Abstract
Hepatic veno-occlusive disease (VOD) is a frequent and severe complication after bone marrow transplantation (BMT). We previously have described plasminogen activator inhibitor-1 (PAI-1) as a possible marker of VOD. To confirm the significance of this finding, we now determined PAI-1 levels in 31 of 186 consecutive patients undergoing BMT who developed hyperbilirubinemia greater than 3 mg/dL for various reasons. Diagnoses were made by clinical criteria and confirmed by biopsy in 23 of 31 patients. They included VOD (n = 7), acute graft-versus-host disease (GVHD) of the liver (n = 7), and other hepatic injury (n = 17). PAI-1 (mean ± SD) was significantly (P < .001) elevated in patients with VOD (321.6 ± 161.2 ng/mL) as compared with patients with GVHD (22.8 ± 8.4 ng/mL) or other hepatic damage (32.8 ± 30.8 ng/mL) at the timepoint of bilirubin increase. At the peak bilirubin concentration, the corresponding PAI-1 levels were 426.1 ± 230.0 ng/mL in patients with VOD, 41.0 ± 20.6 ng/mL in patients with GVHD, and 44.6 ± 32.9 ng/mL in patients with other hepatic injury (P < .001 VOD v GVHD/other hepatic injury). Our results underline the relevance of PAI-1 in the differential diagnosis of hyperbilirubinemia after BMT and its significance as a sensitive and specific marker of severe VOD.
VENO-OCCLUSIVE disease (VOD) was initially described after the ingestion of bush teas containing pyrrolizidine alkaloids.1 The intoxication leads to an obstruction of small intrahepatic venules. The deposition of fibrinogen and von Willebrand factor and fibrous vessel obliteration have been shown histologically.2 Recently, clinically severe VOD was shown to be significantly correlated with the histologic findings of venous occlusion, zone 3 hepatocyte necrosis, sinusoidal fibrosis, and excentric luminal narrowing or phlebosclerosis.3
Typical clinical features of VOD are hyperbilirubinemia, ascites, right upper quadrant pain, unexplained weight gain, and hepatomegaly.4,5 Today, VOD is most often observed in patients undergoing bone marrow transplantation (BMT) after myeloablative radiochemotherapy. The toxicity of the conditioning regimen and resulting endothelial cell damage is held responsible for the pathogenesis of vessel obstruction. In BMT recipients, after sepsis and acute graft-versus-host disease (GVHD), VOD is the third most important reason for fatal outcome.5 Early detection of the syndrome is essential, because thrombolytic therapy represents a promising tool possibly enabling fast resolution of symptoms.6
An enhancement of the aminopropeptide of type III collagen7-9 and a reduction of protein C10-12 have been reported as useful laboratory parameters for the diagnosis of VOD. We have previously described the main inhibitor of the fibrinolytic system, plasminogen activator inhibitor-1 (PAI-1), which is synthesized and released by endothelial cells, as a further specific and early marker of VOD.13 14 To validate our results, we now determined PAI-1 levels in 31 consecutive BMT recipients with posttransplant hyperbilirubinemia.
PATIENTS AND METHODS
Citrated blood samples (3.8% sodium citrate 9:1) of 186 consecutive patients were collected following a standardized protocol on the morning between 7 and 8 AM before (day −8), during (day −5), and after conditioning (day −1); before BMT (day 0); and thereafter weekly (days 7 through 35). Samples were cooled immediately after collection (4°C) and centrifuged within 2 hours (2,000g at 4°C for 15 minutes). Aliquots were frozen and stored at −70°C until assayed. PAI-1 antigen was determined in all patients with hyperbilirubinemia exceeding 3 mg/dL by a commercially available enzyme immunoassay using monoclonal antibodies (Asserachrom PAI-1; Diagnostica Stago; supplied by Boehringer Mannheim, Mannheim, Germany; normal range, 4 to 43 ng/mL). Bilirubin was measured by routine clinical methods.
Hyperbilirubinemia occurred in 31 patients (17 females and 14 males) who received transplants for hematologic malignancies. The mean age was 39.6 years (±8.6 years; range, 23 to 53 years). Diagnoses included chronic myelogenous leukemia (n = 17), acute myelogenous leukemia (n = 8), acute lymphoblastic leukemia (n = 2), chronic lymphocytic leukemia (n = 1), severe aplastic anemia (n = 1), lymphoblastic lymphoma (n = 1), and myelodysplastic syndrome (n = 1). All patients received allogeneic transplants, with 18 patients receiving them from related donors and 13 patients receiving them from unrelated donors. The following preparative regimens were used: Bu4/Cy4 (busulphan at 16 mg/kg for 4 days and cyclophosphamide at 200 mg/kg for 4 days; n = 10); TBI (total body irradiation 3 × 4 Gy in 3 days)/CY4 (n = 20); and CVB (cyclophosphamide at 6 g/m2 for 4 days, BCNU at 300 mg/m2 for 1 day, and VP-16 at 600 mg/m2 for 3 days; n = 1). Cyclosporin and a short course of methotrexate (MTX) were used as prophylaxis for GVHD.
The underlying diagnosis leading to hyperbilirubinemia was made by clinical criteria and confirmed by biopsy in 23 patients. In 8 patients, the diagnosis was based on clinical criteria only. Seven patients were classified as having VOD, 7 patients as having GVHD, and 17 patients as having other diseases.
In those patients whose diagnosis was made on the basis of clinical criteria only, 1 patient had VOD as defined by the classification of Jones et al5 and 1 patient had acute GVHD of the liver as defined by the criteria of Glucksberg et al.15 Other hepatic damage was diagnosed clinically in 6 patients in whom no signs of VOD or GVHD could be detected.
Patients who had samples analyzed by biopsy were grouped as having VOD or GVHD according to the criteria of Shulman et al.3 One patient classified as having VOD represented an overlap between VOD and GVHD. At presentation, centrilobular hepatocyte necrosis, occlusion of central venules, and phlebosclerosis were found histologically along with the destruction of small bile ducts and moderate infiltration of mononuclear cells.
If the patients did not fulfill histologic criteria for VOD or GVHD,3 they were classified as having other causes of liver disease. Venous occclusion or zone 3 changes including hepatocyte necrosis were not present in these biopsies. Several phenomena, such as drug toxicity (eg, antibiotics and immunosuppressive agents), infections, extrahepatic biliary obstruction, and, in some cases, total parenteral nutrition, were presumably responsible for hyperbilirubinemia in this group.16
The Mann-Whitney U-test was used to assess differences between the three groups.
RESULTS
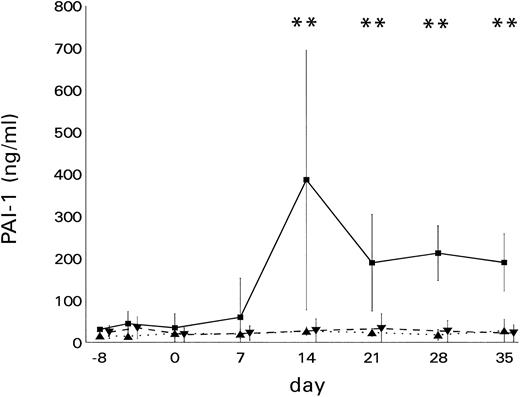
Before conditioning and at the time of BMT, only marginal differences existed between the patient groups. When bilirubin levels exceeded 3 mg/dL for the first time (Figs 1 and 2), a highly significant (P < .001) elevation of PAI-1 (mean ± SD) could be shown in patients with VOD (321.6 ± 161.2 ng/mL; median, 260.0 ng/mL) as compared with patients with GVHD (22.8 ± 8.4 ng/mL; median, 20.2 ng/mL) or other hepatic damage (32.8 ± 30.8 ng/mL; median, 19.0 ng/mL).
The course of PAI-1 levels in BMT recipients with hyperbilirubinemia. PAI-1 levels (mean ± SD) were elevated significantly (**P < .01) in patients with (▪) VOD (n = 7) as compared with patients with (▴) GVHD (n = 7) or (▾) other liver damage (n = 17)
The course of PAI-1 levels in BMT recipients with hyperbilirubinemia. PAI-1 levels (mean ± SD) were elevated significantly (**P < .01) in patients with (▪) VOD (n = 7) as compared with patients with (▴) GVHD (n = 7) or (▾) other liver damage (n = 17)
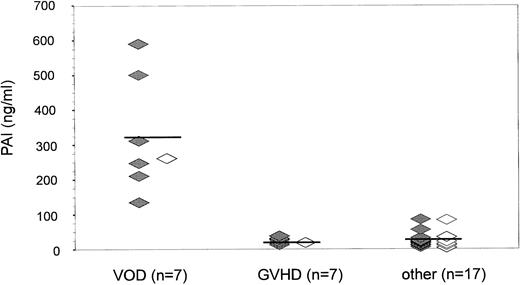
PAI-1 antigen levels at onset of bilirubin increase after BMT (n = 31). As bilirubin levels began to increase (<3 mg/dL), PAI-1 antigen (mean ± SD) was found to be significantly elevated in patients with VOD (321.6 ± 161.2 ng/mL) as compared with patients with GVHD (22.8 ± 8.4 ng/mL; P < .001) or with other liver damage (32.8 ± 30.8 ng/mL; P = .0001). (⋄) Diagnosis by clinical criteria only; (♦) diagnosis confirmed histologically.
PAI-1 antigen levels at onset of bilirubin increase after BMT (n = 31). As bilirubin levels began to increase (<3 mg/dL), PAI-1 antigen (mean ± SD) was found to be significantly elevated in patients with VOD (321.6 ± 161.2 ng/mL) as compared with patients with GVHD (22.8 ± 8.4 ng/mL; P < .001) or with other liver damage (32.8 ± 30.8 ng/mL; P = .0001). (⋄) Diagnosis by clinical criteria only; (♦) diagnosis confirmed histologically.
The decrease of PAI-1 levels within the VOD group between days 21 and 35 as compared with the level on day 14 was not statistically significant (Fig 1).
Considering a cutoff level of 200 ng/mL, the sensitivity of PAI-1 for diagnosing VOD was 85.7% and the specificity was 100%. At a cutoff level of 100 ng/mL, both the sensitivity and specificity was 100%.
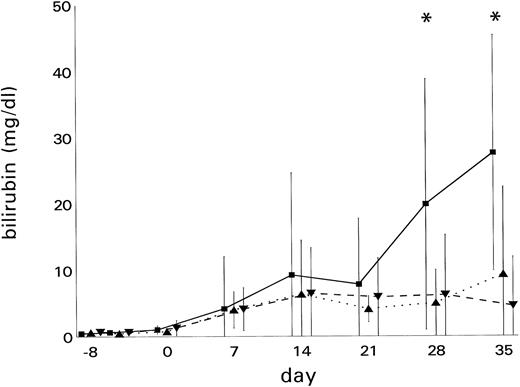
Bilirubin levels (mean ± SD) are indicated in Fig 3. The bilirubin peak levels (mean ± SD) amounted to 18.0 ± 18.9 mg/dL (median, 14.6 mg/dL) in the VOD group, 12.4 ± 13.0 mg/dL (median, 9.9 mg/dL) in the GVHD group, and 7.6 ± 8.7 mg/dL (median, 8.5 mg/dL) in patients with other hepatic injury (VOD v other hepatic injury, P = .047).
Development of hyperbilirubinemia in BMT recipients. Bilirubin levels (mean ± SD) were significantly elevated (*P < .05) in patients with (▪) VOD (n = 7) as compared with patients with (▴) GVHD (n = 7) or (▾) other liver damage (n = 17)
Development of hyperbilirubinemia in BMT recipients. Bilirubin levels (mean ± SD) were significantly elevated (*P < .05) in patients with (▪) VOD (n = 7) as compared with patients with (▴) GVHD (n = 7) or (▾) other liver damage (n = 17)
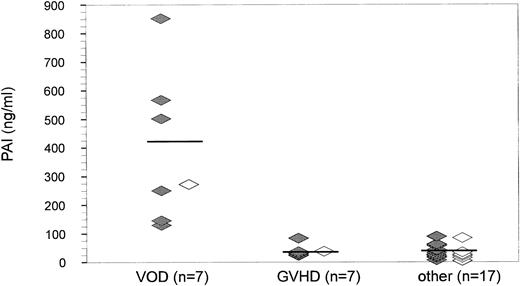
At the time of peak bilirubin values, PAI-1 (mean ± SD) was also significantly (P = .0001, Fig 4) elevated in the VOD group (426.1 ± 230.0 ng/mL; median, 270.0 ng/mL) as compared with patients with GVHD (41.0 ± 20.6 ng/mL; median, 25.6 ng/mL) or other hepatic damage (44.6 ± 32.9 ng/mL; median, 19.1 ng/mL). The sensitivity and specificity at the 200 ng/mL cutoff level was 71.4% and 100%, respectively, and 100% for both at a cutoff level of 100 ng/mL.
PAI-1 antigen levels at the peak of hyperbilirubinemia after BMT (n = 31). At the time when bilirubin levels reached a maximum, PAI-1 antigen (mean ± SD) was found to be significantly elevated in patients with VOD (426.1 ± 230.0 ng/mL) as compared with patients with GVHD (41.0 ± 20.6 ng/mL; P < .001) or with other liver damage (44.6 ± 32.9 ng/mL; P = .0001). (⋄) Diagnosis by clinical criteria only; (♦) diagnosis confirmed histologically.
PAI-1 antigen levels at the peak of hyperbilirubinemia after BMT (n = 31). At the time when bilirubin levels reached a maximum, PAI-1 antigen (mean ± SD) was found to be significantly elevated in patients with VOD (426.1 ± 230.0 ng/mL) as compared with patients with GVHD (41.0 ± 20.6 ng/mL; P < .001) or with other liver damage (44.6 ± 32.9 ng/mL; P = .0001). (⋄) Diagnosis by clinical criteria only; (♦) diagnosis confirmed histologically.
In 5 of 7 patients with VOD, the PAI-1 increase (>100 ng/mL) was observed concomitantly with the onset of bilirubin elevation, whereas in 1 patient, PAI-1 levels increased before bilirubin. In 1 patient, PAI-1 could not be determined the week before bilirubin increased due to a missing sample.
Temperatures did not differ significantly between the groups except on day 14, when patients with GVHD had a significantly (P < .05) higher temperature (38.9°C ± 1.2°C) than did patients with VOD (36.5°C ± 0.7°C) or other hepatic injuries (37.5°C ± 2.4°C).
DISCUSSION
The fate of BMT recipients with VOD depends on the extent of necrosis and fibrosis in acinar zone 3, which is significantly correlated with severe VOD.3 The mortality ranges from 9% among patients with mild VOD to 98% among patients with severe disease.17
Because thrombolytic therapy can lead to a fast removal of symptoms,6 early diagnosis is essential. VOD manifests mostly within the first 3 weeks after transplantation, when thrombocytopenia is present and coagulation is impaired due to the disturbed liver function.
Although biopsies most reliably confirm the diagnosis, the accompanying risk of hemorrhagic complications warrants the search for laboratory markers.
Endothelial cell damage is thought to be an early event in the pathogenesis of VOD.18 PAI-1 is synthesized and released by endothelial cells and represents the main inhibitor of the fibrinolytic system. We have previously described normal PAI-1 levels in BMT recipients without complications; however, a significant increase existed in patients with VOD. On the other hand, the fibrinolytic tissue plasminogen activator (tPA) remained normal in these patients, suggesting a shift towards hypofibrinolysis.13 14
The present data strongly support this hypothesis. Furthermore, because tPA is the physiologic antagonist of PAI-1, our results support the concept of treatment of VOD with tPA.6
Bilirubin levels continued to increase after day 14 in VOD patients, whereas PAI-1 levels showed a tendency to decrease, although not at a level that is statistically significant. It can be speculated that endothelial cell injury is maximal at the time of clinical manifestation, while, as a consequence, liver damage continues and leads to increasing bilirubin levels in this cohort of patients with severe VOD.
Although our data imply that PAI-1 is a sensitive and specific marker for the diagnosis of severe VOD, its value is limited in patients with septic shock and may be limited in patients with severe sepsis. An elevation of PAI-1 has been described in nonleukopenic patients with severe sepsis and septic shock.19-24 Also, in leukopenic patients with septic shock an increase of PAI-1 has been observed.25 26 The increase of PAI-1 in septic shock was also shown in 1 patient of our study classified as suffering from other liver damage. An increase of previously normal PAI-1 levels to 263 ng/mL was found when the patient developed septic shock 2 days after the investigational period.
On the other hand, it has been reported recently that, in leukopenic patients with severe sepsis, PAI-1 levels usually lay within the normal range and were significantly lower than PAI-1 levels of patients suffering from septic shock.25 An influence of leukocytes on PAI-1 levels is also suggested by the observation that, when comparing leukopenic and nonleukopenic sepsis patients without shock, PAI-1 levels were significantly lower in the leukopenic group.26 Because of a lack of sufficient material, we were not able to determine PAI-1 levels in nonjaundiced BMT recipients with severe sepsis. Therefore, the possible significance of PAI-1 for diagnosing VOD in BMT recipients with severe sepsis remains to be established.
Our view that the determination of PAI-1 is relevant for diagnosing VOD is also supported by the course of PAI-1 in 1 patient who is not included in this investigation because VOD manifested as late as day 40. This patient underwent liver transplantation. PAI-1 levels increased with the occurrence of VOD, normalized after transplantation, and reached another peak when the patient suffered from septic shock shortly before she died.27
However, in our cohort, 6 of 7 VOD patients had a temperature equal to or less than 38°C at the onset of hyperbilirubinemia. On the contrary, a significant increase in temperature was found in patients with hepatic GVHD. Additionally, in this group, an increasing bilirubin level (>3 mg/dL) was measured as early as day 7 in 5 patients and on day 21 in 2 patients of the GVHD group. Because the increase on day 7 can hardly be attributed to GVHD effects alone, we assume that the early increase can most probably be explained by overlapping toxic effects of radiochemotherapy.
Chemoirradiation-induced alterations in glutathione levels leading to enhanced vulnerability of the endothelium have been proven to be linked to endothelial cell damage.28 Another mechanism to explain the pathogenesis of VOD focusses on tumor necrosis factor α (TNFα) as a central mediating substance. TNFα induces enhanced tissue factor expression and elevation of PAI-1 in vitro29 and in vivo30 and exerts a net procoagulant effect. The significant correlation of PAI-1 with occurrence of VOD supports the concept of an involvement of cytokines in the pathogenesis of VOD as suggested by our previous studies on systemic release of TNFα.31 Local cytokine production by Kupffer cells seems likely, because TNFα production was accompanied by increased interleukin-6 release, especially in patients with hepatic complications.32
Although we cannot provide evidence that the delayed increase of PAI-1 after chemoirradiation and BMT does not simply reflect progressive endothelial cell injury, considering the data of Deleve28 and the close correlation of increases in PAI-1 with onset of hyperbilirubinemia in our study, we suggest a two-step pathogenesis of VOD. We hypothesize that endothelial cell damage, initiated by the pretransplant conditioning therapy, is followed by a second pathogenetic event resulting in Kupffer cell activation and cytokine-mediated release of PAI-1. Because VOD occurs predominantly during the aplastic phase, translocated bacteria from the damaged gastrointestinal mucosa that deliver endotoxin might be involved in this second step. If possible, the next steps to validate the suggested significance of PAI-1 are to determine PAI-1 levels in a larger number of patients with histologically confirmed VOD in a multicenter study as well as to perform immunohistologic and in vitro studies analyzing PAI-1 expression in endothelial cells after cytotoxic treatment to clarify the exact sequence of events.
In conclusion, our data strongly suggest that analysis of PAI-1 in patients with hyperbilirubinemia after BMT might allow diagnosis of VOD without invasive and potentially life-threatening techniques in the future.
ACKNOWLEDGMENT
We thank Dr K. Schotten (Institute of Biomathematics and Epidemiology, Ludwig-Maximilians-Universität Munich, Munich, Germany) for statistical advice. We thank Dr KE Stötzer (Boehringer Mannheim) for providing PAI-1 enzyme-linked immunosorbent assay kits and K. Hiller for reading the manuscript. We gratefully acknowledge the kind cooperation of the staff of the BMT unit L 21.
Address reprint requests to Christoph Salat, MD, Department of Hematology and Oncology, Klinikum Grosshadern der Ludwig Maximilians-Universität, Marchioninistr, 15, 81377 Munich, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal