Abstract
The use of umbilical cord blood as a source of marrow repopulating cells for the treatment of pediatric malignancies has been established. Given the general availability, the ease of procurement, and progenitor content, cord blood is an attractive alternative to bone marrow or growth factor mobilized peripheral blood cells as a source of transplantable hematopoietic tissue. However, there is a major potential limitation to the widespread use of cord blood as a source of hematopoietic stem cells for marrow replacement and gene therapy. There may be enough hematopoietic stem cells to reconstitute children, but the ability to engraft an adult might require ex vivo manipulations. We describe an in vitro system in which the growth of cord blood CD34+ cells is sustained and greatly expanded for more than 6 months by the simple combination of two hematopoietic growth factors. Progenitors and cells belonging to all hematopoietic lineages are continuously and increasingly generated (the number of colony-forming unit–granulocyte-macrophage [CFU-GM] present at the end of 6 months of culture are well over 2,000,000-fold the CFU-GM present at the beginning of the culture). Very primitive hematopoietic progenitors, including long-term culture-initiating cells (LTC-ICs) and blast cell colony-forming units, are also greatly expanded (after 20 weeks of liquid culture, LTC-IC number is over 200,000-fold the initial number). The extremely prolonged maintenance and the massive expansion of these progenitors, which share many similarities with murine long-term repopulating cells, suggest that extensive renewal and little differentiation take place. This system might prove useful in diverse clinical settings involving treatment of grown-up children and adults with transplantation of normal or genetically manipulated hematopoietic stem cells.
HEMATOPOIETIC tissues contain a small population of primitive, totipotent stem cells capable of self-renewal and of generating committed progenitors of the different myeloid and lymphoid compartments.1-4 In the semisolid assays, hematopoietic progenitors in the presence of specific growth factors proliferate and differentiate to give origin to mature cells.5 In this system, depending on the combination of growth factors, different classes of multipotent committed progenitors (colony-forming units–granulocyte-macrophage [CFU-GM], burst-forming units-erythroid [BFU-E], and colony-forming units-megakaryocyte [CFU-Mk]) with a high differentiation but a negligible self-renewal capacity are identified.1-6
Several culture systems to identify early progenitor/stem cells have been described. In the human system, long-term culture-initiating cell assay (LTC-IC), which detects cells that can generate myeloid clonogenic cells (colony-forming cells [CFCs]) in long-term cultures for a minimum of 5 weeks, appears to be useful in this regard.7 Indeed, human LTC-ICs share a number of features with in vivo murine long-term repopulating cells.8 A number of other assays have been advanced as potentially having some correlations with long-term murine repopulating cells. These include assays for cobblestone areas colony-forming cells (CAFC),9 high proliferative potential colony-forming cells (HPP-CFC),10 mixed colony-forming units granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM),11 and blast cell colony-forming units (CFU-Bl).12,13 The development of assays suitable for the detection of human stem cells and their distinction from other hematopoietic cells with more restricted proliferative and differentiation potentials, therefore, has represented so far an important task. Many attempts to identify culture conditions able to support a prolonged and significant expansion of primitive stem cells have been disappointing, despite considerable success with mature committed progenitors. Indeed, although committed progenitor production can be quite impressive, its sharp decline after some weeks suggests that differentiation and not self-renewal predominates.14,15 These systems can, therefore, be very useful to expand the committed progenitor population and hopefully to shorten the early phase of cytopenia that follows bone marrow16,17 or cord blood18-20 transplantation.
However, their usefulness appears more uncertain in those situations, such as cord blood (CB) transplantation, in which the limited number of stem cells seems to question its feasibility in grown-up children and adults and when a consistent period of pancytopenia is frequent before graft taking.21
We describe here a stroma-free cell culture system in which the combined use of FLT3 ligand (FL)22-24 and thrombopoietin (TPO)25-29 proved able to maintain for more than 6 months an inexhaustible production of committed hematopoietic progenitors belonging to all hematopoietic lineages. The extremely prolonged maintenance and massive expansion of hematopoietic progenitors (including LTC-IC and CFU-Bl) suggest that extensive renewal, with a limited differentiation, take place. This system might prove very useful to expand primitive hematopoietic progenitor/stem cells and to widen the feasibility of cord blood transplantation.
MATERIALS AND METHODS
Cell Sources
Bone marrow (BM) was obtained by aspiration from the posterior iliac crest of fully informed hematologically normal donors. Umbilical cord blood (CB) was obtained at the end of full-term deliveries, after clamping and cutting of the cord by drainage of blood into sterile collection tubes containing the anticoagulant citrate-phosphate dextrose.
CD34+ Cell Purification
Mononuclear cells (MNC) were isolated from CB using Ficoll Hypaque (density, 1077; Nyegaard, Oslo, Norway) density centrifugation. Cells were subjected to two cycles of plastic adherence (60 minutes each); they were then were washed with Hanks' Balanced Salt Solution (GIBCO BRL, Grand Island, NY).
The CD34+ MNC fraction was isolated with superparamagnetic microbeads selection using high-gradient magnetic field and miniMACS columns (Miltenyi Biotech, Glodbach, Germany).
The efficiency of the purification was verified by flow cytometry counterstaining with a CD34-phycoerythrin (PE; HPCA-2; Becton Dickinson, Mountain View, CA) antibody. In the cell fraction containing purified cells, the percentage of CD34+ cells ranged from 90% to 98%.
Recombinant Human Cytokines
The following recombinant purified human cytokines were used in these studies: recombinant human (rh) stem cell factor or c-kit ligand (rhKL) and recombinant human granulocyte colony-stimulating factor (rhG-CSF ) were from Genzyme (Cambridge, MA); recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF ), recombinant human interleukin-6 (rhIL-6), and rhIL-3 were from Sandoz AG (Basel, Switzerland); recombinant human erythropoietin (rhEPO; EPREX) was from Cilag (Milan, Italy); recombinant human FLT3-ligand (rhFL) was obtained from SD Lyman (Immunex Corp, Seattle, WA); rhTPO was obtained from D.C. Foster (Zymogenetics, Seattle, WA); rhIL-7 and rhIL-12 were from R&D Systems Inc (Minneapolis, MN).
Progenitor Assays
LTC-ICs.LTC-ICs were performed as follows. BM stroma was produced by culturing 107 fresh BM MNC in a T25 flask for at least 2 weeks in 5 mL stromal medium (12.5% horse serum [HS] and 12.5% fetal calf serum [FCS]; both from Hyclone [Logan, UT]), Iscove's modified Dulbecco's medium (IMDM; GIBCO BRL), 2-mercaptoethanol (Sigma, St Louis, MO), 10−6 mol/L hydrocortisone (Sigma), and penicillin/streptomycin. One or 2 days before cocultures, BM stroma was irradiated with 15 Gy and plated at 7 × 103/cm2 in 24-well plates to form preestablished stromal layers for the long-term cultures. Limiting dilutions (1 to 1,000 CD34+ CB cells) and equivalent aliquots (ie, limiting dilutions) of the cells grown in long-term stroma-free cultures after 2 to 20 weeks were seeded on top of the irradiated BM stroma in culture medium containing IMDM, 12.5% HS, 12.5% FCS, 2-mercaptoethanol, and 10−6 mol/L hydrocortisone for 5 weeks at 33°C with weekly changes of half the medium. At the end of the 5-week LTC-IC assay period, nonadherent cells were removed from the cultures, combined with the corresponding trypsinized adherent cells, washed, and assayed for cells colony forming units (CFU-Cs) in methylcellulose medium containing 1.3% methylcellulose (Fluka Chemika Biochemika, Buchs, Switzerland), 30% FCS, EPO (3 U/mL), IL-3 (20 ng/mL), G-CSF (20 ng/mL), GM-CSF (20 ng/mL), and KL (50 ng/mL). These cultures were incubated at 37°C and colonies were scored 2 to 3 weeks later. LTC-IC enumeration was based on the number of CFU-C scored in the limiting dilution assays (LDA).
Stroma-free long-term cultures.Stroma-free long-term cultures were performed as follows. Two to ten thousand CD34+ CB cells were cultured in quadruplicate flat-bottomed 24-well plates in 1 mL of IMDM supplemented with 10% FCS and the following cytokines: IL-3 (10 ng/mL), IL-6 (10 ng/mL), KL (50 ng/mL), FL (50 ng/mL), and TPO (10 U/mL), which were added, alone or in a combination of two or more factors, to each series of microwells twice a week. The wells were grown at 37°C.
At initiation of the culture, the number of CFU-GM present in 1 mL of a single well was determined by agar culture,30 the number of CFU-Mk by plasma-clot technique,31 and the number of BFU-E and CFU-GEMM by methylcellulose assay.11 All the assays were performed in quadruplicate.
Every week all the wells were demidepopulated by removal of one half the culture volume, which was replaced with fresh medium and growth factors. Cells of the harvested media were counted and suitable aliquots of the all suspension were assayed for CFU-GM and CFU-Mk every week and for BFU-E, for CFU-GEMM, for CFU-Bl, for LTC-IC, and for immunophenotype analysis every 2 or 3 weeks.
The total number of CFU-GM, CFU-MK, BFU-E, and CFU-GEMM present in each LTC well was determined by using the following equation: CFU-E or BFU-E per milliliter of culture = y (observed CFU-C × 2n), where n is the number of previous demidepopulations31 32 and y is the number corresponding to the fraction of 1 mL cell suspension (ie, 1/62 = 15.6 μL and 1/34 = μL) that was plated in semisolid assays.
Clonogenic assays.The clonogenic assays were performed as follows. For CFU-GM, 5 × 102 CD34+ CB cells of the initial cell suspension or aliquots of the stroma-free long-term cultures were cultured at 4 plates per point in 3% agar, 15% FCS in IMDM (GIBCO). For CFU-Mk, the same number of cells was cultured in plasma clot assay (4 dishes per point) as previously described.31 32 For BFU-E and CFU-GEMM, 5 × 102 CD34+ CB cells/dish in at least 4 plates per point were cultured in 1.3% methylcellulose (Fluka), 30% FCS in IMDM at 37°C in a humidified atmosphere at 5% CO2 in air. Colony scoring was performed on day 12 for CFU-MK (at the immunofluorescent microscope after staining with a fluorescein isothiocyanate [FITC]-conjugated MoAb recognizing GPIIbIIIa) and on day 14 for CFU-GM, BFU-E, and CFU-GEMM. Several growth factors were added at optimum concentrations to sustain the formation of BFU-E and CFU-GEMM: IL-3 (20 ng/mL), GM-CSF (10 ng/mL), Epo (3 U/mL), and KL (50 ng/mL). For CFU-GM, G-CSF (20 ng/mL), GM-CSF (20 ng/mL), IL-3 (20 ng/mL), and KL (50 ng/mL) were added. For CFU-MK, IL-3 (5 ng/mL) was added.
CFU-Bl.Blast cell colony assay was performed according to the method described by Leary and Ogawa13 by plating 1 × 103 CD34+ CB cells of the initial purified population. IL-3 (10 ng/mL) and IL-6 (10 ng/mL) were added on day 14.
Colonies were scored after 7 to 10 days of incubation at 37°C. When identified, blast cell colonies containing 25 or more cells were replated in secondary cultures for confirmation of their ability to form secondary colonies. For this purpose, blast cell colonies were individually plucked from the culture and suspended in 100 μL IMDM. The samples were gently pipetted to obtain a single cell suspension and then added to 0.9 mL of methylcellulose medium containing 30% FCS, 1% deionized bovine serum albumin (Sigma), 3 U EPO, 20 ng/mL IL-3, 20 ng/mL GM-CSF, 20 ng/mL G-CSF, and 50 ng/mL KL. Cultures were incubated at 37°C for 14 additional days and all the colonies were enumerated. Smears were prepared using a cytospin for morphologic examinations.
Every 2 to 3 weeks aliquots of the harvested liquid cultures were assayed for CFU-Bl. Secondary replating was performed on at least 20 colonies, as described above.
Immunophenotyping by flow cytometry.After purification, aliquots of CD34+ CB cells were incubated with mouse IgG (Fluka) to block nonspecific binding to Fc receptor and then stained with a panel of monoclonal antibodies (FITC- or PE-conjugated CD34, CD38, CD33, CD14, CD2, CD19, and CD41 [all from Becton Dickinson] and antiglycophorin [Dakopatts, Glostrup Denmark]). FITC- and PE-conjugated mouse isotype control antibodies were used for each culture.
Every 2 to 3 weeks aliquots of cultured cells were counted, washed, and then subjected to the same procedure. The only difference was that cells were also incubated with propidium iodide (1 mg/mL) to analyze only viable cells.
Flow cytometric analysis was performed using a FACScan cytometer (Becton Dickinson). At least 10,000 events were acquired for each analysis.
RESULTS
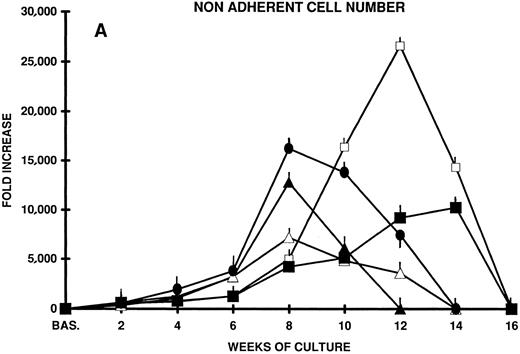
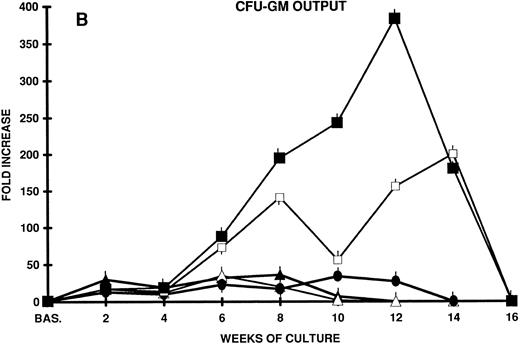
In an effort to induce the long-term proliferation and, possibly, the amplification of primitive hematopoietic progenitors contained in CD34+ CB cells, we set up stroma-free suspension cultures in which a number of cytokines known to be active on more immature hematopoietic progenitors (IL-3 and IL-6)14,15,33 in combination with early acting cytokines (kit ligand or stem cell factor [KL]14,15,34-36 and FLT3 ligand [FL])22-24 37-41 were used. Although cellular proliferation occurred (7,000- to 12,000-fold the input cell number after 8 weeks of culture with IL-3 and KL or IL-3 and FL), by week 10 the total cell population began to decrease and soon disappeared. Accordingly, the output of committed progenitors (CFU-C) after an initial increase (36-fold the input CFU-C after 8 weeks of culture) by week 10 progressively decreased (Fig 1A and B).
(A) Nonadherent cell expansion from CD34+ CB cells. Two thousand CB cells in 1 mL were plated in quadruplicate 24-well plates on day 0. Cells were cultured in stroma-free in LTC medium in the presence of FL, KL, IL-3, and IL-6 alone or combined. Every week half the culture volume was removed from cell culture and the cells were counted. The mean fold increase over cell number plated on day 0 is shown at each time point of LTC. Mean ± SEM from three separate experiments performed in quadruplicate. (B) CFU-GM expansion from CD34+ CB cells in stroma-free LTC. Two thousand CD34+ CB cells were plated in quadruplicate 24-well plates in the presence of the factors reported above. The content of CFU-GM of each well was established at the beginning of the LTC by agar assays. Every week all the wells were demidepopulated by removal of one half the culture volume, which was replaced with freshly medium and growth factors. Cells of the harvested media were counted and suitable aliquots of the cell suspension were assayed for CFU-GM in quadruplicate dishes. The results represent the mean ± SEM of three separate experiments performed in quadruplicate. (▴) IL-3 + FL; (▵) IL-3 + KL; (•) IL-3 + KL + FL; (□) IL-6 + FL + KL; (▪) KL + FL.
(A) Nonadherent cell expansion from CD34+ CB cells. Two thousand CB cells in 1 mL were plated in quadruplicate 24-well plates on day 0. Cells were cultured in stroma-free in LTC medium in the presence of FL, KL, IL-3, and IL-6 alone or combined. Every week half the culture volume was removed from cell culture and the cells were counted. The mean fold increase over cell number plated on day 0 is shown at each time point of LTC. Mean ± SEM from three separate experiments performed in quadruplicate. (B) CFU-GM expansion from CD34+ CB cells in stroma-free LTC. Two thousand CD34+ CB cells were plated in quadruplicate 24-well plates in the presence of the factors reported above. The content of CFU-GM of each well was established at the beginning of the LTC by agar assays. Every week all the wells were demidepopulated by removal of one half the culture volume, which was replaced with freshly medium and growth factors. Cells of the harvested media were counted and suitable aliquots of the cell suspension were assayed for CFU-GM in quadruplicate dishes. The results represent the mean ± SEM of three separate experiments performed in quadruplicate. (▴) IL-3 + FL; (▵) IL-3 + KL; (•) IL-3 + KL + FL; (□) IL-6 + FL + KL; (▪) KL + FL.
The addition of both KL and FL to IL-3 further enhanced cell proliferation and CFU-C output, but, again, without substantial effects on the long-term maintenance of primitive stem cells.
In contrast, KL plus FL, in the absence of IL-3 or in the presence of IL-6, enhanced progenitor cell proliferation to a much greater extent and for a longer time (after 12 to 14 weeks of culture, the cell output was >10,000- to 20,000-fold the input value, and CFU-C output was greater than 200- to 300-fold). However, at week 14, progenitor cell proliferation slowed down and by week 16 disappeared completely (Fig 1A and B).
c-mpl ligand (TPO) is a likely growth factor also for primitive stem cells26-29,42-45; therefore, we sought whether the combination of TPO with another early acting cytokine would support the long-term proliferation and perhaps the renewal of primitive human CB hematopoietic stem cells.
In an attempt to carefully investigate the proliferative events occurring during the long-term cultures, different stages of hematopoiesis were analyzed.
CD34+ Cell Proliferation
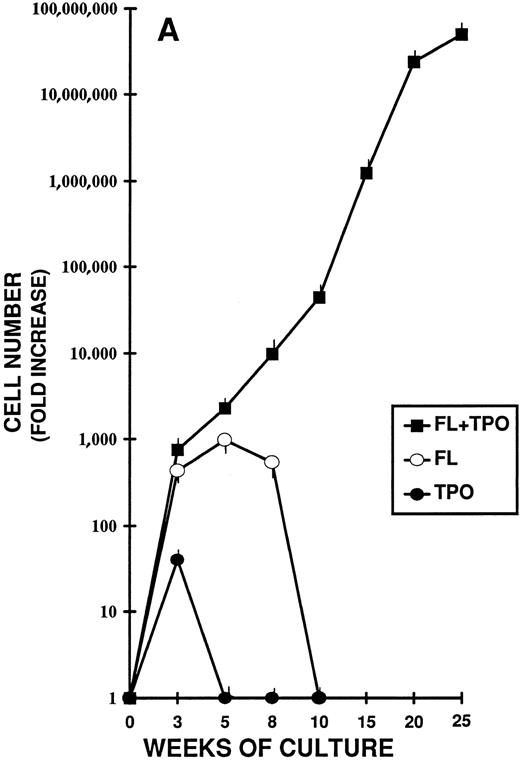
Highly purified CD34+ CB cells were cultured in stroma-free liquid cultures with FL and TPO, alone or combined, for more than 6 months (Fig 2A). The total cell counts were performed weekly. In the cultures without cytokines, a rapid decrease in cell number was observed during the first 3 weeks and by day 21 no cells were alive. Cultures set up with either TPO or FL alone lasted, respectively, 4 and 10 weeks.
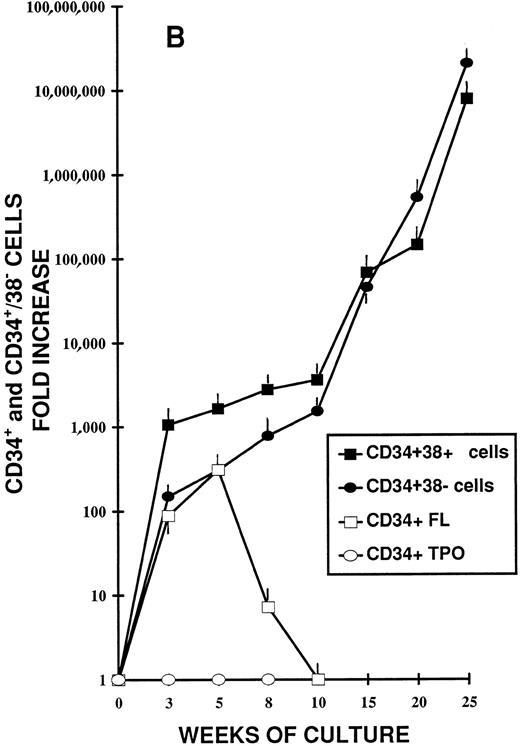
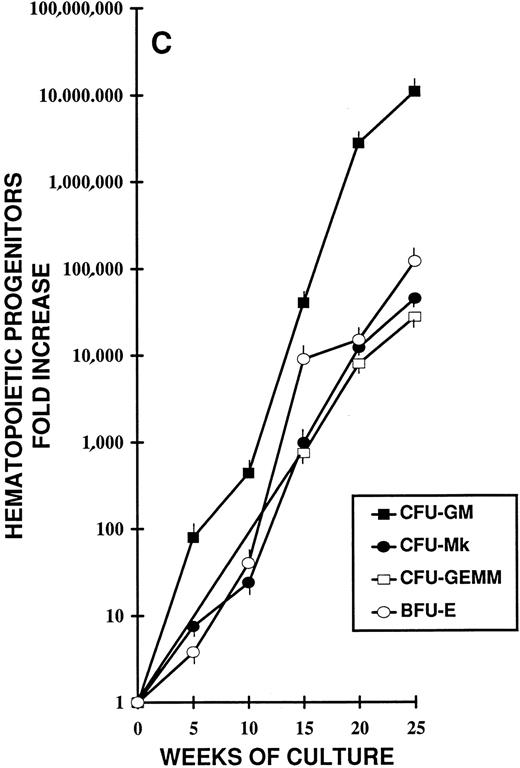
Different stages of hematopoiesis in stroma-free LTC of CB CD34+ cells. (A) Expansion of hematopoietic cells in stroma-free cultures stimulated by TPO (10 U/mL) alone (•), by FL (50 ng/mL) alone (○), and by the combination of TPO (10 U/mL) + FL (50 ng/mL) (▪). The starting population was represented by 2 × 103 CD34+ CB cells in 1 mL in 24-well plates. Fresh growth factors were added twice a week. Cells were counted weekly from quadruplicate wells and the fold increase was obtained by dividing the number of cells of each well counted each week by the number of the starting population. Represented here is the mean ± SEM of four different experiments (each performed in quadruplicate). (B) Expansion of the CD34+ (▪) and the CD34+ CD38− (•) cell populations during LTC with FL + TPO. CB CD34+ cells were grown as described in Fig 1 for 25 weeks. Every 2 to 3 weeks the cells harvested were stained with FITC- and PE-conjugated anti-CD34 and anti-CD38 MoAb. The total number of each subpopulation was obtained by multiplying the percentage of each subset by the number of cells contained in each well. Results are expressed as the fold increase (compared with the starting subpopulation) and represent the mean ± SEM of analyses performed on quadruplicate wells in four separate experiments. (□) CD34+ cells in LTC grown in the presence of FL alone. (○) CD34+ cells in LTC grown in the presence of TPO alone. (C) Production of hematopoietic progenitors in LTC in the presence of FL + TPO. CD34+ CB cells were grown for 25 weeks as described in Fig 1. CFU-GM (▪), CFU-MK (○), BFU-E (•), and CFU-GEMM (□) were cultured as described in Fig 1. Results represent the fold increase of each class of progenitors present in each well in determined periods of LTC, compared with the number of progenitors of the beginning of the cultures. Mean ± SEM of four wells per point in four separate experiments.
Different stages of hematopoiesis in stroma-free LTC of CB CD34+ cells. (A) Expansion of hematopoietic cells in stroma-free cultures stimulated by TPO (10 U/mL) alone (•), by FL (50 ng/mL) alone (○), and by the combination of TPO (10 U/mL) + FL (50 ng/mL) (▪). The starting population was represented by 2 × 103 CD34+ CB cells in 1 mL in 24-well plates. Fresh growth factors were added twice a week. Cells were counted weekly from quadruplicate wells and the fold increase was obtained by dividing the number of cells of each well counted each week by the number of the starting population. Represented here is the mean ± SEM of four different experiments (each performed in quadruplicate). (B) Expansion of the CD34+ (▪) and the CD34+ CD38− (•) cell populations during LTC with FL + TPO. CB CD34+ cells were grown as described in Fig 1 for 25 weeks. Every 2 to 3 weeks the cells harvested were stained with FITC- and PE-conjugated anti-CD34 and anti-CD38 MoAb. The total number of each subpopulation was obtained by multiplying the percentage of each subset by the number of cells contained in each well. Results are expressed as the fold increase (compared with the starting subpopulation) and represent the mean ± SEM of analyses performed on quadruplicate wells in four separate experiments. (□) CD34+ cells in LTC grown in the presence of FL alone. (○) CD34+ cells in LTC grown in the presence of TPO alone. (C) Production of hematopoietic progenitors in LTC in the presence of FL + TPO. CD34+ CB cells were grown for 25 weeks as described in Fig 1. CFU-GM (▪), CFU-MK (○), BFU-E (•), and CFU-GEMM (□) were cultured as described in Fig 1. Results represent the fold increase of each class of progenitors present in each well in determined periods of LTC, compared with the number of progenitors of the beginning of the cultures. Mean ± SEM of four wells per point in four separate experiments.
By contrast, in the long-term cultures (LTC) containing the association of FL and TPO, a progressive, steady increase of cell production was seen. After 5 weeks the cell output was greater than 1,000-fold the input number; after 15 weeks, it was greater than 1,000,000-fold and at week 25 the cell output was greater than 50,000,000-fold the input number.
Immunophenotype of Cultured Cells
Cells harvested weekly were assayed by flow cytometry for surface antigen expression using essentially viable cells (Fig 2B). The CD34+ cell population, in the presence of FL + TPO kept increasing throughout the LTC period. After 5 weeks, CD34+ cells were close to 1,600-fold the initial value; after 20 weeks, they reached 146,000-fold the initial value and kept growing.
Previous studies have shown that CD34+ CD38− cells constitute a small population of primitive hematopoietic stem cells enriched for LTC-IC.46 47 We thus wanted to see whether this subpopulation persisted during the LTC period. As shown in Fig 2B, the CD34+ CD38− subpopulation not only persisted, but underwent a continuous expansion. Indeed, after 5 weeks of culture, it was 300-fold the input value; at week 20, it was greater than 500,000-fold the input number and kept expanding. The complete immunophenotyping of cultured cells showed that the most of the cells produced in LTC belonged to the granulocyte-macrophage, the erythroid, and the megakaryocyte lineages. CD2+ and CD19+ cells represented only 0.2% and 0.012% of the total cell population.
CFC Content and Amplification in LTCs
To measure the progenitor content of the cell expansion occurring throughout the LTC, semisolid assays for CFU-GM and CFU-Mk were performed weekly and for BFU-E and for CFU-GEMM were performed every 2 to 3 weeks. Figure 2C shows the fold increase, over time, of CFU-GM, CFU-Mk, BFU-E, and CFU-GEMM generated during 6 months of liquid cultures.
After 5 weeks of culture, CFU-GM output was greater than 70-fold and by week 25 was greater than 6,000,000-fold the input value. Besides CFU-GM, during the entire culture period, CFU-Mk, BFU-E, and CFU-GEMM were generated by the expanded cells. Indeed, CFU-Mk generated after 15 weeks of culture were 1,000-fold the number of CFU-Mk present in the inoculum at the beginning of the suspension cultures. After 25 weeks, they were well over 45,000-fold the input number. Similarly, BFU-E and CFU-GEMM were generated during the entire culture period (at week 15, they were, respectively, >9,000- and >850-fold; at week 25, they were >122,000-fold and >27,000-fold the input number, respectively).
In an effort to show that, among the cells growing in the LTC, totipotent stem cells were present, we sought to find out whether T and B cells had been generated. At the beginning of the cultures, the frequency of T and B cells in the CD34+ cell population was very low. After several weeks of liquid culture, T and B cells were barely detectable (CD2+ and CD19+ cells represented, respectively, 0.2% and 0.012%). However, after incubation for an additional 14 days in the presence of IL-7, IL-12, and FL, the percentage of CD2+ cells ranged from 24.98% to 33.8% and CD19+ cells ranged from 0.78% to 3.75% (Table 1).
Appearance of CD2+ Cells After 12 Weeks of LTC
| Experiment . | % CD2+ Cells . | % CD19+ Cells . | ||
|---|---|---|---|---|
| No. . | Untreated* . | +FL + IL-7 + . | Untreated* . | +FL + IL-7 + . |
| . | . | IL-12 . | . | IL-12 . |
| 1 | 0.12 ± 0.01 | 33.8 ± 1.1 | 0.012 ± 0 | 1.4 ± 0.3 |
| 2 | 0.24 ± 0.03 | 42.36 ± 2.3 | 0.01 ± 0 | 0.78 ± 0.1 |
| 3 | 0.18 ± 0.08 | 24.98 ± 2.1 | 0.02 ± 0 | 3.75 ± 0.2 |
| Experiment . | % CD2+ Cells . | % CD19+ Cells . | ||
|---|---|---|---|---|
| No. . | Untreated* . | +FL + IL-7 + . | Untreated* . | +FL + IL-7 + . |
| . | . | IL-12 . | . | IL-12 . |
| 1 | 0.12 ± 0.01 | 33.8 ± 1.1 | 0.012 ± 0 | 1.4 ± 0.3 |
| 2 | 0.24 ± 0.03 | 42.36 ± 2.3 | 0.01 ± 0 | 0.78 ± 0.1 |
| 3 | 0.18 ± 0.08 | 24.98 ± 2.1 | 0.02 ± 0 | 3.75 ± 0.2 |
CD34+ CB cells (2 × 103) were grown in stroma-free LTC containing FL + TPO for 12 weeks and then were analyzed for CD2 and CD19 expression (untreated). Analogous replicate samples were incubated for additional 14 to 21 days in the presence of 50 ng/mL FL, 100 U/mL IL-7, and 100 U/mL IL-12. The cells were then harvested and analyzed for CD2 and CD19 expression. The results are the mean ± SEM of analyses performed in triplicate wells.
LTC-IC Content and Amplification in Suspension Cultures
The frequency of LTC-IC in the initial CD34+ CB cells (input value) was 0.6% ± 0.4%. This was determined by LDA by plating from 1 to 1,000 CD34+ cells onto preestablished irradiated feeder layers from normal BM and then assessing the number of CFC present 5 weeks later.
After 2 weeks of liquid culture, the LTC-IC content, determined by LDA as for input LTC-IC by evaluating the number of CFC generated after 5 additional weeks in the standard BM stroma assay, was found to be 160-fold the input value. After 10 weeks of LTC, the LTC-IC number was 1,000-fold and by week 20 was over 200,000-fold the input value (Table 2).
LTC-IC Amplification in CD34+ CB Cells Cultured in LTC in the Presence of Various Cytokine Combinations
| Factors Added . | No. of Cells/100 Initial Nucleated Cells . | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Nonadherent Cells . | CFU-C . | LTC-IC . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||||
| . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Med | 100 | 380 | 0 | 0 | 0 | 0 | 13.2 | 0 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
| FL | 100 | 8,400 | 97,500 | 51,000 | 0 | 0 | 13.2 | 144 | 847 | 0 | 0 | 0 | 0.6 | 0.75 | 0.63 | 0 | 0 | 0 | |||||||||||||||
| TPO | 100 | 4,800 | 2,000 | 0 | 0 | 0 | 13.2 | 51.2 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
| FL + TPO | 100 | 9,400 | 197,000 | 1,025,000 | 89,700,000 | 1,074,790,000 | 13.2 | 206 | 2,947 | 154,091 | 2,873,468 | 21,936,205 | 0.6 | 96 | ND | 599 | 1,975 | 83,886 | |||||||||||||||
| KL + FL + TPO | 100 | 25,000 | 227,000 | 2,514,000 | 124,110,000 | 1,454,895,000 | 13.2 | 202 | 835 | 58,655 | 2,714,461 | 17,469,274 | 0.6 | 76.8 | ND | ND | ND | 31,457 | |||||||||||||||
| IL-3 + FL + TPO | 100 | 19,400 | 185,000 | 128,000 | 0 | 0 | 13.2 | 157 | 1,597 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0 | 0 | |||||||||||||||
| Factors Added . | No. of Cells/100 Initial Nucleated Cells . | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Nonadherent Cells . | CFU-C . | LTC-IC . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||||||||
| . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | Start . | 2 wk . | 5 wk . | 10 wk . | 15 wk . | 20 wk . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Med | 100 | 380 | 0 | 0 | 0 | 0 | 13.2 | 0 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
| FL | 100 | 8,400 | 97,500 | 51,000 | 0 | 0 | 13.2 | 144 | 847 | 0 | 0 | 0 | 0.6 | 0.75 | 0.63 | 0 | 0 | 0 | |||||||||||||||
| TPO | 100 | 4,800 | 2,000 | 0 | 0 | 0 | 13.2 | 51.2 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
| FL + TPO | 100 | 9,400 | 197,000 | 1,025,000 | 89,700,000 | 1,074,790,000 | 13.2 | 206 | 2,947 | 154,091 | 2,873,468 | 21,936,205 | 0.6 | 96 | ND | 599 | 1,975 | 83,886 | |||||||||||||||
| KL + FL + TPO | 100 | 25,000 | 227,000 | 2,514,000 | 124,110,000 | 1,454,895,000 | 13.2 | 202 | 835 | 58,655 | 2,714,461 | 17,469,274 | 0.6 | 76.8 | ND | ND | ND | 31,457 | |||||||||||||||
| IL-3 + FL + TPO | 100 | 19,400 | 185,000 | 128,000 | 0 | 0 | 13.2 | 157 | 1,597 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0 | 0 | |||||||||||||||
Differential ability of FL, TPO, KL, and IL-3, alone or combined, to support different stages of hematopoiesis in long term stroma-free cultures suspension cultures of CD34+ CB cells. Cultures were initiated with 2 × 103 highly purified CD34+ CB cells and maintained as described in the Materials and Methods. Results show the mean of three to five experiments performed in duplicate after normalization of each result per 100 cells seeded into each type of culture. The column on the left shows the number of cells generated after progressively longer periods of time in liquid cultures. The central column shows the committed progenitor production in the same liquid cultures. The right-side column shows the generation and expansion of primitive stem cells (identified as LTC-IC).
CFU-Bl
Primitive hematopoietic progenitors capable in vitro of producing colonies composed of blast cells (CFU-Bl) have been described.13 These cells possess many features of stem cells in that they are capable of self-renewal and also of commitment towards a number of hematopoietic lineages.
In our hands, at the beginning of long-term suspension cultures, 63% ± 5.4% of the CFU-Bl were capable of generating secondary colonies after replating. Each blast colony generated a mean of 5.6 ± 1.2 secondary colonies. During the LTC period, CFU-Bl underwent a progressive expansion paralleling with that of other more mature committed progenitors (Table 3). Thus, after 5 weeks of LTC, the CFU-Bl colony number was 43-fold the input number and at week 20 reached 305,000-fold the number present in the initial inoculum. In addition, expanded CFU-Bl still retained their self-renewal capacity (or, at least, maintained the capacity to differentiate toward multiple lineages). When the blast cell colonies were replated in methylcellulose containing IL-3 + IL-6, new blast cell colonies were generated, which, in turn, were capable of generating tertiary colonies (data not shown).
Progressive Expansion of CFU-Bl in LTCs
| Weeks of . | Blast Cell Colonies . | ||
|---|---|---|---|
| LTC . | Fold Increase . | % Recloning . | No. of Secondary Colonies/ . |
| . | . | Efficiency3-150 . | Blast Colony . |
| 0 | 1 | 63 ± 5.4 | 5.6 ± 1.2 |
| +3 | 9.24 ± 0.6 | 55 ± 2.6 | 5.2 ± 0.4 |
| +5 | 43 ± 2.7 | ND | ND |
| +8 | 421 ± 11 | ND | ND |
| +10 | 4,200 ± 97 | 61 ± 3.1 | 4.3 ± 1.2 |
| +12 | 5,407 ± 145 | ND | ND |
| +15 | 28,672 ± 1,200 | 58 ± 2.3 | 6 ± 0.8 |
| +18 | 65,556 ± 2,406 | ND | ND |
| +20 | 305,420 ± 12,400 | 53 ± 4.6 | 6.7 ± 1.2 |
| Weeks of . | Blast Cell Colonies . | ||
|---|---|---|---|
| LTC . | Fold Increase . | % Recloning . | No. of Secondary Colonies/ . |
| . | . | Efficiency3-150 . | Blast Colony . |
| 0 | 1 | 63 ± 5.4 | 5.6 ± 1.2 |
| +3 | 9.24 ± 0.6 | 55 ± 2.6 | 5.2 ± 0.4 |
| +5 | 43 ± 2.7 | ND | ND |
| +8 | 421 ± 11 | ND | ND |
| +10 | 4,200 ± 97 | 61 ± 3.1 | 4.3 ± 1.2 |
| +12 | 5,407 ± 145 | ND | ND |
| +15 | 28,672 ± 1,200 | 58 ± 2.3 | 6 ± 0.8 |
| +18 | 65,556 ± 2,406 | ND | ND |
| +20 | 305,420 ± 12,400 | 53 ± 4.6 | 6.7 ± 1.2 |
Ability of FL + TPO to support CFU-Bl in LTC. CFU-Bl generated by 1,000 CD34+ CB cells at the beginning of the LTC (time 0) and secondary colonies after replating (mean ± SEM values from 3 separate experiments). Secondary clones comprised all types of colonies (GM, G, erythroid, burst, and GEMM clones represented the total numbers of colonies, respectively).
The recloning efficiency is the percentage of colonies that generates secondary colonies upon replating. Results represent the mean ± SEM of three separate experiments.
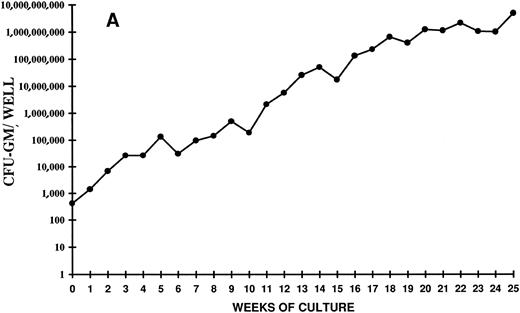
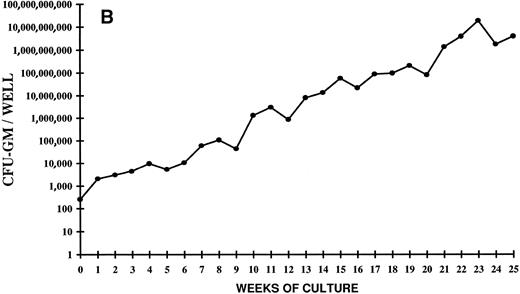
Analysis of the time course of CFC expansion showed that the generation of CFU-GM did not show an entirely linear progression (Fig 3A and B). Indeed, three to four waves of colony generation were evident at certain time points in all the experiments. The earliest peak was seen around 5 weeks of culture and, since then, a peak was clearly detectable every 4 to 5 weeks. The last peak occurred at week 25 (Fig 3A). Overall, the picture seems to reflect a production of clonogenic precursors from more and more primitive stem cell subpopulations, which are driven into proliferation after progressively longer periods of time. Alternatively, it might also indicate that, during such a long period of liquid culture, self-renewal of primitive stem cells has occurred.
Time course of CFU-GM expansion from CD34+ CB cells. Two thousand CD34+ cells were plated on day 0 in duplicate in 24 wells in the presence of FL + TPO. Every week the content of CFU-GM per well was determined. The vertical axis represents the CFU-GM expansion during 25 weeks of LTC. Two independent experiments of four are shown.
Time course of CFU-GM expansion from CD34+ CB cells. Two thousand CD34+ cells were plated on day 0 in duplicate in 24 wells in the presence of FL + TPO. Every week the content of CFU-GM per well was determined. The vertical axis represents the CFU-GM expansion during 25 weeks of LTC. Two independent experiments of four are shown.
The colonies generated in semisolid assays, in identical culture conditions, and in the presence of identical growth factors, after several months of liquid culture, were much larger (up to 1,000 cells), as if committed progenitors possessed an higher proliferative potential.
DISCUSSION
Our data demonstrate that the simple combination of two hematopoietic growth factors (FL and TPO), in the absence of stromal cells, is capable of sustaining for more than 6 months both proliferation and renewal of CB primitive stem cells, without any sign of their exhaustion.
The progenitor cells and the cells produced in this system possess both antigenic and growth characteristics of all hematopoietic lineages, including those of T and B cells; thus, the cells assayed during the extended period of LTC are likely to represent primitive stem cells. The progressively higher and higher waves of CFU-C generation that can be identified during the entire period of LTC suggest the presence of numerous populations of primitive, deeply quiescent stem cells, which begin to proliferate only after more and more prolonged periods of time, well after that of the standard LTC-ICs and the extended LTC-ICs (ELTC-ICs).48 Consistent with the very prolonged and massive progenitor output, which is still expanding after 5 months of liquid culture (CFU-GM number after 25 weeks of LTC is over 6,000,000-fold the initial number), the LTC-IC number is also in continuous expansion (after 20 weeks it is over 200,000-fold the input number).
It has recently been shown that the CD34+ CD38− immunophenotype defines a rare and primitive subpopulation of hematopoietic progenitor cells in BM and CB enriched for LTC-IC.46 47 Our starting population was composed of both CD34+ CD38+ and CD34+ CD38− CB cells. It is possible that the early wave of CFU-C production is sustained by the CD34+ CD38+ population and that the later waves, after 8 to 10 weeks of culture, are generated by the CD34+ CD38− subpopulation.
Besides the LTC-ICs, which reflect the presence of primitive, in vivo long-term repopulating stem cells, other assays have been used in this study to measure generation and amplification of primitive stem cells. CFU-Bl12 13 possess many features of stem cells in that they are capable of self-renewal and also commitment toward a number of hematopoietic lineages. In the LTC described here, the population of CFU-Bl not only is maintained, but also is greatly expanded. What is more, the expanded population, after several weeks of liquid culture, still retains its capability to self-renew and to generate colonies of cells belonging to granulocyte-macrophage, erythroid, and megakaryocyte lineages and colonies of blast cells, which, in turn are capable of tertiary replating. This represents an additional proof of the capability of this culture system to sustain and expand also primitive progenitors.
So far, LTC systems capable to support human hematopoiesis for extended periods of time, without exhaustion of primitive stem cells, are all dependent on the presence of marrow stromal feeder layers.14,15,40,46,48 At the present time, the molecular mechanisms that mediate stromal cell-based stimulation of LTC-IC proliferation and differentiation are not known. Previous studies showing the ability of immortalized murine fibroblasts to substitute for human marrow feeder layers in human LTC-IC assays, including fibroblasts genetically unable to produce KL, have suggested that novel growth factors may be involved.49 The possibility that FLT3 ligand may play a role in this regard has emerged.22-24 Moreover, it has been recently reported that incubation of single CD34+ CD38− BM cells in medium supplemented with FL, KL, IL-3, IL-6, G-CSF, and nerve growth factor resulted in the proliferation of initial cells to produce clones containing LTC-IC. A 50-fold overall expansion in a 3- to 5-week time frame was observed, indicating the ability of that cytokine mixture to stimulate primitive cells.39
The simple combination of the two growth factors used here proved able to maintain for at least 6 months a very large and continuously increasing production of hematopoietic progenitors belonging to all lineages. This extremely prolonged maintenance and the massive expansion of primitive hematopoietic progenitors, including CFU-Bl and LTC-IC, suggest that extensive renewal, with little differentiation, takes place.
FLT3 ligand was a likely candidate for supporting primitive hematopoietic stem cell growth22-24; however, in our assay, FL, by itself, was not sufficient to sustain long-term hematopoiesis. Indeed, previous studies showed its ability to act synergistically with more lineage-restricted growth factors and other early acting cytokines, such KL.37-41 In our system, the association of FL with IL-3 or IL-6 resulted in an impressive CFU-GM output that, on the other hand, was not accompanied by maintenance of primitive stem cells.
Also, TPO by itself proved unable to sustain long-term hematopoiesis. Indeed, most studies initially suggested that TPO was a lineage-restricted regulator with little or no activity on nonmegakaryocitic lineages.25-29 Nevertheless, in populations of mouse fetal liver enriched for primitive hematopoietic cells, a significant proportion of cells express c-mpl.50 Moreover, recent data suggest that, in combination with EPO, TPO may augment growth of erythroid progenitors42 and cells of human nonmegakaryocitic leukemias can proliferate in response to TPO.51 An elevation of the number of CFU-GEMM has also been observed upon TPO administration in monkeys.52 Finally, c-mpl–deficient mice displayed reduced total hematopoietic progenitor cell numbers, including multipotential, blast, and committed progenitors of multiple lineages.43
Thus, the simple combination of FL and TPO resulted to be both necessary and sufficient to sustain long-term stem cell proliferation and renewal. The clinical applications seem very exciting: transplantation of adults and grown-up children could be extremely facilitated if CB hematopoietic progenitor stem cells can be readily expanded. Moreover, the ability to control and amplify pluripotent stem cell renewal and expansion will provide a major boon to stem cell gene therapeutics and could permit increased levels of stem cell transduction, thus allowing diverse applications of stem cell modification.
ACKNOWLEDGMENT
We thank Stewart D. Lyman (Immunex) for providing FLT3 ligand and Donald C. Foster (Zymogenetics) for providing TPO.
Support for this work was provided by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy) and Consiglio Nazionale delle Ricerche (CNR; Rome, Italy) to special project ACRO (Grant No. 01062.PF399) to M.A. and from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica to W.P. and to M.A. L.G. and A.S. are recipients of the “G. Ghicotti Foundation,” Ses. Piemonte grants.
Address reprint requests to Wanda Piacibello, MD, Clinica Medica I, Department of Biomedical Sciences and Human Oncology, Via Genova 3, 10126 Torino, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal