Abstract
Mast cells represent a potential source of interleukin-6 (IL-6) and other cytokines that have been implicated in host defense, tissue maintenance/remodeling, immunoregulation, and many other biologic responses. In acquired immune responses to parasites or allergens, the extensive IgE-dependent activation of mast cells via FcεRI can result in the release of large quantities of biogenic amines that are stored in the cells' cytoplasmic granules as well as the production of lipid mediators and many cytokines; these products together can orchestrate an intense inflammatory response. We now report that activation of mouse mast cells via c-kit, the receptor for the pleiotropic survival/growth factor, stem cell factor (SCF ), can induce the release of IL-6. Upon challenge with SCF, bone marrow-derived cultured mouse mast cells (BMCMCs) released amounts of IL-6 that were greater than 100-fold more than those produced by unstimulated cells, but that were substantially less than those produced in response to IgE and specific antigen. Moreover, BMCMCs released IL-6 upon challenge with concentrations of SCF that resulted in little or no detectable release of tumor necrosis factor-α, leukotriene C4 , histamine, or serotonin. These findings indicate that SCF, a widely expressed protein that is critical for mast cell development and survival, can also regulate the differential release of mast cell mediators.
MAST CELLS ARE widely distributed throughout vascularized tissues and certain epithelia, where they function as major effector cells in IgE-dependent immune responses to parasites and disorders of immediate hypersensitivity.1-9 Recent evidence suggests that mast cells can also contribute importantly, as a component of native immunity, in host resistance to certain bacterial pathogens.9-11 In addition, mast cells have been implicated in the pathogenesis of a diverse group of immunologically nonspecific inflammatory responses.5-8 12
The expression of mast cell function in these settings is thought to reflect, largely if not entirely, the actions of the mediators released by these cells in response to their activation by immunologically specific or nonspecific stimuli.1-12 The most extensively studied stimulus, activation of the cells by aggregation of their high-affinity IgE receptors (FcεRI), eg, by IgE and specific antigen,3,4 results in the release of biogenic amines and other mediators that are stored preformed in the cells' cytoplasmic granules, the generation of newly synthesized products of the cyclooxygenase and/or lipoxygenase pathways of arachidonic acid metabolism, and the secretion of many immunoregulatory and/or proinflammatory cytokines.1-9,12,13 When released together, rapidly and in large quantities, these mast cell-derived mediators can orchestrate intense inflammatory responses.1-9,12 13
However, it has been proposed that expressions of mast cell function distinct from those observed in IgE-dependent responses might be elicited in response to agents that induce a pattern of mast cell mediator release that is qualitatively or quantitatively different from that of cells that have been activated via the FcεRI.5-9,12 In light of the potential ability of mast cell-derived cytokines to influence diverse biologic responses,5-13 the identification of agents that can promote the relatively selective release of mast cell-derived cytokines has been of particular interest.
Notably, only a few actual examples of such agents have yet been reported, and all of these factors induce mast cells to produce interleukin-6 (IL-6). Thus, Leal-Berumen et al14-16 have shown that lipopolysaccharide (LPS),14 prostaglandin E2 ,15 and cholera toxin16 each can stimulate IL-6 secretion, but not detectable histamine release, from highly purified populations of rat peritoneal mast cells. However, there have been no reports showing that endogenous mammalian proteins can promote mast cells to secrete IL-6 in preference to preformed mediators such as histamine or serotonin.
In this report, we show that stem cell factor (SCF ), the major growth factor for mast cell development and survival,17,18 can induce bone marrow-derived cultured mouse mast cells to release IL-6 to a greater extent than tumor necrosis factor-α (TNF-α), leukotriene C4 (LTC4 ), histamine, or serotonin; that the amounts of IL-6 or TNF-α released by these mast cells in response to SCF are substantially less than those that can be released in response to IgE and specific antigen; and that this SCF-induced mast cell cytokine production requires a functionally adequate SCF receptor (c-kit [or SCFR], the product of the c-kit protooncogene).17 18 These findings thus identify SCF as a product that can promote the differential release of mast cell mediators as well as regulate mast cell development and survival.
MATERIALS AND METHODS
Mice.BALB/c and C57BL/6 mice (Charles River Laboratory, Wilmington, MA) and normal WBB6F1-+/+ mice and the congenic genetically mast cell-deficient WBB6F1-KitW/KitW-v (KitW/KitW-v) mice (Jackson Laboratory, Bar Harbor, ME) were purchased at 4 to 6 weeks of age. KitW/KitW-v mice, which contain less than 1.0% the number of dermal mast cells present in the skin of the congenic normal (+/+) mice,19 were locally and selectively repaired of their mast cell deficiency in the left ear (+/+ MC→KitW/KitW-v mice) by the intradermal injection of +/+ bone marrow-derived cultured mouse mast cells (BMCMCs) at least 8 weeks before their use in experiments, as described.12 20-22 All animal care and experimentation was conducted in accord with current National Institutes of Health and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee guidelines.
Mast cells.BMCMCs were obtained by maintaining the femoral BM cells of 4- to 6-week-old BALB/c, WBB6F1-+/+ or WBB6F1-KitW/KitW-v mice in suspension in IL-3–containing conditioned medium, consisting of 10% heat-inactivated fetal calf serum (FCS; Sigma Chemical Co, St Louis, MO), 5 × 10−5 mol/L 2-mercaptoethanol (Sigma), and 2 mmol/L L-glutamine (GIBCO Laboratories, Grand Island, NY) in Dulbecco's modified Eagle's medium (GIBCO Laboratories; complete medium) supplemented with 20% (vol/vol) of either supernatants from Concanavalin A (Con A)-activated spleen cells (for BALB/c BMCMCs23,24 ) or WEHI-3 cell-conditioned medium (for WBB6F1-+/+ or -KitW/KitW-v BMCMCs24,25 ). The cells were resuspended in fresh conditioned medium 1 to 2 times a week. After 4 to 5 weeks, at least 95% of cells that remained in the cultures were identifiable as mast cells, as determined by neutral red or May-Grünwald/Giemsa staining.23-25 Cl.MC/C57.1 cells, a cloned growth factor-independent mouse mast cell line26 of BALB/c origin,27 were maintained in complete medium.26
Activation of mast cells in vivo.WBB6F1-+/+ or -KitW/KitW-v mice were injected intradermally (ID) with 20 μL of recombinant rat SCF (rrSCF; 30 μg/kg/site) into the left ears (L) or vehicle alone (0.9% sterile, pyrogen-free NaCl) into the right (R) ears.22 To assess whether mast cells could influence the expression of responses to rrSCF in the skin of KitW/KitW-v mice, rrSCF was injected into both the L (mast cell-reconstituted) and R (mast cell-deficient) ears of +/+ MC→KitW/KitW-v mice.22 The rrSCF preparations that were injected into the ears of our mice to provide 30 μg of rrSCF/kg/site also provided approximately 13 pg/kg/site of LPS, as determined using the Limulus amebocyte assay (LAL Assay; Whitaker Bioproducts, Inc, Walkersville, MD). Accordingly, in some experiments, we injected mice ID with E Coli bacterial LPS (Sigma) at 13 pg/kg/ear site (in 20 μL of 0.9% sterile, pyrogen-free NaCl).
Ear swelling was measured with a micrometer 2 hours after rrSCF, LPS, or vehicle injection, and swelling was expressed as the increment of thickness (postinjection value minus preinjection baseline value) in units of 10−4 inch.22 Central strips of the ears were obtained after the mice were killed 2 hours after injection and processed into 1-μm Epon-embedded, Giemsa-stained sections for morphometric assessment of mast cell degranulation as extensive (≥50% of the cytoplasmic granules exhibiting fusion, staining alterations, and/or extrusion from the cell), moderate (10% to 50% of the granules exhibiting changes), or none (<10% of granules exhibiting changes).22 The rest of the tissue was stored at −80°C until processing for RNA extraction.
Reverse transcriptase-polymerase chain reaction (RT-PCR) and Southern hybridization.Total RNA was isolated from ear tissue according to the manufacturer's specifications using Ultraspec RNA isolation solution (Biotecx, Houston, TX). Semiquantitative RT-PCR was performed using serial dilution of heparinase-treated RNA with primers specific for mouse IL-6 and G3PDH (Clontech, Palo Alto, CA).25 RT-PCR products were analyzed on 1% agarose gels and then transferred to a nylon membrane and hybridized with a 32P-labeled IL-6 or G3PDH cDNA probe.
Activation of mast cells in vitro.BMCMCs derived from BALB/c, WBB6F1-+/+, or WBB6F1-KitW/KitW-v mice were washed, resuspended at 3 × 106 cells/mL of complete medium, and then stimulated for 2 hours with various concentrations of rrSCF (Escherichia coli-derived recombinant rat SCF164; Amgen Inc, Thousand Oaks, CA; as in Tsai et al24 ) or E Coli bacterial LPS (Sigma). For analysis of the kinetics of IL-6 release in response to challenge with rrSCF, LPS, or IgE and specific antigen, Cl.MC/C57.1 mast cells or BALB/c BMCMCs were stimulated with rrSCF at 50 or 250 ng/mL or LPS at 250 mg/mL or were sensitized for 2 hours at 37°C with ascites fluid containing a mouse monoclonal anti-DNP IgE28 at approximately 8 μg/mL and then washed and resuspended in complete medium containing DNP30-40-HSA (Sigma) at 10 ng/mL. Supernatants were collected at various intervals after stimulation and frozen at −80°C until assayed for cytokines and mediators. Cell pellets were stored at −80°C until processing for RNA isolation or (after 3 cycles of freezing and thawing) assay for IL-6 protein (see below).
Measurement of mediators.IL-6 and TNF-α were assayed using enzyme-linked immunosorbent assay (ELISA) kits (ENDOGEN, Boston, MA). Histamine and LTC4 were assayed using a radioimmunoassay kit (Immunotech Inc, Westbrook, ME) or an enzyme immunoassay kit (Cayman Chemical Co, Ann Arbor, MI), respectively, according to the manufacturers' instructions. Serotonin release was measured by the specific release of [3H]-hydroxytryptamine creatinine sulfate (3H-5HT; NEN, Boston, MA).29
Measurement of IL-6 bioactivity.The IL-6–dependent 7TD1 mouse B-cell hybridoma cell line was maintained in IL-6–containing medium as previously described30 and was deprived of IL-6 for 3 days before experiments. Supernatants collected 2 hours after rrSCF (50 ng/mL) or IgE/DNP-HSA (10 ng/mL) stimulation of BALB/c BMCMCs were diluted and added to 96-well plates containing 2 × 103 7TD1 cells/well. Aliquots of the supernatants were preincubated with 10 μg/mL of a rabbit antimouse IL-6 polyclonal neutralizing antibody (R&D Systems, Minneapolis, MN) for 1 hour before addition to the cells. The plates were incubated at 37°C for 24 hours, 3H-thymidine ([3H]-TdR, 6.7 Ci/mmol/L; NEN) was then added to a final activity of 2.0 μCi/mL for 4 hours, and cultures were then collected onto glass filter strips using a PHD cell harvester and counted by liquid scintillation. rrSCF induced no significant proliferation of 7TD1 cells.
Northern hybridization.Total RNA was prepared according to the manufacturer's specifications using Ultraspec RNA isolation solution (Biotecx); 10 or 15 μg of total RNA was loaded per lane and electrophoresed in 1% agarose-formaldehyde denaturing gel and then transferred onto Zetabind nylon membrane (Cuno, Meriden, CT). RNA blots were hybridized at 42°C for 16 to 18 hours with 106 cpm/mL of 32P-labeled IL-6, TNF-α, or G3PDH cDNA probes, washed at 42°C to a final stringency of 0.2× SSC, then exposed to Kodak XAR-5 films (Eastman Kodak, Rochester, NY) at −80°C. The films were scanned using a densitometer (Molecular Dynamics, Sunnyvale, CA) and the specific signals were quantitated by ImageQuant software (Molecular Dynamics). Relative IL-6 or TNF-α intensities were calculated after normalization with the corresponding G3PDH signals.
Statistical analysis.The results were analyzed for statistical significance by the unpaired Student′s t-test (two-tailed; for the ear swelling assay or differences in mediator release), or the χ2 test (for the extent of mast cell degranulation). All results are expressed as the arithmetic mean ± SEM.
RESULTS AND DISCUSSION
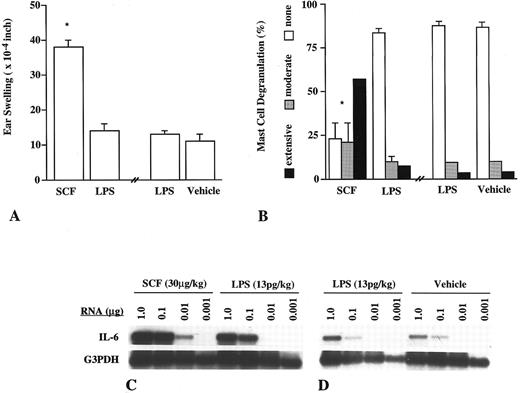
SCF induces mast cell-dependent enhancement of IL-6 mRNA expression in vivo.We first wished to assess whether the SCF-induced, c-kit–dependent mast cell degranulation that can be elicited in mouse skin in vivo22 is associated with evidence of mast cell-dependent cytokine production. We therefore confirmed that intradermal injection of recombinant rat SCF164 (rrSCF ), at 30 μg/kg/site, can induce a significant local tissue swelling response in normal (WBB6F1-+/+) mice but not in the congenic mast cell-deficient WBB6F1-KitW/KitW-v mice (Fig 1A). To demonstrate that this rrSCF-induced tissue swelling is mast cell-dependent, we showed that injection of rrSCF induced a swelling response in ears of KitW/KitW-v mice that had been locally and selectively repaired of their mast cell deficiency, but not in the contralateral, mast cell-deficient, ears of the same mice (Fig 1B). Morphometric analysis of the ears of the mice shown in Fig 1A and B indicated that injection of rrSCF resulted in extensive degranulation of dermal mast cells in the ears of WBB6F1-+/+ mice and in the mast cell-reconstituted ears of WBB6F1-KitW/KitW-v mice (+/+ MC→KitW/KitW-vmice), whereas little mast cell degranulation was observed in the vehicle-injected control ears of WBB6F1-+/+ mice (Fig 1C). At the time interval examined (2 hours), little or no leukocyte recruitment was observed at sites of ID injection of rrSCF or vehicle (data not shown).
Induction of ear swelling (A and B) and mast cell activation (C) by intradermal injection of rrSCF (30 μg/kg/site) or vehicle alone in the ear skin of WBB6F1-KitW/KitW-v mice or KitW/KitW-v mice that had been selectively and locally reconstituted, in the left ear, with BMCMCs derived from the congenic normal +/+ mice (+/+ MC → KitW/KitW-v mice). (A) Ear swelling measured 2 hours after injection of rrSCF or vehicle. *P < .001 versus values for vehicle-injected +/+ mice or SCF-injected KitW/KitW-v mice. (B) Ear swelling determined 2 hours after injection of rrSCF into the left (mast cell-reconstituted) or right (mast cell-deficient) ears of +/+ MC → KitW/KitW-v mice. *P < .005 versus values for contralateral mast cell-deficient ears. (C) Extent of mast cell activation in the ear skin of +/+ mice after injection of SCF or vehicle or in the left (mast cell-reconstituted) SCF-injected ears of +/+ MC → KitW/KitW-v mice. *P < .0001 versus values for the SCF-injected +/+ mice or SCF-injected +/+ MC → KitW/KitW-v mice. The differences between values for SCF-injected +/+ or +/+ MC → KitW/KitW-v mice are not statistically significant (P < .1).
Induction of ear swelling (A and B) and mast cell activation (C) by intradermal injection of rrSCF (30 μg/kg/site) or vehicle alone in the ear skin of WBB6F1-KitW/KitW-v mice or KitW/KitW-v mice that had been selectively and locally reconstituted, in the left ear, with BMCMCs derived from the congenic normal +/+ mice (+/+ MC → KitW/KitW-v mice). (A) Ear swelling measured 2 hours after injection of rrSCF or vehicle. *P < .001 versus values for vehicle-injected +/+ mice or SCF-injected KitW/KitW-v mice. (B) Ear swelling determined 2 hours after injection of rrSCF into the left (mast cell-reconstituted) or right (mast cell-deficient) ears of +/+ MC → KitW/KitW-v mice. *P < .005 versus values for contralateral mast cell-deficient ears. (C) Extent of mast cell activation in the ear skin of +/+ mice after injection of SCF or vehicle or in the left (mast cell-reconstituted) SCF-injected ears of +/+ MC → KitW/KitW-v mice. *P < .0001 versus values for the SCF-injected +/+ mice or SCF-injected +/+ MC → KitW/KitW-v mice. The differences between values for SCF-injected +/+ or +/+ MC → KitW/KitW-v mice are not statistically significant (P < .1).
Preliminary studies with in vitro-derived mouse mast cells indicated that rrSCF can induce a marked increase in the cells' steady state level of IL-6 mRNA, as well as the release of IL-6 protein, at concentrations that produce little or no release of TNF-α. We therefore used RT-PCR to evaluate levels of IL-6 mRNA in serial dilutions of total RNA isolated from the ears of the same mice shown in Fig 1. We found that intradermal injection of rrSCF induced more substantial increases in levels of IL-6 mRNA in the skin of WBB6F1-+/+ normal mice (Fig 2A) or WBB6F1-KitW/KitW-v mice that had undergone selective local repair of their dermal mast cell deficiency (Fig 2C), than in the skin of mast cell-deficient WBB6F1-KitW/KitW-v mice (Fig 2B). However, injection of rrSCF did not detectably increase local levels of TNF-α mRNA (not shown).
Detection of IL-6 mRNA by RT-PCR in serial dilutions of RNA from the ear skin of the same WBB6F1-+/+ (A), WBB6F1-KitW/KitW-v (B), and +/+ MC→KitW/KitW-v (C) mice that are shown in Fig 1. Note that +/+ MC → KitW/KitW-v mice (C) were injected with SCF in both the L (mast cell-reconstituted) and R (mast cell-deficient) ears.
Detection of IL-6 mRNA by RT-PCR in serial dilutions of RNA from the ear skin of the same WBB6F1-+/+ (A), WBB6F1-KitW/KitW-v (B), and +/+ MC→KitW/KitW-v (C) mice that are shown in Fig 1. Note that +/+ MC → KitW/KitW-v mice (C) were injected with SCF in both the L (mast cell-reconstituted) and R (mast cell-deficient) ears.
To asses the possibility that our results may have been influenced by the small amount of LPS in our rrSCF preparations, we injected some C57BL/6 mice in the left ears with rrSCF (30 μg/kg/site) and in the contralateral (right) ears with the amount of LPS (13 pg/kg/site) that was present in the solution of rrSCF that had been injected into the left ears. Another group of C57BL/6 mice were injected in the left ears with LPS (13 pg/kg/site) and in the right ears with vehicle (0.9% NaCl) alone. As assessed at 2 hours after challenge, rrSCF induced tissue swelling responses (Fig 3A) and levels of mast cell degranulation (Fig 3B), which were similar to those observed in identically challenged WBB6F1-+/+ or +/+ MC→KitW/KitW-v mice (Fig 1A through C), whereas the sites injected with LPS exhibited responses that were statistically indistinguishable from those in sites that had been injected with vehicle alone (Fig 3A and B). In accord with these results, our RT-PCR analysis indicated that rrSCF induced a more substantial increase in cutaneous levels of IL-6 mRNA than did LPS (Fig 3C). Indeed, in a separate experiment, the IL-6 mRNA signal in ears that had been injected with LPS was similar to that obtained in the contralateral, vehicle-injected ears (Fig 3D).
Induction of ear swelling (A), mast cell activation (B), and IL-6 mRNA expression (C and D) by ID injection of rrSCF (30 μg/kg/site), LPS (13 pg/kg/site), or vehicle alone in the ear skin of C57BL/6 mice (n = 5 to 10 per treatment group). (A) Ear swelling measured 2 hours after injection of rrSCF into the left ears or LPS alone (in the same amount as was present in the rrSCF solution) into the right ears or 2 hours after injection of LPS into the left ears and vehicle alone into the right ears. *P ≤ .001 versus values for LPS-injected or vehicle-injected ears. (B) Extent of mast cell activation in the rrSCF-, LPS-, or vehicle-injected ear skin. *P < .0001 versus LPS- or vehicle-injected ears. The differences between values for LPS- or vehicle-injected ears (in A or B) are not statistically significant (P < .1).
Induction of ear swelling (A), mast cell activation (B), and IL-6 mRNA expression (C and D) by ID injection of rrSCF (30 μg/kg/site), LPS (13 pg/kg/site), or vehicle alone in the ear skin of C57BL/6 mice (n = 5 to 10 per treatment group). (A) Ear swelling measured 2 hours after injection of rrSCF into the left ears or LPS alone (in the same amount as was present in the rrSCF solution) into the right ears or 2 hours after injection of LPS into the left ears and vehicle alone into the right ears. *P ≤ .001 versus values for LPS-injected or vehicle-injected ears. (B) Extent of mast cell activation in the rrSCF-, LPS-, or vehicle-injected ear skin. *P < .0001 versus LPS- or vehicle-injected ears. The differences between values for LPS- or vehicle-injected ears (in A or B) are not statistically significant (P < .1).
These findings indicate that LPS contamination of our rrSCF preparations accounted for neither the ability of these preparations to induce extensive mast cell degranulation or tissue swelling nor their ability to induce local upregulation of tissue levels of IL-6 mRNA. Our results instead suggest that rrSCF, and perhaps other agents, may be able to induce increased local levels of IL-6 mRNA in proportion to their ability to promote cutaneous mast cell degranulation. In accord with this hypothesis, we found that rrSCF that had been boiled for 20 minutes, when injected at 30 μg/kg/site, also induced extensive mast cell degranulation, significant tissue swelling, and increased levels of IL-6 mRNA (as detected by RT-PCR), whereas a low dose of unboiled rrSCF (0.3 μg/kg/site) induced neither significant mast cell degranulation, increased tissue swelling, nor detectably enhanced levels of IL-6 mRNA (data not shown).
rrSCF induces mast cells to release IL-6 in vitro.The results shown in Figs 1-3 indicate that rrSCF can induce elevations in levels of IL-6 mRNA in the skin of mice and that such rrSCF-induced increases in IL-6 mRNA levels are both mast cell-dependent and associated with morphologic evidence of mast cell degranulation. However, these findings do not prove that rrSCF can directly induce the production of IL-6 protein from mast cells. For example, even though little or no leukocyte recruitment occurred within 2 hours of ID injection of rrSCF, we cannot exclude the possibility that mediators released from the mast cells that degranulated in response to rrSCF directly or indirectly enhanced levels of IL-6 mRNA in other cell types that are normally resident in the skin. This in vivo system also cannot be readily used to quantify the amounts of IL-6 protein (or other mediators) that might be produced in this context.
To assess whether rrSCF can directly induce mast cells to release IL-6 protein and to determine the extent to which such cytokine release is associated with degranulation and release of preformed or lipid mediators, we analyzed the responses of BMCMCs in vitro. In BALB/c BMCMCs, rrSCF induced markedly increased steady-state levels of IL-6 mRNA (not shown) as well as the dose-dependent release of IL-6 protein (Fig 4A). In confirmation of a previous report,31 rrSCF also induced BMCMCs to release LTC4 (Fig 4B), albeit at higher concentrations than those required to induce IL-6 production (Fig 4A) and in smaller amounts than those produced in response to IgE and specific antigen (Fig 4, legend). Analysis of the same BMCMCs shown in Fig 4A and B showed that rrSCF challenge resulted in only low-level release of two preformed mediators, histamine (Fig 4A), or serotonin (5-hydroxytryptamine [5-HT]; Fig 4B).
Release of (A) IL-6 and histamine and (B) [3H]-5HT and LTC4 from BALB/c BMCMCs that had been stimulated with various concentrations of rrSCF. Release of IL-6, histamine, and LTC4 was measured at 2 hours (from the same aliquots of cells), whereas [3H]-5HT release was measured at 10 minutes (in separate aliquots of the same cell preparations, but which had been incubated before challenge with [3H]-5HT; see the Materials and Methods). #P < .05 or *P < .001 versus values for unstimulated control cells. After sensitization with anti-DNP IgE and challenge with DNP30-40-HSA at 10 ng/mL (see the Materials and Methods), the same population of BMCMCs shown in (A) and (B) released 5,510 ± 270 pg IL-6 and 165 ± 10 ng LTC4 per 106 cells (at 2 hours) and gave 20% ± 2.3% specific release of histamine (at 2 hours) and 38% ± 0.7% specific release of [3H]-5HT (at 10 minutes). Data are shown as mean ± SEM (n = 4 to 5 per point) but, in many instances, error bars are too small to be seen in the figures.
Release of (A) IL-6 and histamine and (B) [3H]-5HT and LTC4 from BALB/c BMCMCs that had been stimulated with various concentrations of rrSCF. Release of IL-6, histamine, and LTC4 was measured at 2 hours (from the same aliquots of cells), whereas [3H]-5HT release was measured at 10 minutes (in separate aliquots of the same cell preparations, but which had been incubated before challenge with [3H]-5HT; see the Materials and Methods). #P < .05 or *P < .001 versus values for unstimulated control cells. After sensitization with anti-DNP IgE and challenge with DNP30-40-HSA at 10 ng/mL (see the Materials and Methods), the same population of BMCMCs shown in (A) and (B) released 5,510 ± 270 pg IL-6 and 165 ± 10 ng LTC4 per 106 cells (at 2 hours) and gave 20% ± 2.3% specific release of histamine (at 2 hours) and 38% ± 0.7% specific release of [3H]-5HT (at 10 minutes). Data are shown as mean ± SEM (n = 4 to 5 per point) but, in many instances, error bars are too small to be seen in the figures.
In all, we performed 12 experiments with BALB/c BMCMCs and 3 experiments with an IL-3–independent mouse mast cell clone of BALB/c origin (Cl.MC/C57.1) to assess the ability of rrSCF, in concentrations of up to 250 or 1,250 ng/mL, to induce the release of IL-6 as opposed to histamine or 5-HT. In each of these experiments, rrSCF either failed to increase significantly the release of histamine or 5-HT or did so only modestly and at the higher concentrations of rrSCF tested. However, the amounts of IL-6 release detected at 2 hours after rrSCF challenge varied in different experiments. For example, in 9 experiments using BALB/c BMCMCs that had been derived in medium that contained supernatants of Con A-stimulated spleen cells as a source of IL-3, rrSCF (at 250 ng/mL) induced the production of 19 ± 1 to 841 ± 30 pg of IL-6/106 cells (mean ± SEM = 297 ± 88 pg/106 cells) at 2 hours after challenge.
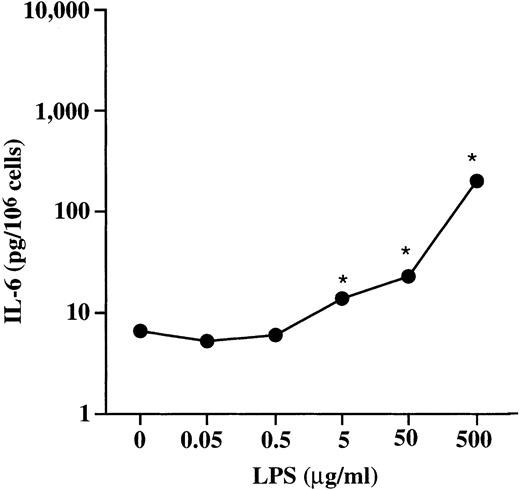
When administered in the presence of FCS, bacterial LPS can induce IL-6 release from rat peritoneal mast cells in the absence of detectable histamine release.14 According to analysis of our reagents with the Limulus amebocyte assay (LAL Assay; Whitaker Bioproducts, Inc), none of the mast cells stimulated with rrSCF in vitro could have been exposed to greater than 10 pg of LPS/mL. Nevertheless, in 2 experiments, we challenged BMCMCs for 6 and/or 2 hours with LPS (in medium containing 10% FCS) at a range of doses from 50 ng to 500 μg/mL. Incubation of BMCMCs with LPS at 50 μg/mL for 2 hours induced release of low levels of IL-6 (22.9 ± 0.8 pg/106 cells; Fig 5) but no specific release of 5-HT (not shown); no increased release of IL-6 was detected from cells incubated with LPS at ≤0.5 μg/mL. When aliquots of these same BMCMC populations were challenged with rrSCF at 50 ng/mL for 2 hours, they released 253 ± 9 pg of IL-6/106 cells. Thus, on a weight basis, rrSCF in this experiment was at least 1,000 times more potent than LPS as an inducer of IL-6 release from BMCMCs.
Dose-dependent release of IL-6 in BALB/c BMCMCs stimulated for 2 hours with LPS. *P < .001 versus values for unstimulated control cells (n = 4 to 5 per point).
Dose-dependent release of IL-6 in BALB/c BMCMCs stimulated for 2 hours with LPS. *P < .001 versus values for unstimulated control cells (n = 4 to 5 per point).
Comparison of the kinetics and magnitude of mast cell IL-6 release in response to rrSCF, LPS, or IgE and specific antigen.To assess the kinetics of IL-6 production in response to different types of mast cell stimulation and to evaluate the extent to which the IL-6 produced in response to various types of challenge was cell-associated, we evaluated BALB/c BMCMCs that had been derived in complete medium supplemented with supernatants from Con A-stimulated spleen cells. BMCMCs stimulated with rrSCF (250 ng/mL) or LPS (250 μg/mL) released much less IL-6 than did cells activated via the FcεRI (Fig 6A). However, for all three stimuli, substantial IL-6 production was detectable in the cell supernatants at 2 hours and near maximal levels were present by 6 hours (Fig 6A). No IL-6 was detectable in the cell pellets of the control BMCMCs that had been incubated in vehicle alone (Fig 6B), indicating that little or no preformed IL-6 was present in these cells. However, cell-associated IL-6 was detectable in BMCMCs after their activation by rrSCF, LPS, or IgE and specific antigen (Fig 6B). At 2 hours after addition of the stimuli, this cell-associated IL-6 represented approximately 33% of total IL-6 production in BMCMCs that had been stimulated with rrSCF, approximately 28% in cells that had been stimulated with LPS, and approximately 15% in cells that had been challenged with IgE and antigen (Fig 6C). The proportion of total IL-6 production that was present in the cell pellet fraction had declined to 5.5% or less by 6 hours after challenge and decreased to even lower proportions of the total IL-6 at later intervals (Fig 6B and C).
Kinetics of IL-6 production, as assessed by measurements of (A) supernatant- or (B) cell-pellet-associated IL-6, or (C) total (supernatant-plus cell-pellet-associated) IL-6 in BALB/c BMCMCs after stimulation with rrSCF (250 ng/mL; ▪), LPS (250 μg/mL; ▧), IgE and DNP-HSA (10 ng/mL; ▧), or vehicle alone (□). *P < .001 versus corresponding values for vehicle-challenged cells (n = 3 to 5 per point). Please note that IL-6 associated with cell pellets from vehicle-challenged cells was below the limit of detection of the ELISA assay (ie, < 1 pg/106 cells).
Kinetics of IL-6 production, as assessed by measurements of (A) supernatant- or (B) cell-pellet-associated IL-6, or (C) total (supernatant-plus cell-pellet-associated) IL-6 in BALB/c BMCMCs after stimulation with rrSCF (250 ng/mL; ▪), LPS (250 μg/mL; ▧), IgE and DNP-HSA (10 ng/mL; ▧), or vehicle alone (□). *P < .001 versus corresponding values for vehicle-challenged cells (n = 3 to 5 per point). Please note that IL-6 associated with cell pellets from vehicle-challenged cells was below the limit of detection of the ELISA assay (ie, < 1 pg/106 cells).
We also found that, for all three types of stimulation, total IL-6 measured 24 hours after challenge was actually less than that measured at the peak of the response for that type of stimulation (42% less for rrSCF-challenged cells at 24 v 6 hours [P < .2), 10% less for LPS-stimulated cells at 24 v 12 hours [P < .3], and 20% less for IgE and specific antigen-challenged cells at 24 v 6 hours [P < .03]). These results might be due to any of a number of factors. For example, some of the released IL-6 might have been degraded over time (eg, by mast cell-derived proteases). In addition, we removed the BMCMCs from IL-3–containing conditioned medium before challenging them for measurement of IL-6 production. At late intervals after their removal from IL-3–containing medium, such BMCMC populations undergo apoptosis.32 33
In light of the finding of substantial release of IL-6 as early as 2 hours after mast cell activation, we performed additional experiments to examine the kinetics of IL-6 release at early intervals after challenge with rrSCF or IgE and specific antigen in both BALB/c BMCMCs and a growth factor-independent cloned mast cell line of BALB/c origin, Cl.MC/C57.1 (Fig 7). The kinetics of rrSCF-induced or FcεRI-dependent release of IL-6 were similar in both BMCMCs and Cl.MC/C57.1 cells, with release significantly above that from vehicle-treated control cells detectable as early as 30 minutes after challenge and with levels of IL-6 in the cells' supernatants increasing steadily thereafter (Fig 7).
Kinetics of IL-6 production in (A) BALB/c BMCMCs or (B) Cl.MC/C57.1 cells that had been stimulated with rrSCF (50 ng/mL; ▪) or IgE and DNP-HSA (10 ng/mL; ▧; n = 4 to 5 per point). *P < .001 versus values for unstimulated control cells (□; n = 4 to 5 per point); †P < .0001 versus values for IgE/DNP-HSA–stimulated cells.
Kinetics of IL-6 production in (A) BALB/c BMCMCs or (B) Cl.MC/C57.1 cells that had been stimulated with rrSCF (50 ng/mL; ▪) or IgE and DNP-HSA (10 ng/mL; ▧; n = 4 to 5 per point). *P < .001 versus values for unstimulated control cells (□; n = 4 to 5 per point); †P < .0001 versus values for IgE/DNP-HSA–stimulated cells.
However, as in our other experiments (eg, Fig 6), the magnitude of cytokine release with these two types of signaling was very different (note that IL-6 production is shown on a log10 scale). In 5 or 8 independent experiments, the amount of IL-6 released from BMCMCs 2 hours after stimulation with rrSCF at 50 or 250 ng/mL was 37- ± 13-fold (mean ± SEM, n = 8) or 166- ± 85-fold (n = 5) higher than baseline release from control cells, but only 2.6% ± 1.0% or 7.0% ± 3.0% that released by cells sensitized with IgE anti-DNP antibody and then stimulated for 2 hours with DNP-HSA at 10 ng/mL. In growth factor-independent cloned mast cells (Cl.MC/C57.1 cells; Fig 7B), both baseline and rrSCF-induced levels of IL-6 production (and also levels of IL-6 production in response to IgE and specific antigen) were higher than those observed in BMCMCs, but the extent to which rrSCF stimulation increased levels of IL-6 release over those observed in vehicle-treated cells was less in Cl.MC/C57.1 cells than in BMCMCs (compare Fig 7A and B). Moreover, the proportional increases in levels of IL-6 secretion over time, in response to either rrSCF or IgE and specific antigen, occurred somewhat more slowly in Cl.MC/C57.1 cells than in BMCMCs. Nevertheless, as in BMCMCs, levels of secretion of IL-6 by Cl.MC/C57.1 cells in response to rrSCF were much lower than those observed in cells activated via the FcεRI (Fig 7B).
Thus, although rrSCF challenge markedly increased the release of IL-6 compared with that observed in unstimulated control mast cells, the amount of IL-6 produced by mast cells in response to rrSCF generally represented only a small fraction of that released by mast cells in response to challenge with IgE and specific antigen.
To determine whether the relatively small amounts of IL-6 that can be released from mast cells by rrSCF are sufficient to express biologic activity, we assessed the ability of supernatants derived from rrSCF-challenged mast cells to induce proliferation in the IL-6–dependent B-cell hybridoma cell line, 7TD1. As shown in Fig 8, the amounts of IL-6 detected by ELISA in supernatants of BMCMCs stimulated with rrSCF at 50 ng/mL were biologically active. Moreover, virtually all of this bioactivity was abolished by a neutralizing antibody against mouse IL-6.
IL-6 bioactivity, measured by [3H]-TdR uptake in 7TD1 cells, in supernatants from BALB/c BMCMCs that had been stimulated for 2 hours with rrSCF or IgE/DNP; supernatants were tested with (+) or without (−) prior incubation with an anti–IL-6 antibody. The mean baseline value for 7TD1 cells incubated without a source of IL-6 (898 cpm) was subtracted before showing cpm data as mean ± SEM (n = 3). *P < .05, **P < .005 versus corresponding values without anti–IL-6 antibody; †, not significant (P < .05) versus baseline values (898 ± 114 cpm) for negative control 7TD1 cells incubated without IL-6.
IL-6 bioactivity, measured by [3H]-TdR uptake in 7TD1 cells, in supernatants from BALB/c BMCMCs that had been stimulated for 2 hours with rrSCF or IgE/DNP; supernatants were tested with (+) or without (−) prior incubation with an anti–IL-6 antibody. The mean baseline value for 7TD1 cells incubated without a source of IL-6 (898 cpm) was subtracted before showing cpm data as mean ± SEM (n = 3). *P < .05, **P < .005 versus corresponding values without anti–IL-6 antibody; †, not significant (P < .05) versus baseline values (898 ± 114 cpm) for negative control 7TD1 cells incubated without IL-6.
rrSCF-dependent release of mast cell IL-6 Is c-kit–dependent.To evaluate the mechanism of rrSCF-induced cytokine release from BMCMCs, we analyzed BMCMCs derived from WBB6F1-KitW/KitW-v mice and the congenic normal (WBB6F1-+/+) mice. WBB6F1-KitW/KitW-v BMCMCs express normal amounts of c-kit receptors (SCFR) encoded by the KitW-v allele; these receptors have a wild-type extracellular ligand-binding domain, but have a Thr660 → Met substitution in the tyrosine kinase domain that results in markedly diminished ligand-dependent c-kit autophosphorylation and signal transduction.34 In two experiments, one of which is shown in Fig 9, rrSCF (in concentrations of up to 250 ng/mL) induced little or no specific release of 5-HT from BMCMCs derived from either WBB6F1-+/+ or WBB6F1-KitW/KitW-v mice (Fig 9A). By contrast, both types of BMCMCs released 5-HT in response to FcεRI-dependent challenge (Fig 9A). However, WBB6F1-+/+ BMCMCs expressed increased amounts of IL-6 (Fig 9C and D) and, to a lesser extent, TNF-α (Fig 9F and G) mRNA in response to rrSCF and also released the corresponding proteins (Fig 9B and E), whereas, by comparison, WBB6F1-KitW/KitW-v BMCMCs exhibited very attenuated responses (Fig 9B through G). WBB6F1-KitW/KitW-v BMCMCs did not have a global defect in receptor-dependent enhancement of cytokine production, in that WBB6F1-KitW/KitW-v or -+/+ BMCMCs released very similar amounts of IL-6 (Fig 9B) or TNF-α (Fig 9E) in response to FcεRI-dependent activation.
Release of (A) 3H-serotonin, (B) IL-6, and (E) TNF-α (all shown as mean ± SEM, n = 4 to 5 per point) or expression of mRNA for IL-6 (C and D) or TNF-α (F and G) by rrSCF or IgE/DNP-HSA-stimulated BMCMCs derived from WBB6F1-KitW/KitW-v mice or the congenic +/+ mice. (A) Release of serotonin (assessed as percentage of specific release of [3H]-5HT) at 10 minutes after challenge. (B) IL-6 production 2 hours after challenge. (C) Northern blot of IL-6 mRNA induction 2 hours after stimulation. (D) Densitometric analysis of IL-6 mRNA expression, presented as the ratio of density of IL-6 mRNA and G3PDH mRNA in the same specimens shown in (C). (E) TNF-α production 2 hours after challenge. (F ) Northern blot of TNF-α mRNA induction 2 hours after stimulation. (G) Densitometric analysis of TNF-α mRNA expression (ratio of density of TNF-α v G3PDH mRNA) in the same specimens shown in (F ). †P < .001 versus values for KitW/KitW-v BMCMCs.
Release of (A) 3H-serotonin, (B) IL-6, and (E) TNF-α (all shown as mean ± SEM, n = 4 to 5 per point) or expression of mRNA for IL-6 (C and D) or TNF-α (F and G) by rrSCF or IgE/DNP-HSA-stimulated BMCMCs derived from WBB6F1-KitW/KitW-v mice or the congenic +/+ mice. (A) Release of serotonin (assessed as percentage of specific release of [3H]-5HT) at 10 minutes after challenge. (B) IL-6 production 2 hours after challenge. (C) Northern blot of IL-6 mRNA induction 2 hours after stimulation. (D) Densitometric analysis of IL-6 mRNA expression, presented as the ratio of density of IL-6 mRNA and G3PDH mRNA in the same specimens shown in (C). (E) TNF-α production 2 hours after challenge. (F ) Northern blot of TNF-α mRNA induction 2 hours after stimulation. (G) Densitometric analysis of TNF-α mRNA expression (ratio of density of TNF-α v G3PDH mRNA) in the same specimens shown in (F ). †P < .001 versus values for KitW/KitW-v BMCMCs.
Conclusions.We have found that rrSCF can promote IL-6 release from mouse mast cells via a cognate interaction with c-kit (the SCFR), even at concentrations that produce little or no detectable release of LTC4 or biogenic amines. rrSCF can also induce the c-kit–dependent release of TNF-α, but this was detectable only at the higher concentrations of rrSCF tested. Moreover, rrSCF can induce IL-6 release in both primary populations of mouse BMCMCs and in Cl.MC/C57.1 mouse mast cells, a cloned cell line devoid of even small numbers of contaminating cell types. These findings add SCF, the major endogenous regulator of murine and human mast cell survival and development,17,18,35 to the relatively short list of agents that can induce the differential release of mast cell cytokines. Leal-Berumen et al14-16 have reported that LPS,14 prostaglandin E2 ,15 and cholera toxin16 can induce IL-6 secretion in the absence of detectable histamine release in highly purified preparations of rat peritoneal mast cells.
We are not aware of reports of other endogenous mammalian proteins that can induce mast cells to release IL-6 preferentially with respect to preformed mediators such as histamine or 5-HT. However, after the submission of this manuscript, Lu-Kuo et al36 reported that high levels of IL-6 (∼9 ng/106 cells) could be released from IL-3–derived mouse BMCMCs that had been stimulated for 7 to 24 hours with a combination of SCF (50 ng/mL), IL-10 (20 U/mL), and IL-1β (5 ng/mL). Lu-Kuo et al36 also noted that substantially less IL-6 (∼0.2 ng/106 BMCMCs) was released by such cells in response to SCF and IL-10 without IL-1β and that negligible amounts of IL-6 (data not shown) were released in response to challenge with each of these three cytokines when they were tested individually. However, that study did not report the amounts of IL-6 that were released by mast cells challenged with SCF alone or the extent to which such IL-6 secretion was associated with the release of preformed or lipid mediators.
Similarly, Hültner and Moeller37 reported that a growth factor-dependent mouse mast cell line and a growth factor-independent malignant mouse mast cell line could produce IL-6 in response to IL-9 and that the growth factor-dependent cell line also secreted IL-6, albeit in smaller amounts, in response to IL-4. However, the effects of IL-9 (or IL-4) on the release of other mediators from these mast cell lines were not reported. Nevertheless, the findings of Hültner and Moeller,37 when taken together with those of Lu-Kuo et al36 and our results, raise the possibility that the differential, low-level release of IL-6 may represent part of the response of mast cells to many growth factors that can influence the cells' development, survival, or phenotype.
In BMCMCs and Cl.MC/C57.1 cells, rrSCF induced significant release of IL-6 at concentrations that resulted in little or no release of histamine or serotonin. However, it may well be that the extent to which mast cells can exhibit differential mediator release in response to c-kit–dependent activation (like many other aspects of mast cell phenotype) is developmentally and/or microenvironmentally regulated. Mouse BMCMCs are immature, lineage-committed mast cells with some similarities to mucosal mast cells.1,17,23 Whereas rrSCF induced relatively low levels of degranulation and release of biogenic amines in our BMCMC populations, recombinant SCF can promote more extensive degranulation and/or release of biogenic amines from certain mature mast cells, including human38 or mouse (Fig 1C and Wershil et al22 ) dermal mast cells.
Accordingly, it will be of interest to assess the ability of SCF to induce IL-6 production in various distinct mast cell populations and to investigate the mechanisms (such as the presence or absence of IL-4, IL-9, IL-10, IL-1β, or other cytokines36,37 ) that might determine the extent to which SCF-induced IL-6 production in these cells is associated with the release of other mediators, including the cytoplasmic granule-associated preformed products, the lipid mediators, and additional cytokines. In this context, it should be noted that the expression of IL-6 in mast cells may be regulated differently than that of other cytokines. For example, we previously showed that phorbol myristate acetate can induce increased levels of IL-6 mRNA in mouse mast cell lines under conditions that do not result in corresponding changes in the mRNA of several other cytokines,39 and we found in this study that rrSCF can induce BMCMCs to release IL-6 at concentrations that promote little or no detectable release of TNF-α.
Although the in vivo relevance of our findings remain to be determined, the observation that SCF can induce mast cells to release low levels of IL-6 and, to a lesser extent, TNF-α nevertheless has several potentially important implications. Certain reactions to pathogenic bacteria in mice are associated with marked increases in the local production of SCF.40 Moreover, mast cell-deficient WBB6F1-KitW/KitW-v mice exhibit increased morbidity and mortality in several models of bacterial infection,10,11,40 at least in part because of their lack of mast cell-derived cytokines.10,11 When taken together with these observations, our results suggest that SCF-induced mast cell cytokine production may contribute to the expression of host responses to bacteria and perhaps other pathogens. Our findings also raise the possibility that the clinical use of recombinant human SCF41 may result in effects on mast cell cytokine production and function, even in those subjects who do not exhibit evidence of widespread SCF-dependent mast cell degranulation and histamine release.35 Moreover, in light of the findings of Hültner and Moeller37 and Lu-Kuo et al,36 it seems likely that the magnitude and nature of the effects of SCF on mast cell production of IL-6 and other mediators may be significantly modulated by the local levels of other cytokines, whether in the context of host responses to pathogens or during the therapeutic use of rrSCF.
Our observations also offer a new perspective about the potential physiologic functions of mast cells. Tissue mast cells that have not been extensively activated, eg, by IgE and specific antigen, are often regarded as resting or functionally quiescent. However, an alternative hypothesis is that, under physiologic conditions, mast cells can release small amounts of some of the same mediators that they produce in large quantities during pathologic or immunologic responses. The findings presented here identify the interaction between c-kit and its ligand, which is already regarded as critical for mast cell development, survival, and proliferation,17,18,35 as a candidate mechanism to regulate the physiologic, relatively low-level production of IL-6 and perhaps other mediators (eg, TNF-α) by tissue mast cells. Although IL-6 can mediate a broad spectrum of biologic functions,42,43 examples of potential consequences of c-kit–dependent IL-6 production by mast cells include autocrine effects on mast cell development44 as well as diverse paracrine effects on the development or function of additional cell types, such as hematopoietic or lymphoid cells42,43 and vascular endothelial cells.45
NOTE ADDED IN PROOF
After stimulation for 6 hours with rrSCF (50 ng/mL), IL-10 (40 ng/mL), and IL-1β (5 ng/mL) (as in Luo-Kuo et al36 ), BALB/c BMCMCs derived in either ConA-activated spleen cell- or WEHI-3 cell-conditioned medium released 14.1 ± 0.7 (n = 5) or 7.8 ± 0.8 (n = 3) ng of IL-6/106 cells, amounts that were much greater (by ∼57- to 228-fold) than those produced by aliquots of the same BMCMCs in response to rrSCF (50 ng/mL) alone (P ≤ .001); however, neither SCF nor SCF + IL-10 + IL-1β induced substantial specific release of 3H-5HT (≤2.0% ± 1.1% at 10 minutes).
ACKNOWLEDGMENT
We thank Mary Miyamoto, Zhen-sheng Wang, and Sue Fish for technical assistance; Fu-Tong Liu and David H. Katz for providing H1 DNP-ε-26 hybridoma cells; K.E. Langley of Amgen Inc for rrSCF164; and B.K. Wershil and J.-P. Kinet for helpful discussions.
E.G. and M.T. are co-first authors.
Supported by US Public Health Service Grants No. CA/AI-72074, AI/GM-23990, and AI-31982 (to S.J.G.); by Amgen Inc; and by the Beth Israel Hospital Pathology Foundation. S.J.G. has performed research funded by Amgen Inc and consults for Amgen Inc under terms that are in accord with Beth Israel Deaconess Medical Center and Harvard Medical School conflict-of-interest guidelines.
Address reprint requests to Stephen J. Galli, MD, Division of Experimental Pathology, Department of Pathology — Research North, Room 227, Beth Israel Deaconess Medical Center-East Campus, 330 Brookline Ave, Boston, MA 02215.



![Fig. 4. Release of (A) IL-6 and histamine and (B) [3H]-5HT and LTC4 from BALB/c BMCMCs that had been stimulated with various concentrations of rrSCF. Release of IL-6, histamine, and LTC4 was measured at 2 hours (from the same aliquots of cells), whereas [3H]-5HT release was measured at 10 minutes (in separate aliquots of the same cell preparations, but which had been incubated before challenge with [3H]-5HT; see the Materials and Methods). #P < .05 or *P < .001 versus values for unstimulated control cells. After sensitization with anti-DNP IgE and challenge with DNP30-40-HSA at 10 ng/mL (see the Materials and Methods), the same population of BMCMCs shown in (A) and (B) released 5,510 ± 270 pg IL-6 and 165 ± 10 ng LTC4 per 106 cells (at 2 hours) and gave 20% ± 2.3% specific release of histamine (at 2 hours) and 38% ± 0.7% specific release of [3H]-5HT (at 10 minutes). Data are shown as mean ± SEM (n = 4 to 5 per point) but, in many instances, error bars are too small to be seen in the figures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2654/4/m_bl_0057f4.jpeg?Expires=1767743139&Signature=RvQxTJcxh1GNDabmt9m9aeDprQUMpSRk0eTwtYl45cW~0Q7UBt4sjxXfp5FysHzmF6--W1YOD54WUae792o0AcDAsIJrVuAYaQvnhhxDGNWFdKMbjClHw1I9MTkujcBT~U1ceaLYkK-8KKRSp6L9AYxIpAa6JrUK5Zc9ypzq46vTrMt4XXQNHPPKo9T-8bup2xDBZwVljmiZ7bOkcV~uV518Ip~vQ3udAAOO62RLWU4kCFNIsjwdUkd7FNybRjKTDZF10v3pDfCmng6yZ7qgWny6dZ5xnqnkEg1ha-PGeaQqn7hcQ8lPfnla4PG6luBKNlYUv~rs85nPvyoC7XnBbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 8. IL-6 bioactivity, measured by [3H]-TdR uptake in 7TD1 cells, in supernatants from BALB/c BMCMCs that had been stimulated for 2 hours with rrSCF or IgE/DNP; supernatants were tested with (+) or without (−) prior incubation with an anti–IL-6 antibody. The mean baseline value for 7TD1 cells incubated without a source of IL-6 (898 cpm) was subtracted before showing cpm data as mean ± SEM (n = 3). *P < .05, **P < .005 versus corresponding values without anti–IL-6 antibody; †, not significant (P < .05) versus baseline values (898 ± 114 cpm) for negative control 7TD1 cells incubated without IL-6.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2654/4/m_bl_0057f8.jpeg?Expires=1767743139&Signature=CijcxFvoTwz99nFEuaJZnl4IbzhxUfpbErWbLvZMH~9qSXEKjI9bzNI2wXNCGB54~VMVaQrzzVw7N-njRdEUktKUn5~J5UWaPKW7vfBUaJYW4Dpr3uNvrRCVovsDxz1Fx1e~tpLIUzLlLs81LG~CZ~bo-leqwvHgsDXd4KRKUAcOtxKfC1e0uSnL6ehjgTpxrGpanxPf-41R0rdb4vjQWlEZggpX1wrLcX3u18PzEwxSm7XiC4lNi-59Apka2CjaGMqxJoSv0w5vH0uvtt6s95~hNAPtdKM6CkJSv5VSUWDMm6AlpNkWpWFHyEkQqjQTZ60ERt1EhjIhfviJIdyUjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Release of (A) 3H-serotonin, (B) IL-6, and (E) TNF-α (all shown as mean ± SEM, n = 4 to 5 per point) or expression of mRNA for IL-6 (C and D) or TNF-α (F and G) by rrSCF or IgE/DNP-HSA-stimulated BMCMCs derived from WBB6F1-KitW/KitW-v mice or the congenic +/+ mice. (A) Release of serotonin (assessed as percentage of specific release of [3H]-5HT) at 10 minutes after challenge. (B) IL-6 production 2 hours after challenge. (C) Northern blot of IL-6 mRNA induction 2 hours after stimulation. (D) Densitometric analysis of IL-6 mRNA expression, presented as the ratio of density of IL-6 mRNA and G3PDH mRNA in the same specimens shown in (C). (E) TNF-α production 2 hours after challenge. (F ) Northern blot of TNF-α mRNA induction 2 hours after stimulation. (G) Densitometric analysis of TNF-α mRNA expression (ratio of density of TNF-α v G3PDH mRNA) in the same specimens shown in (F ). †P < .001 versus values for KitW/KitW-v BMCMCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2654/4/m_bl_0057f9.jpeg?Expires=1767743139&Signature=ATEuPjEhPjQNzg95y08ryNatqFUjCm3kroFfkbsV0-F1vj9AQT~MtC9yWxOJH~kTqshQ~am7sea-1nggnIHqHtcJDBKxU8JZhGlcKYS13O7KRJanarm0e5FqykDfRZbbGkSNDDGmCsSuMVaURAtuClOHoGsodjqyjNAZ6nNHGGjgylRF0GNKJto42zPF7vVht2M5YK658ZNpGh3XMcTC35PSaR9YzFn7V6xN2s8H2SqIu0vmr28ObO0nH~POHQD-f2NbZ83tWgqt1WEF~BhrPdMzIgZKUsUpYUX1vNQMOfFcBvEZjcfDUz5cDPv-nBb4xVe4Vgxf4Dudp7uEZge1dg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal