Abstract
Yolk sac hematopoiesis is characterized by restricted hematopoietic cell differentiation. Although multipotent hematopoietic progenitor cells have been identified in the early yolk sac, long-term multilineage repopulating (LTMR) hematopoietic stem cell (HSC) activity has not been demonstrable before day 11 postcoitus (PC) using standard transplantation assays. In the present study, day-10 PC yolk sac hematopoietic cells were infused into myeloablated congenic newborn pups and donor cell engraftment and multilineage reconstitution of peripheral blood cells for at least 11 months in primary recipients was observed. In contrast, transplantation of day-10 PC yolk sac cells into congenic adult recipients did not result in engraftment despite pretransplant conditioning of the recipients or use of recipients that were genetically deficient in stem cells. Although fresh yolk sac cells were incapable of reconstitution when injected into adult recipient mice, yolk sac donor-derived cells residing in the bone marrow of primary newborn transplant recipients were capable of efficient reconstitution of conditioned secondary recipient adult mice. Primary newborn and secondary adult recipient animals engrafted with yolk sac cells were observed to have normal peripheral blood white blood cell counts. Lymphocyte subsets in peripheral blood, thymus, and spleen were also similar to control animals. The distribution and frequency of lineage-restricted progenitors derived from bone marrow of secondary transplant recipients were normal. These results indicate that day-10 PC yolk sac HSCs are capable of engrafting and reconstituting the hematopoietic system of conditioned newborn but not adult recipient animals. Furthermore, the ability of the yolk sac HSCs to differentiate into all hematopoietic lineages in these recipients strongly suggests that the local cellular microenvironment plays a prominent role in regulating yolk sac HSC differentiation.
THE PREDOMINANT anatomic site of hematopoiesis changes during murine and human ontogeny.1-3 In the mouse, blood cells appear in yolk sac blood islands on day 7.5 postcoitus (PC),4 multipotent hematopoietic progenitor cells are simultaneously identifiable in the yolk sac and para-aortic splanchnopleure on day 8.5 PC,5 and fetal liver hematopoiesis commences on day 10.6,7 Hematopoietic stem cells (HSCs) in the fetal liver subsequently migrate via the circulation to the bone marrow and contribute to life-long hematopoiesis.8 It remains unclear whether hematopoiesis in the fetal liver is derived from stem cells that originate in situ or from cells that have migrated from extraembryonic or intraembryonic sites.
HSCs capable of long-term multilineage repopulating (LTMR) of conditioned adult recipients reportedly are present in the day-10 PC aorta-gonad-mesonephros region (AGM) but not the day-10 PC yolk sac and fetal liver.9 On day 11 PC, HSCs capable of the LTMR of adult recipients are concurrently present in the yolk sac, AGM, and the fetal liver.9 We and others9,10 have postulated that the failure of less than day-11 PC yolk sac cells to engraft in conditioned adult recipients may occur because of an inability of the yolk sac cells to home to a supportive hematopoietic microenvironment in the adult recipient. Because the sequence of hematopoiesis in murine ontogeny begins in the yolk sac, proceeds to the fetal liver, and is sustained throughout life in the spleen and bone marrow, we have hypothesized that yolk sac cells would engraft in conditioned newborn mice, because the liver, spleen, and bone marrow concurrently produce blood cells at this stage of murine ontogeny.11
The molecular determinants regulating HSC differentiation throughout ontogeny also remain unclear.12,13 HSC differentiation in the yolk sac is restricted and only macrophages and primitive nucleated erythrocytes (synthesizing embryonic hemoglobins) are present as mature blood cells.2 During the early phase (days 12 through 14 PC) of fetal liver hematopoiesis, erythropoiesis exceeds granulopoiesis.14,15 In addition, certain thymocyte precursor cells and a B-lymphocyte–macrophage lineage-restricted progenitor cell are demonstrable in the fetal liver but not in adult bone marrow and spleen, suggesting that the potential of HSC changes during ontogeny.16 It is unclear if hematopoietic cell fate decisions are determined by intrinsic ontogenic HSC differentiation programs17 or if HSC differentiation is modulated by local cellular microenvironments.11 18
We report that HSCs capable of the LTMR of conditioned newborn recipients are present in the yolk sac on day 10 PC. These HSCs do not engraft lethally myeloablated or congenitally anemic adult recipients. In contrast to the highly restricted pattern of yolk sac hematopoietic cell differentiation normally observed, yolk sac donor cells reconstituted multiple blood cell lineages in the newborn recipients, resulting in peripheral blood cell counts and lymphocyte subsets that were similar to those of age-matched control animals. These results suggest that yolk sac HSCs may require a unique microenvironment for graft survival that is present in the newborn but not in the adult animal and strongly support the hypothesis that local microenvironmental signals play a significant role in the differentiation of HSCs.
MATERIALS AND METHODS
Yolk sac cell isolation.Yolk sac cells were obtained as previously described.19 Briefly, timed-mated C57BL/6J females were killed by cervical dislocation. The uterine horns were removed and washed extensively in Hank's Balanced Salt Solution (HBBS), and the day-10 PC embryos (26 to 28 somites) were harvested. The yolk sacs were dissected free and drawn through a 23-guage needle before transferring to a 10-cm dish (Corning, Corning, NY) and incubated with 0.1% collagenase (Sigma, St Louis, MO) in 20% serum (fetal calf serum) and HBSS for 60 minutes at 37°C. After the mild digestion, dispersed yolk sac cells were drawn through the 23-guage needle into a syringe, deposited into a polystyrene tube, and pelleted at 500g for 10 minutes. The single-cell suspension of yolk sac cells was counted and viability was tested via Trypan blue exclusion criteria.
Transplantation assay.Transplant recipient pups were drawn from our breeding colony of B6 hemoglobin diffuse (Hbbd/Hbbd), glucose phosphate isomerase-1a (Gpi-1a/Gpi-1a) mice. These animals are congenic with the C57BL/6J donors who are Hbbs/Hbbs (hemoglobin single) and Gpi-1b/Gpi-1b. We administered busulfan (20 mg/mL in 1% carboxymethylcellulose; Sigma) at a dose of 15 mg/kg to pregnant B6.Hbbd/Hbbd, Gpi-1a/Gpi-1a dams on day 18 PC via subcutaneous injection (SQ) to condition the newborn animals for improved donor cell engraftment as recently described.20 Conditioned congenic newborn pups were injected with a 1-embryo equivalent dose of donor yolk sac cells. One embryo equivalent is equal to the average number of cells isolated from a day-10 PC embryo (140,800 ± 6,640 cells, mean ± SD, n = 40 embryos). The yolk sac cells were suspended in 25 μL HBBS and drawn into a 100-μL glass syringe adapted with an injection guide (Hamilton, Reno, NV). The cell suspension was directly injected into the fetal liver by tracking a 30-guage needle (Becton Dickinson, Franklin Lakes, NJ) SQ between the scapula of the pup and along the thoracic cage to penetrate the liver capsule below the right costal margin. In an alternate approach, the yolk sac cells were injected into the facial vein of the conditioned newborn recipient animals. In other experiments, 5 or 10 embryo equivalent doses of day-10 PC yolk sac cells were intravenously injected into B6.F1 W/Wv recipient adult animals. B6.F1W/Wv animals are genetically anemic and generally accept grafts from C57BL/6J fetal and adult donors without pretransplant conditioning.21,22 Yolk sac cells were also injected via intravenous, intraperitoneal, or intrahepatic routes into lethally myeloablated congenic adult recipients that were conditioned as previously described.23 Finally, to control for the possibility of maternal peripheral blood contaminating the yolk sac preparation, 25 μL of fresh whole peripheral blood from C57BL/6J pregnant dams was directly injected into the liver of 8 conditioned congenic newborn pups. Peripheral blood of all transplanted recipient animals was sampled from 1 to 11 months posttransplant for assay of donor-type hemoglobin or Gpi-1 isoenzyme chimerism.
When primary recipient animals had reached 4 months of age, 6 animals were killed and bone marrow cells were isolated as previously described. Low-density mononuclear cells (LDMN) were recovered and 3 × 106 cells were intravenously injected into secondary recipient B6.Hbbd/Hbbd, Gpi-1a/Gpi-1a adult animals that had been conditioned with total body irradiation (TBI; 11 Gy γ irradiation administered at 96 cGy/min in 2 doses divided by 4 hours using a 137Cs irradiator [Nordion, Kanata, Canada]). Peripheral blood of secondary recipient animals was obtained at intervals from 1 to 6 months posttransplant and analyzed for the presence of donor-type Gpi-1 isoenzyme (see below), peripheral white blood cell (WBC) counts, and lymphocyte subset analysis. Differences in peripheral WBC counts, the percentage of hemoglobin or Gpi-1 isoenzyme type, and lymphocyte subset percentages between primary or secondary transplant recipients and normal control animals were determined using the Student's t-test with the level of significance set at P < .01.
Peripheral blood cell isolation.Peripheral blood was drawn via the tail vein of the transplant recipient animals into three heparinized capillary hematocrit tubes (Monoject, St Louis, MO). The whole blood was spun in a hematocrit tube centrifuge (IEC MB Centrifuge; IEC Equipment, Needham Heights, MA) for 2 minutes. The white blood cell buffy coat was cut from the capillary tube, placed in a 3-mL polypropylene tube, and washed with buffer (HBBS + 0.5% bovine serum albumin + 5 mmol/L EDTA). The WBCs were pelleted and aliquoted into three tubes in which 1 μg of rat antimouse Gr-1 (granulocytes), B220 (B lymphocytes), or CD4/CD8 (T lymphocytes) monoclonal antibodies (Pharmingen, San Diego, CA) were individually added for 15 minutes of incubation on ice. The individually stained cell populations were pelleted (500g for 10 minutes), washed with buffer, repelleted, and resuspended in 80 μL of buffer. To each tube, 1 μL of goat antirat IgG magnetic microbeads (Miltenyi Biotec, Sunnyvale, CA) was added for 15 minutes of incubation on ice. The cell suspensions were pelleted, washed with buffer, repelleted, and resuspended in 500 mL of buffer. The suspended cells were added to individual prefilled and washed columns (Miltenyi Biotic) attached to the magnetic separation device (Miltenyi Biotec). Each column was rinsed with 2,000 μL of buffer and the effluent was collected for discarding. Each column was then removed from the magnet and rinsed with 500 μL of buffer to collect the antigen-positive cells. B and T lymphocytes and granulocytes of ≥92% purity by fluorescent activated cell sorter analysis were routinely obtained in this manner, as previously described.20
Glucose phosphate isomerase-1 and hemoglobin assay.The isolated red blood cells were pipetted into microcentrifuge tubes and lysed in a cystamine solution as previously described.20 The B and T lymphocytes and granulocytes were deposited in separate microcentrifuge tubes and pelleted, and the cells were lysed by freeze-thawing in distilled water. The cell lysates were applied to Titan III Zip Zone cellulose acetate plates (Helena, Beaumont, TX) using a Super Z 0.5-μL 8-well applicator (Helena). The loaded gels were run for 30 minutes in an electrophoresis chamber at 300 V. The Gpi-1 isoenzymes and hemoglobin were detected as recently described.20 Stained gels were scanned with a Rep clinical densitometer (Helena) for quantitation. The assays are sufficiently sensitive to detect 2% donor-type hemoglobin or Gpi-1 isoenzyme chimerism when donor cells are mixed with recipient cells.20
Hematopoietic progenitor cell assay.Bone marrow LDMN cells were isolated from the femurs of secondary recipient mice that were killed 6 months posttransplantation or from normal age-matched control animals. Yolk sac cells were isolated as described above. The cells (100,000/plate for bone marrow cells and 12,500 to 25,000/plate for yolk sac cells) were plated in progenitor cell methylcellulose colony assays as previously described.24 Briefly, the cells were suspended in 10 × 35 mm gridded culture dishes (Lux; Miles Scientific, Naperville, IL) with 1% methylcellulose (Fluka, Huappage, NY), 30% fetal calf serum, 10−5 mol/L 2-mercaptoethanol (Sigma), 2 mmol/L glutamine (GIBCO, Grand Island, NY), 4 U recombinant human erythropoietin (Amgen, Thousand Oaks, CA), 20 U recombinant murine interluekin-3 (Genzyme, Cambridge, MA), and 100 ng recombinant rat stem cell factor (gift of Amgen). The cells were plated in triplicate, incubated in a 5% O2 , 10% CO2 , and 85% N2 humidified environment at 37°C for 7 days and groups of more than 50 cells were scored as colonies.
Lymphocyte subset analysis.Peripheral blood, spleen, thymus, and bone marrow LDMN cells were incubated with 1 μg/106 cells rat antimouse CD16/32 (Pharmingen) for 10 minutes on ice to block nonspecific (Fc) binding of lineage-specific monoclonal antibodies. The cells were pelleted, resuspended, and incubated with rat phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies (1 mg/106 cells) to murine markers, including B220, IgG, IgM, CD43, CD4, CD8, and CD3, or isotype control antibodies (Pharmingen). Cell analysis was accomplished using a dual-laser FACStar PLUS (Becton Dickinson, San Jose, CA) equipped with an argon laser. Dead cells were gated on the basis of forward and side scatter.
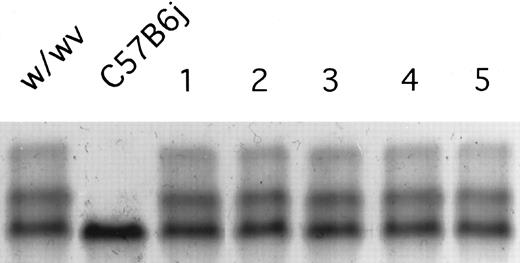
A hemoglobin-stained cellulose acetate electrophoresis gel loaded with peripheral blood from a control B6.W/Wv (w/wv) and a donor C57BL/6J (C57B6j) animal are depicted. B6.W/Wv are heterozygous for the hemoglobin alleles expressing both hemoglobin single (Hbbs) and hemoglobin diffuse (Hbbd), whereas the donor C57BL/6J animals are homozygous for hemoglobin single (Hbbs/Hbbs). Five representative B6.W/Wv recipient animals receiving transplants of 10 embryo equivalent doses (1.4 × 106 cells) of day-10 PC C57BL/6J yolk sac cells were also bled and the hemoglobin bands were stained are indicated in lanes 1 through 5. Because the intensity of the Hbbs band did not increase in proportion to that of the Hbbd bands in these five recipients, there was no evidence of engraftment.
A hemoglobin-stained cellulose acetate electrophoresis gel loaded with peripheral blood from a control B6.W/Wv (w/wv) and a donor C57BL/6J (C57B6j) animal are depicted. B6.W/Wv are heterozygous for the hemoglobin alleles expressing both hemoglobin single (Hbbs) and hemoglobin diffuse (Hbbd), whereas the donor C57BL/6J animals are homozygous for hemoglobin single (Hbbs/Hbbs). Five representative B6.W/Wv recipient animals receiving transplants of 10 embryo equivalent doses (1.4 × 106 cells) of day-10 PC C57BL/6J yolk sac cells were also bled and the hemoglobin bands were stained are indicated in lanes 1 through 5. Because the intensity of the Hbbs band did not increase in proportion to that of the Hbbd bands in these five recipients, there was no evidence of engraftment.
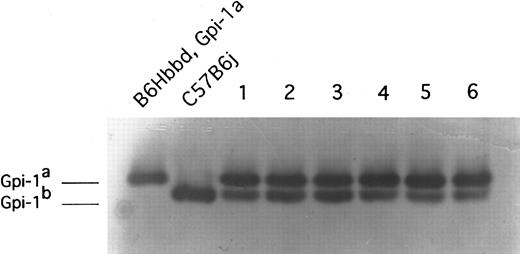
The results of Gpi-1 analysis of the peripheral blood of recipient newborn animals 4 months posttransplant. These animals received transplants of 1 embryo equivalent of day-10.0 PC C57BL/6J yolk sac cells. Recipient animal (B6Hbbd, Gpi-1a) cells express the Gpi-1a isoenzyme and donor animal (C57B6j) cells express the Gpi-1b isoenzyme. Donor-type Gpi-1b enzyme was observed in peripheral red blood cells in these six representative animals and in all 16 animals tested.
The results of Gpi-1 analysis of the peripheral blood of recipient newborn animals 4 months posttransplant. These animals received transplants of 1 embryo equivalent of day-10.0 PC C57BL/6J yolk sac cells. Recipient animal (B6Hbbd, Gpi-1a) cells express the Gpi-1a isoenzyme and donor animal (C57B6j) cells express the Gpi-1b isoenzyme. Donor-type Gpi-1b enzyme was observed in peripheral red blood cells in these six representative animals and in all 16 animals tested.
RESULTS
Preliminary experiments were conducted to determine if day-10 PC yolk sac cells from the C57Bl6J strain engraft in congenic adult recipients. No evidence of donor type hemoglobin was detectable in the transplanted adult B6.F1W/Wv recipients that received 5 (n = 12 recipients) or 10 (n = 8 recipients) embryo equivalent doses of donor day-10 PC yolk sac cells (Fig 1). Furthermore, no evidence of donor cell engraftment (analyzed for donor-type hemoglobin or Gpi-1 b isoenzyme) was identified in 45 congenic adult recipient animals (conditioned with TBI or busulfan) receiving intravenous, intraperitoneal, or intrahepatical transplantation of 5 to 10 embryo equivalent doses of yolk sac cells (data not shown). In total, none of 65 adult animals showed engraftment. These results are consistent with previous reports that yolk sac cells less than day-11 PC fail to engraft in conditioned adult recipient animals.25 26
In contrast to a lack of donor yolk sac cell engraftment in conditioned adult recipients, donor yolk sac cells engrafted in conditioned recipient newborn animals (Fig 2). A progressive increase in the percentage of donor-type hemoglobin was observed in the peripheral blood of the conditioned newborn recipient animals (n = 16) receiving a transplantation of 1 embryo equivalent of day-10 PC yolk sac cells for the first 4 months after transplantation (Table 1). After the donor contribution stabilized at 4 months, no significant difference in the percentage of donor-type hemoglobin was seen. In all recipient animals, the percentage of donor-type Gpi-1b values present in red blood cell lysates was concordant with the percentage of donor-type hemoglobin during the 11-month study period (data not shown); therefore, Gpi-1b isoenzyme evidence of donor engraftment is presented hereafter. Donor-type Gpi-1b enzyme was observed in peripheral blood B and T lymphocytes and granulocytes in all 16 animals tested (Table 2). There was no significant difference in the contributions of yolk sac donor cells to each lineage and no differences were noted over time within a lineage. Day-10 PC yolk sac cells (1 embryo equivalent dose) also engrafted in the conditioned newborn pups when administered via intravenous injection (Table 2), although only 5 of 8 animals engrafted. None of the conditioned pups (n = 8) receiving a transplant of pregnant adult peripheral whole blood showed evidence of donor engraftment in the conditioned recipient newborns, indicating that maternal blood contamination was not playing a role in the reconstitution of the recipients with donor yolk sac cells (data not shown).
Percentage of Donor-Type Hemoglobin in Primary Recipients Receiving Transplants of Day-10 PC Yolk Sac Cells
| Donor Cells . | Months Posttransplant . | |||||
|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 6 . | 11 . |
| Day-10 PC yolk sac | 2 ± 2 | 10 ± 5 | 20 ± 4 | 35 ± 6 | 38 ± 8 | 37 ± 9 |
| Donor Cells . | Months Posttransplant . | |||||
|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 6 . | 11 . |
| Day-10 PC yolk sac | 2 ± 2 | 10 ± 5 | 20 ± 4 | 35 ± 6 | 38 ± 8 | 37 ± 9 |
Data are presented as the mean ± SD percentage of donor hemoglobin of 16 recipient animals.
Percentage of Donor-Type Gpi-1 Isoenzyme Present in the Peripheral Blood Cells of Primary Recipients Receiving Transplants at Birth of Day-10 PC Yolk Sac Cells Via Intraliver or Intravenous Routes
| Cell Type . | Months Posttransplant . | ||
|---|---|---|---|
| . | 4 . | 6 . | 11 . |
| B lymphocyte | |||
| IL | 36 ± 5 | 35 ± 7 | 36 ± 6 |
| IV | 15 ± 1 | 12 ± 4 | ND |
| T lymphocyte | |||
| IL | 26 ± 7 | 28 ± 4 | 32 ± 6 |
| IV | ND | 10 ± 6 | ND |
| Granulocyte | |||
| IL | 33 ± 9 | 34 ± 10 | 31 ± 10 |
| IV | 16 ± 9 | 19 ± 7 | ND |
| Red blood cell | |||
| IL | 35 ± 6 | 39 ± 8 | 37 ± 9 |
| IV | 23 ± 5 | 25 ± 10 | ND |
| Cell Type . | Months Posttransplant . | ||
|---|---|---|---|
| . | 4 . | 6 . | 11 . |
| B lymphocyte | |||
| IL | 36 ± 5 | 35 ± 7 | 36 ± 6 |
| IV | 15 ± 1 | 12 ± 4 | ND |
| T lymphocyte | |||
| IL | 26 ± 7 | 28 ± 4 | 32 ± 6 |
| IV | ND | 10 ± 6 | ND |
| Granulocyte | |||
| IL | 33 ± 9 | 34 ± 10 | 31 ± 10 |
| IV | 16 ± 9 | 19 ± 7 | ND |
| Red blood cell | |||
| IL | 35 ± 6 | 39 ± 8 | 37 ± 9 |
| IV | 23 ± 5 | 25 ± 10 | ND |
Data are presented as the mean ± SD of 3 representative animals (n = 16 for IL injections and n = 5 for IV injections).
Abbreviations: IL, intraliver; IV, intravenous; ND, not done.
To assess whether the donor yolk sac cells that engrafted in the primary recipients were capable of long-term survival and to determine if these cells were now capable of engrafting in adult recipients, bone marrow cells were harvested from 6 random primary newborn recipients 4 months posttransplant and the marrow from each was transplanted into 3 lethally irradiated secondary recipient adult animals. Donor-type Gpi-1b isoenzyme was observed in peripheral blood B and T lymphocytes, granulocytes, and erythroid cells in all secondary recipients animals at 4 and 6 months posttransplant (Table 3), indicating that engrafted donor yolk sac HSCs in primary recipients were capable of survival and reconstitution of adult secondary hosts.
Percentage of Donor-Type Gpi-1 Isoenzyme Present in the Peripheral Blood of Secondary Recipients Receiving Transplants of Bone Marrow Cells From 4-Month-Old Primary Recipients
| Cell Type . | Months Posttransplant . | |
|---|---|---|
| . | 4 . | 6 . |
| B lymphocyte | 35.3 ± 5.7 | 33.0 ± 6.0 |
| T lymphocyte | 27.0 ± 4.6 | 30.8 ± 9.9 |
| Granulocyte | 30.8 ± 4.7 | 35.5 ± 6.1 |
| Red blood cells | 37.7 ± 5.7 | 35.3 ± 4.8 |
| Cell Type . | Months Posttransplant . | |
|---|---|---|
| . | 4 . | 6 . |
| B lymphocyte | 35.3 ± 5.7 | 33.0 ± 6.0 |
| T lymphocyte | 27.0 ± 4.6 | 30.8 ± 9.9 |
| Granulocyte | 30.8 ± 4.7 | 35.5 ± 6.1 |
| Red blood cells | 37.7 ± 5.7 | 35.3 ± 4.8 |
Data are presented as the mean ± SD of 6 representative animals (n = 18).
The mean peripheral WBC counts and hematocrit levels of primary recipients receiving transplants at birth of day-10 PC yolk sac cells were not significantly different from age-matched control animals not receiving transplants (data not shown). Peripheral WBC count differentials of the primary recipients were also equivalent to age-matched control animals (data not shown). In addition, peripheral blood parameters were normal in secondary recipient animals (data not shown).
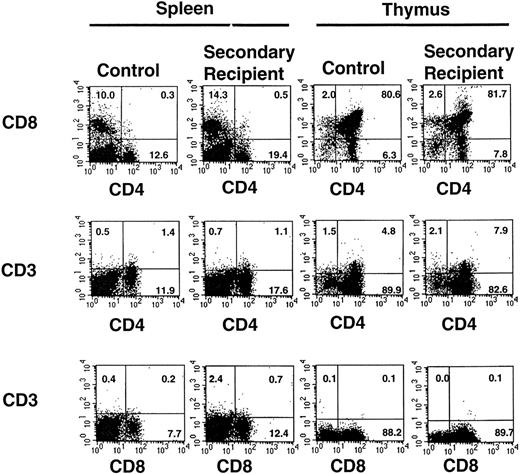
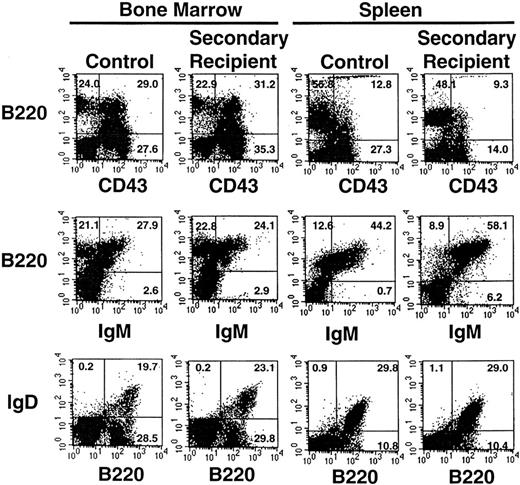
The percentage of peripheral blood lymphocytes comprising T- and B-lymphocyte subsets was determined at 6 months posttransplant in primary and secondary recipients and compared with values obtained in age-matched control mice (Table 4). There was no significant difference in the percentage of double-positive T cells (CD4+C8+) or single-positive CD4+ or CD8+ subsets in these three groups. Likewise, thymic T-cell subsets (Fig 3) were similar in 6-month posttransplant secondary recipient and age-matched control animals. Similarly, the percentage of mature B lymphocytes in peripheral blood (IgM+B220+ or IgD+B220+) was not significantly different in the primary or secondary recipient mice compared with control animals (Table 4). Further analysis indicated that the percentage of cells expressing immature (CD43+B220+) and mature (IgM+B220+, IgD+B220+) B-lymphocyte markers in the spleen and bone marrow were not significantly different in 6-month posttransplant secondary recipient animals compared with age-matched control animals (Fig 4). When splenic, bone marrow, and thymic lymphocyte subsets from 6-month posttransplant secondary recipient animals were isolated and analyzed for donor-type Gpi-1b isoenzyme (Table 5), the percentage of chimerism was similar to the percentage of chimerism determined for peripheral blood B and T cells in these animals (Table 3). In summary, yolk sac HSCs appear capable of contributing to full T- and B-lymphoid differentiation in vivo.
Percentage of Peripheral Blood Lymphocytes Expressing Selected Subset Markers 6 Months After Transplantation
| Cell Source . | CD4+CD8+ . | CD4+ . | CD8+ . | B220+IgD+ . |
|---|---|---|---|---|
| Primary transplants | 0.2 ± 0.1 | 11.6 ± 1.4 | 7.4 ± 1.4 | 26.7 ± 5.0 |
| Secondary transplants | 0.6 ± 0.3 | 15.0 ± 2.8 | 6.1 ± 2.4 | 22.7 ± 6.0 |
| Control mice | 0.2 ± 0.1 | 15.7 ± 3.0 | 7.8 ± 2.1 | 32.3 ± 6.1 |
| Cell Source . | CD4+CD8+ . | CD4+ . | CD8+ . | B220+IgD+ . |
|---|---|---|---|---|
| Primary transplants | 0.2 ± 0.1 | 11.6 ± 1.4 | 7.4 ± 1.4 | 26.7 ± 5.0 |
| Secondary transplants | 0.6 ± 0.3 | 15.0 ± 2.8 | 6.1 ± 2.4 | 22.7 ± 6.0 |
| Control mice | 0.2 ± 0.1 | 15.7 ± 3.0 | 7.8 ± 2.1 | 32.3 ± 6.1 |
Data are presented as the mean ± SD percentage of total lymphocytes from 6 representative animals.
Expression of T-lymphocyte subset markers on cells isolated from the thymus and spleen of one representative secondary recipient animal and an age-matched control animal. Gates were set to identify populations of positive cells in the normal animals (compared with isotype controls) and then were applied to identify positive cells recovered from the secondary recipient animal. The percentages of positive cells are indicated. A total of 8 secondary recipient animals were analyzed with similar results.
Expression of T-lymphocyte subset markers on cells isolated from the thymus and spleen of one representative secondary recipient animal and an age-matched control animal. Gates were set to identify populations of positive cells in the normal animals (compared with isotype controls) and then were applied to identify positive cells recovered from the secondary recipient animal. The percentages of positive cells are indicated. A total of 8 secondary recipient animals were analyzed with similar results.
Expression of B-lymphocyte subset markers on cells isolated from the spleen and bone marrow of one representative secondary recipient animal and an age-matched control animal. Gates were set to identify populations of positive cells in the normal animals (compared with isotype controls) and then were applied to identify positive cells recovered from the secondary recipient animal. The percentages of positive cells are indicated. A total of 8 secondary recipient animals were analyzed with similar results.
Expression of B-lymphocyte subset markers on cells isolated from the spleen and bone marrow of one representative secondary recipient animal and an age-matched control animal. Gates were set to identify populations of positive cells in the normal animals (compared with isotype controls) and then were applied to identify positive cells recovered from the secondary recipient animal. The percentages of positive cells are indicated. A total of 8 secondary recipient animals were analyzed with similar results.
Percentage of Donor-Type Gpi-1 Isoenzyme Present in B- and T-Lymphocyte Subsets From 6-Month-Old Secondary Recipient Animals Receiving Transplants of Bone Marrow Cells From Primary Newborn Recipients of Day-10 Yolk Sac Cells
| Lymphocyte Subset . | Percentage of Donor Gpi-1 . |
|---|---|
| Thymus | |
| CD4+ CD8+ | 28 |
| CD4− CD8− | 26 |
| CD4+ | 31 |
| CD8+ | 25 |
| Bone marrow | |
| B220+ CD43+ | 38 |
| B220+ IgG+ | 24 |
| B220+ IgM+ | 30 |
| B220+ | 29 |
| CD43+ | 35 |
| Spleen | |
| B220+ IgG+ | 20 |
| B220+ IgM+ | 30 |
| B220+ CD43+ | 35 |
| CD4+ | 29 |
| CD8+ | 31 |
| Lymphocyte Subset . | Percentage of Donor Gpi-1 . |
|---|---|
| Thymus | |
| CD4+ CD8+ | 28 |
| CD4− CD8− | 26 |
| CD4+ | 31 |
| CD8+ | 25 |
| Bone marrow | |
| B220+ CD43+ | 38 |
| B220+ IgG+ | 24 |
| B220+ IgM+ | 30 |
| B220+ | 29 |
| CD43+ | 35 |
| Spleen | |
| B220+ IgG+ | 20 |
| B220+ IgM+ | 30 |
| B220+ CD43+ | 35 |
| CD4+ | 29 |
| CD8+ | 31 |
Data are presented as the mean values for two experiments.
Culture of yolk sac hematopoietic progenitor cells in methylcellulose with recombinant hematopoietic growth factors showed a dramatic difference in both the number and type of lineage-restricted and multipotent progenitor colonies compared with cultures initiated with adult bone marrow cells (Table 6). Significantly more total colony-forming cells, multipotent progenitors, and committed erythroid and macrophage progenitors were cultured from the freshly isolated yolks sac cells compared with the bone marrow cells from long-term engrafted secondary recipient animals and the age-matched control animals (Table 6). However, more striking was the observation that no granulocyte lineage-restricted progenitors were cultured from yolk sac cells, whereas these lineage-restricted progenitors were a predominant population in normal adult bone marrow. In data that parallel the findings of all other transplantation studies, the differences in the frequency and distribution of progenitor populations (fresh yolk sac v normal adult bone marrow) were no longer identifiable when bone marrow cells from secondary recipient animals, which contained progeny derived from donor yolk sac cells, were used to initiate progenitor cultures. The frequency and distribution of bone marrow colony-forming cells in the secondary recipients was comparable with normal age-matched control animals (Table 6). The percentage of secondary recipient bone marrow granulocyte-macrophage, granulocyte, and burst-forming unit-erythroid progenitor colonies expressing donor-type Gpi-1b was 28%, 36%, and 42%, respectively, when 40 to 50 colonies from triplicate plates were plucked and analyzed (2 experiments). This level of donor cell chimerism was similar to the percentage of donor cell chimerism identified in the peripheral blood myeloid lineages of secondary recipient animals (Table 3). Thus, the behavior of the donor yolk sac cells is altered upon engraftment in the conditioned newborn animals and recovered bone marrow cells appear to function in a pattern not different from normal adult bone marrow cells when engrafted in secondary recipient animals.
Progenitor Cell Content in Freshly Isolated Yolk Sacs and in Bone Marrow From Secondary Recipient and Normal Control Animals
| Cell Source . | Colonies/100,000 Cells Plated . | ||||
|---|---|---|---|---|---|
| . | E . | G . | M . | GM . | GEMM . |
| Day-10 yolk sac | 236 ± 366-150 | 0 | 163 ± 196-150 | 22 ± 8 | 121 ± 126-150 |
| Secondary host | 33 ± 17 | 121 ± 21 | 95 ± 26 | 16 ± 9 | 67 ± 25 |
| Normal control | 39 ± 12 | 116 ± 18 | 101 ± 12 | 28 ± 13 | 59 ± 21 |
| Cell Source . | Colonies/100,000 Cells Plated . | ||||
|---|---|---|---|---|---|
| . | E . | G . | M . | GM . | GEMM . |
| Day-10 yolk sac | 236 ± 366-150 | 0 | 163 ± 196-150 | 22 ± 8 | 121 ± 126-150 |
| Secondary host | 33 ± 17 | 121 ± 21 | 95 ± 26 | 16 ± 9 | 67 ± 25 |
| Normal control | 39 ± 12 | 116 ± 18 | 101 ± 12 | 28 ± 13 | 59 ± 21 |
Burst-forming unit-erythroid (E) and colony-forming units-granulocyte (G), -macrophage (M), –granulocyte-macrophage (GM), and mixed lineage (GEMM) were scored after 7 days of culture and colony identity was confirmed by plucking colonies and microscopically identifying the Giemsa-stained mature blood cells present. Data shown are the mean ± SD of triplicate cultures of two experiments.
P < .01 day 10 yolk sac versus secondary host or normal control.
DISCUSSION
Numerous questions regarding the embryonic origin, proliferation, and migration of murine hematopoietic stem cells remain unresolved.1,3 Although some evidence has been reported to show a contribution of day-8 to 10 PC yolk sac donor cells to lymphoid and erythroid lineages or to multilineage progenitor cell colonies,4,5,27-32 no LTMR HSC activity has been detected in the yolk sac before day 11 PC.9,25,26,33 One current hypothesis to explain these data is that LTMR HSC activity first originates in the intraembryonic AGM region of the developing embryo at day 10 PC and that, on day 11 PC, HSC activity also becomes simultaneously evident in the yolk sac and fetal liver.9,34 No LTMR activity has been detectable in peripheral blood cells of the murine embryo on day 10 or 11 PC using adult animals as transplant recipients.10 The present results comprise definitive evidence that HSCs with the capacity to provide LTMR of primary and secondary myeloablated hosts are present as early as day 10 PC in the murine extraembryonic yolk sac.
Despite the highly restricted pattern of blood cell differentiation normally observed in the embryonic yolk sac,1,2 19 HSCs present in the day-10 PC yolk sac donor cell population differentiated in the recipient animals in a pattern not distinguishable from normal animals. Peripheral WBC counts, hematocrits, and lymphocyte subsets were all similar in primary and secondary transplant recipients when compared with normal age-matched control animals, and stable chimerism was maintained for nearly 1 year in the primary recipients and for a minimum of 6 months in secondary recipient animals. Comparison of the frequency and distribution of hematopoietic progenitor cells present in the freshly isolated day-10 PC yolk sac cells with the results obtained from culture of normal adult bone marrow or the bone marrow cells of secondary recipient animals indicates that significant differences in the proliferation and differentiation of the donor yolk sac cells occurs when the cells engraft in the recipient animals. Because nearly one third of the myeloid progenitor cells in the secondary recipients expressed the donor-type Gpi-1b enzyme, a higher frequency of total colony-forming cells, multipotent progenitors, and fewer committed granulocyte progenitors would have been expected if the donor yolk sac cells continued to proliferate and differentiate in vivo in the pattern displayed by freshly isolated day-10 PC yolk sac cells in vitro. In contrast, the frequency and distribution of bone marrow colony-forming cells was similar in the secondary recipient animals and normal age-matched control animals. These results suggest that the in vivo proliferation and differentiation of day-10 PC yolk sac HSC are responsive to and regulated by the cellular microenvironments in which the yolk sac cells ultimately reside.
The inability to detect engraftment of less than day-11 PC yolk sac cells in previous studies contrasts with the high-level engraftment of donor yolk sac cell in newborn hosts presented here. These differences may result from the inability of early yolk sac cells to home and/or engraft in the conditioned adult recipients used in prior experiments. These data agree with our inability to show engraftment of yolk sac cells in genetically anemic or lethally conditioned adult animals in the present studies. During murine ontogeny, hematopoietic stem and progenitor cells are postulated to arise in multiple sites (yolk sac and para-aortic splanchnopleure) before development of an intact circulatory system.1-3 10 After circulation is established (day 9 PC), hematopoietic stem and progenitor cells are postulated to seed the developing liver primordium (day 10 PC); thereafter, multipotent progenitors and stem cells seed other hematopoietic organs. Our data suggest that this sequence of hematopoietic cell migration is critical, although these studies do not specifically identify the target organs or cellular components of the organ microenvironments that are essential for yolk sac cell engraftment.
Differential support of hematopoietic cells by stromal cells derived from hematopoietic microenvironments at different stages of ontogeny has been reported.15-19 Hematopoietic stem and progenitor cell proliferation and differentiation occurs in a complex cellular microenvironment composed of hematopoietic cords, macrophages, reticular fibroblasts, barrier cells, endothelial cells, and bone-forming cells.35-41 Individual stromal cell types or subpopulations of stromal cells of the same type show a differential capacity to support hematopoietic stem cell proliferation and lineage differentiation in vitro.19,41-48 In vivo studies also support the concept of unique cellular microenvironments that differentially support hematopoietic cell proliferation and differentiation when compared at differing stages of ontogeny.35,49-52 The molecular mechanisms regulating differential support of HSC proliferation and differentiation by stromal cells are unknown, but the ability of hematopoietic cells to home to a specific supportive hematopoietic environment via cell-cell interactions through cell surface molecules is likely to be a critical first step in engraftment.10 53-55
Donor cells were delivered directly into the liver of the newborn conditioned hosts in the studies reported here. This method of delivery resulted in higher levels of engraftment than when the same number of yolk sac cells were administered via intravenous injection, suggesting that the newborn liver may play an important role in supporting the proliferation and differentiation of yolk sac HSCs that are capable of homing to and engrafting in this organ. Future experiments designed to determine the site and kinetics of yolk sac cell engraftment in conditioned newborn recipients will improve our understanding of the ontogeny of cell surface molecules that promote HSC homing to hematopoietic microenvironments. Biochemical and molecular dissection of the signals critical for the microenvironment to support differentiation of migrating yolk sac HSCs may also lead to a fundamental understanding of embryonic cell fate decisions.
ACKNOWLEDGMENT
The authors thank P. Fox for manuscript preparation and Drs D. Williams and H. van der Loo for reviewing the manuscript.
Supported in part by Grant No. 6-FY95-0376 from the March of Dimes Birth Defects Foundation.
Address reprint requests to Mervin C. Yoder, MD, Wells Center for Pediatric Research, Indiana University Medical Center, 702 Barnhill Dr, RI 2658, Indianapolis, IN 46202.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal