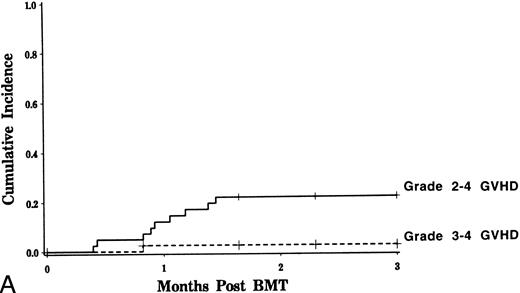
GVHD.Grade 2 to 4 GVHD occurred in nine patients. It was scored as grade 2 in eight, and grade 3 in one (Fig 1A). Eight of nine patients had cutaneous involvement only. The onset of acute GVHD occurred at a median of day +26. The estimated probability of developing grades 2 to 4 GVHD was 15% in recipients of genotypically identical marrow compared with 42% for those receiving genotypically nonidentical marrow (Fisher's exact, P = .01). Univariate analysis was performed evaluating risk factors including HLA genotypic identity, patient/donor age, patient/donor sex, number of nucleated cells infused (total, CD3+, CD56+, CD6+, CD4+, CD8+), TBI dose, diagnosis, disease stage, and use of G-CSF revealed that only the use of nongenotypically HLA identical donors could predict for the development of acute GVHD. Chronic GVHD was observed in five patients, with an estimated probability for those at risk of 14% (Fig 1B). Chronic GVHD arose in the setting of prior acute GVHD in all cases; no patients developed de novo chronic GVHD. Posttransplant treatment with IL-2 did not induce acute or chronic GVHD in any patients.
Incidence of acute and chronic GVHD after CD6 depleted allo-BMT. The estimated probability of developing grades 2 to 4 and 3 to 4 acute GVHD is depicted in (A). The incidence of chronic GVHD over time is shown in (B).
Incidence of acute and chronic GVHD after CD6 depleted allo-BMT. The estimated probability of developing grades 2 to 4 and 3 to 4 acute GVHD is depicted in (A). The incidence of chronic GVHD over time is shown in (B).
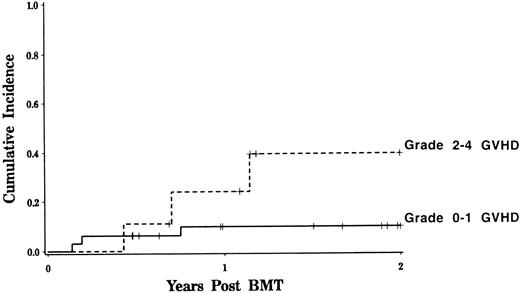
Transplant-related complications.Six patients died as a result of transplant-related complications. Interstitial pneumonitis was the most common direct cause of toxic death, occurring in five patients. In two instances, pneumonitis appeared to be caused by CMV, in one case by toxoplasmosis, and in two cases no organisms could be identified. Fatal EBV lymphoproliferative disease occurred in one patient. Nonfatal veno-occlusive disease of the liver was diagnosed by clinical criteria32 in two patients. Day 100 transplant-related mortality was only 5% due to the low incidence of acute/chronic GVHD and other transplant-related complications such as CMV, EBV, and VOD. Transplant-related mortality at 2 years was 16%. Development of GVHD was the only variable associated with transplant-related mortality (Fig 2, logrank, P = .08). Among patients who did not develop grades 2 to 4 GVHD, overall transplant-related mortality was only 10%. Patient or donor sex, age, diagnosis, use of G-CSF, and dose of TBI did not influence the likelihood of transplant-related death. No patients who received IL-2 suffered a fatal complication posttransplant. Among surviving patients, more than 90% have Karnofsky performance status of 90% or greater.
Transplant-related mortality after CD6 depleted allo-BMT. The estimated probability of death while in remission is depicted for patients with grades 0 to 1 GVHD and compared with those who developed grades 2 to 4 GVHD (logrank, P = .08).
Transplant-related mortality after CD6 depleted allo-BMT. The estimated probability of death while in remission is depicted for patients with grades 0 to 1 GVHD and compared with those who developed grades 2 to 4 GVHD (logrank, P = .08).
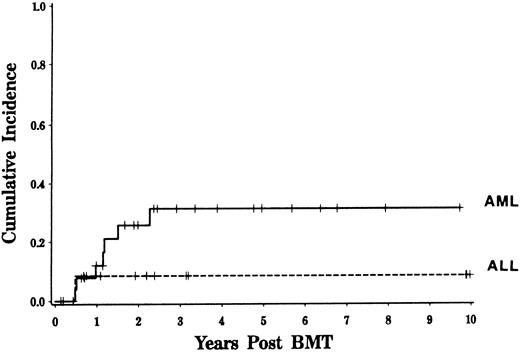
Disease relapse.For patients undergoing CD6-depleted allo-BMT for acute leukemia in first remission, the Kaplan-Meier 3-year estimated incidence of relapse was 25%. The majority of relapses occurred between 6 and 24 months posttransplant. Clinical factors including age, sex, interval between diagnosis and transplant, number of T cells or NK cells infused with the marrow, TBI dose, and development of GVHD did not significantly influence the risk of relapse post-BMT. Six of 32 patients (19%) with grades 0 to 1 GVHD relapsed compared to two of nine patients (22%) with grades 2 to 4 GVHD. Although a greater percentage of patients with AML relapsed than patients with ALL, the estimated likelihood of relapse was not significantly different for the two diseases (31% v 10%, logrank P = .25, Fig 3). All three patients who received IL-2 post-BMT remain alive and free of disease. Two of the patients who relapsed 1 year post-BMT are now disease free following either a second CD6 depleted BMT procedure with busulfan and cyclophosphamide conditioning (4 years after relapse) or CD4 donor lymphocyte infusion (1 year after relapse).
Relapse rate following CD6-depleted BMT for acute leukemia. Kaplan-Meier estimated risk of disease relapse is depicted for patients with AML and ALL (logrank, P = .25).
Relapse rate following CD6-depleted BMT for acute leukemia. Kaplan-Meier estimated risk of disease relapse is depicted for patients with AML and ALL (logrank, P = .25).
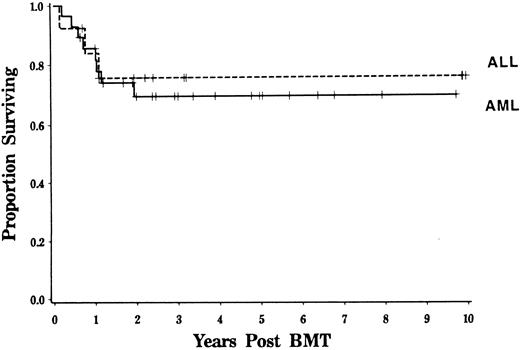
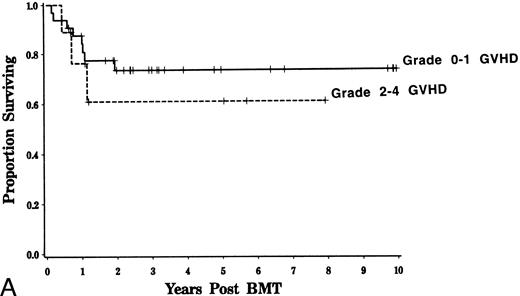
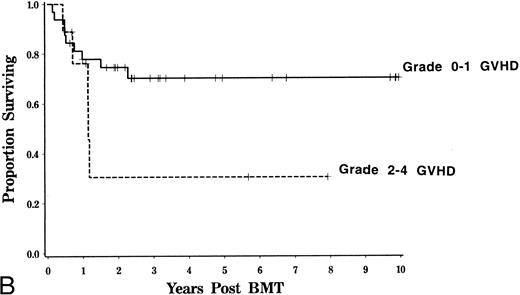
Event-free and overall survival.Overall survival at 4 years was 71% with an estimated event-free survival of 63% for adults. Survival was similar for patients with AML and ALL (Fig 4). Age, TBI dose, HLA disparity, and cellular composition of the marrow did not significantly influence event-free or overall survival. Because of the higher mortality rate associated with GVHD, event-free survival was somewhat inferior for those patients who developed grades 2 to 4 GVHD compared with that of patients in whom grades 2 to 4 GVHD did not develop (logrank, P = .08, Fig 5).
Survival for patients with AML and ALL. Kaplan-Meier estimated probability of survival is shown for patients transplanted in first remission. Results for patients transplanted for AML and ALL are contrasted (P = NS).
Survival for patients with AML and ALL. Kaplan-Meier estimated probability of survival is shown for patients transplanted in first remission. Results for patients transplanted for AML and ALL are contrasted (P = NS).
Influence of GVHD survival and event-free survival. Kaplan-Meier estimated probability of survival (A) and event-free survival (B) is displayed for patients who developed grades 0 to 1 and 2 to 4 GVHD. Event-free survival is somewhat lower for patients with a history of grades 2 to 4 GVHD (logrank, P = .08)
Influence of GVHD survival and event-free survival. Kaplan-Meier estimated probability of survival (A) and event-free survival (B) is displayed for patients who developed grades 0 to 1 and 2 to 4 GVHD. Event-free survival is somewhat lower for patients with a history of grades 2 to 4 GVHD (logrank, P = .08)
DISCUSSION
In most series of patients undergoing unpurged marrow transplantation for acute leukemia in first remission, transplant-related toxicity is the primary reason for treatment failure.16,33,34 Most commonly, fatalities are related to the development of acute and chronic GVHD. GVHD, once established, is difficult to successfully treat and often leads to a fatal outcome.8,10 Ringden et al, reporting for the IBMTR, found a 33% incidence of acute GVHD at 100 days for leukemic patients transplanted in first remission.35 Chronic GVHD occurred in 46% of patients at risk. Transplant-related mortality was 37%.
The risk of transplant-related complications we observed following CD6-depleted allo-BMT for acute leukemia was considerably lower than that previously reported by the IBMTR. Among patients undergoing BMT in first remission, the cumulative incidence of fatal complications was 5% at day +100 and 16% at 2 years. The low risk of transplantrelated mortality in our series is likely related to the low incidence of acute and chronic GVHD. Other serious complications, such as hepatic veno-occlusive disease and EBV lymphoproliferative disease, were rare. In addition, in the absence of immune suppression administered for GVHD, infectious complications were uncommon. In particular, parenchymal infection with CMV was diagnosed in less than 5% of patients. The low incidence of CMV in our series contrasts with the higher likelihood of developing invasive CMV disease (10%-50%) reported after BMT using either other T-cell depletion methods or unpurged marrow.36-39 The relative paucity of opportunistic infections in our series may be related to the elimination of the routine use of immune suppressive medications for GVHD prophylaxis and the steady recovery of circulating T cell number during the first 6 months post-BMT. Although we have observed a variety of abnormalities in T-cell activation and responsiveness following CD6-depleted allo-BMT during the first year posttransplant, the clinical relevance of these defects remains uncertain.40-42
Despite reductions in the incidence of GVHD achieved by several different T-cell depletion methods, this approach has not been widely accepted for use in recipients of HLA-identical sibling marrow because of an increased risk of disease relapse reported by a number of centers.16,17 The observed increase in disease recurrence has been linked, in part, to the reduction in the incidence of GVHD following donor marrow T-cell depletion. The antitumor effect associated with allogeneic marrow and GVHD has been termed the graft-versus-leukemia (GVL) effect.43-45 There is both experimental and clinical evidence, however, that allogeneic marrow can mediate GVL activity in the absence of GVHD.46-49 Data reported by Gale et al suggests that, after adjusting for the development of GVHD, allogeneic BMT results in a lower risk of relapse than syngeneic transplantation.50 Such data imply that effectively preventing GVHD may not eliminate GVL activity in allogeneic transplant recipients. Conversely, induction of GVHD by truncating the prophylactic regimen or by administering donor buffy coat cells at the time of marrow infusion does not necessarily result in GVL activity sufficient to prevent disease relapse.51 It is noteworthy that in our series the development of GVHD did not exert any significant effect on subsequent disease recurrence.
The impact of T-cell depletion on relapse rate depends on the disease for which patients are being transplanted. In virtually all reports of T-cell depletion of donor marrow in patients with chronic myelogenous leukemia (CML), an increased rate of relapse has been observed.16,17 In contrast, the effect of T-cell depletion on recurrence of acute leukemia post-BMT appears less striking. The relapse rate we observed after CD6 depleted allo-BMT was similar to that reported by the IBMTR as well as that of many individual transplant centers for adults with acute leukemia in first remission52-54 even though our patient population was composed of a large fraction of individuals whose leukemia had cytogenetic abnormalities that confer a poor prognosis following transplantation.55
Despite the encouraging results we observed in patients with early leukemia, relapse remains a problem. It is possible that efforts to restore some of the GVL activity potentially lost by donor marrow T-cell depletion with IL-2 therapy might reduce relapse rates and improve disease-free survival in these patients. At this point, it would be premature to draw any conclusions regarding the efficacy of low doses of IL-2 after CD6 depleted allogeneic BMT for patients with acute leukemia in first remission. Both encouraging56 and disappointing57 results of IL-2 therapy have recently been reported following autologous transplantation for AML and ALL, respectively. In addition to IL-2 administration, other immunotherapeutic strategies should be investigated, such as the delayed infusion of defined numbers of either marrow or blood derived T-cell subsets post-BMT47,58 or the use of more selective T-cell depletion methods.49 There is some evidence in patients with CML that depletion of CD8+ cells from donor marrow can reduce the incidence of GVHD without compromising GVL activity. However, the efficacy of this approach by itself for GVHD prophylaxis remains unclear as patients receiving CD8 depleted marrow also received cyclosporine at the time of BMT. Another potential approach to preventing disease relapse after T-cell depleted allo-BMT might be intensification of the ablative regimen. Although dose escalation of ablative regimens has previously been associated with decreases in relapse rates at the cost of increased morbidity and mortality,59 60 this strategy may merit further exploration in a setting where the incidence of GVHD is low and patients do not require hepatotoxic and nephrotoxic immune suppressive agents.
Reducing the incidence of GVHD and its attendant mortality without significantly increasing relapse rates makes CD6 T-cell depletion an attractive means of GVHD prophylaxis for patients undergoing allogeneic BMT for acute leukemia in first remission. Studies in patients with AML and in those with ALL comparing the use of allogeneic marrow transplantation to intensive chemotherapy have suggested that the potential advantage provided by allogeneic BMT is significantly diminished by complications associated with BMT. If CD6-depleted allo-BMT can decrease toxicity without compromising disease eradication, then it might be reasonable to adopt it or similar strategies in the treatment of adults in first remission. Not only do morbidity and mortality appear to be limited by this approach, but hospitalization and the costs associated with it may be reduced compared with conventional unpurged marrow transplantation.
Supported by Grant No. A129530. R.J.S. is a recipient of the Baruj Benacerraf Clinical Scholar Award.
Address reprint requests to Robert J. Soiffer, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal