Abstract
Human umbilical vein endothelial cells (HUVECs) undergo programmed cell death (apoptosis) after coculture with peripheral blood mononuclear cells (PBMCs) preactivated by ionizing radiation (IR) or by bacterial endotoxin (lipopolysaccharide [LPS]). Cell-to-cell contact-mediated apoptosis could be blocked in both cases by anti–tumor necrosis factor-α (anti–TNF-α) monoclonal antibody MAK195 and also by the antagonistic cytokine interleukin-10 (IL-10). Cell-free PBMC supernatants from both preactivation treatments were sufficient to trigger endothelial apoptosis. In contrast, MAK195 and IL-10 were found to be ineffective in this system, suggesting a TNF-α–independent mechanism. However, N-Acetylcystein, an antioxidant, fully abrogated programmed cell death mediated by the supernatant of IR-treated PBMCs, but not of LPS-treated PBMCs. Additionally, we found that coculture and cell-free supernatants of preactivated as well as untreated PBMCs caused cell cycle arrest in proliferating EC in G0/1 , which could be relieved by IL-10, but not by anti–TNF-α. Further analysis showed that transforming growth factor-β, which was constitutively expressed in the supernatant of PBMCs, namely lymphocytes, was responsible for this. These data suggest a pathophysiologic model in which preactivated PBMCs cause EC damage and may prevent blood vessel repair by arresting the proliferation of ECs. This could contribute to the understanding of various clinical endothelial complications that occur after irradiation as well as in cases of endotoxemia or related inflammatory states.
APOPTOSIS OR APOPTOTIC programmed cell death may be one cause of endothelial cell (EC) damage contributing to various inflammatory disorders, including transplant-related complications (TRCs) after bone marrow transplantation (BMT). These TRCs are almost always associated with endothelial complications, such as the endothelial leakage syndrome (ELS) or the veno-occlusive disease (VOD).1 In particular, ionizing radiation (IR) has been found to induce apoptosis in vitro2,3 and in vivo.4,5 Because total body irradiation (TBI) is part of the pretransplant conditioning regimen, it is likely to contribute to the pathophysiology of TRCs.6,7 On the other hand, evidence is accumulating that cytokines are crucially involved in host-related inflammatory processes8 and that tumor necrosis factor-α (TNF-α) plays a key role.9,10 Previous work from our group has shown cultured ECs to undergo apoptosis in response to IR and that this effect can be enhanced by bacterial endotoxin (lipopolysaccharide [LPS]).3 The transmembrane form of TNF-α (mTNF-α) was identified as accounting for this LPS-mediated sensitization of ECs towards IR-induced cell death. Furthermore, the antagonistic cytokine interleukin-10 (IL-10) turned out to be protective by its modulation of mTNF-α expression on LPS+IR-treated EC.3
Using a clinically more relevant model, we have now investigated whether LPS- and/or IR-treated peripheral blood mononuclear cells (PBMCs) interfered with EC viability and their proliferative repair capacity. Preactivated PBMCs caused apoptosis in ECs via mTNF-α in a coculture model as well as after the incubation of ECs with cell-free supernatants of preactivated PBMCs, independent of TNF-α. Concurrently, a constitutively expressed soluble factor in PBMC preparations, most likely transforming growth factor-β (TGF-β), caused cell cycle arrest in ECs in the G0/1 phase. The data presented here may have clinical impact, because they provide evidence of mTNF-α's importance as a trigger of cytotoxic mechanisms in the endothelium, which supports the rationale for therapeutic intervention based on cytokine antagonists such as IL-10.
MATERIALS AND METHODS
Cell culture and reagents.Human umbilical vein ECs (HUVECs; further referred to as ECs throughout this report) were freshly prepared from umbilical cords using the method of Jaffe et al.11 EC cultures were found to be greater than 95% pure, as assessed by staining for factor VIII-related antigen (von Willebrand factor) by indirect immunofluorescence (data not shown). ECs were cultured in low (3%) serum endothelial cell growth medium (EGM; Promocell, Heidelberg, Germany) to avoid undesirable side effects due to possible LPS contamination by the serum. The LPS content of the serum used, as determined by the manufacturer, was less than 3.6 EU/mL, ie, less than 0.26 ng/mL. Only the second to fourth passage cells were used in this study. PBMCs were derived from the heparinized (Novo Nordisk, Mainz, Germany) blood of healthy human blood donors according to a standard protocol using Ficoll hypaque (Pharmacia, Freiburg, Germany) density gradient centrifugation. The monocytic cell line Mono Mac6 was kindly provided by Dr H.W.L. Ziegler-Heitbrock (Ludwig-Maximilians-Universität, München, Germany). All cell culture reagents were purchased from GIBCO (Karlsruhe, Germany), unless stated otherwise. Cytokines, endotoxin, and cytokine antagonists and (monoclonal) antibodies were obtained from the following sources or described in the following references: human recombinant TNF-α (Knoll AG, Ludwigshafen, Germany), LPS (serotype 026:B6 from Escherichia coli; Sigma, Deisenhofen, Germany), human recombinant IL-10 (Biermann, Bad Nauheim, Germany), human recombinant TGF-β1 and -β2 (PBH, Braunschweig, Germany), anti–TGF-β ELISA kit (Quantikine; Biermann), anti–TNF-α monoclonal antibodies (MoAbs) MAK195 and MAK199 (Knoll AG)12 and T1 (hybridoma supernatant; kindly provided by Dr M. Grell, University of Stuttgart, Stuttgart, Germany), anti-CD14 MoAb (Immunotech, Hamburg, Germany), anti–TGF-β MoAbs 1DII.16 (IC Chemikalien, München, Germany) and AB-101-NA (Biermann), and goat antimouse IgG-fluorescein isothiocyanate (FITC)–conjugated antibody F(ab)2 fragment (Dako, Hamburg, Germany). For the inhibition studies, these antibodies were used at a concentration of 20 μg/mL. The optimal antibody dose was determined by dose-response experiments (not shown).
Apoptosis assays.To detect apoptosis of ECs, 1 × 105 ECs/plate were seeded into 35-mm petri dishes (Nunc, Wiesbaden, Germany) and cocultured for 48 hours with PBMCs. PBMCs had been irradiated either with 4 Gy (137Cs γ-radiation source; Atomic Energy of Canada Ltd, Canada) or incubated in the presence or absence of LPS (10 ng/mL) for 4 hours and washed before coculture with ECs. PBMCs were then removed after 48 hours by vigorous washing. Alternatively, PBMCs were treated as stated above, and cell-free supernatants (SN) of these cells were collected after 4 hours for incubation with ECs. To perform the apoptosis assays, ECs were fixed with methanol/acetone (1:1) for 2 minutes. Costaining for factor VIII-related antigen expression (not shown) showed the purity of EC cultures. ECs were then washed once in phosphate-buffered saline (PBS) and stained with 4,6-Diamidino-2-phenylindole (DAPI; 0.5 μg/mL; Sigma), dissolved in 20% glycerin/PBS, mounted, and subjected to microscopic analysis. Nuclear condensation as shown by DAPI staining in the absence of trypan blue uptake is considered characteristic of apoptosis as opposed to necrosis.13 14 The quantitative analysis included counting the number of apoptotic relative to all identifiable cells from at least 10 microscopic fields, with an average of 70 cells per field.
An alternative method for detecting apoptosis in human ECs was performed as previously described15 using flow cytometry (see below). Apoptotic cells were identified by a characteristic side scatter image distinct from that of nonapoptotic cells.
Flow cytometric analyses.Cell surface expression of TNF on activated PBMCs was assessed by indirect immunofluorescence and subsequent flow cytometry using the FACScan flow cytometer and the CellQuest analysis program (Becton Dickinson, Heidelberg, Germany). After treatment and incubation for various time periods, cells were washed once in cold PBS and incubated with anti–TNF-α MoAb for 1 hour on ice. Omission of the first antibody served as the negative control for detecting nonspecific fluorescence (further referred to as nil control). Experiments with isotype-matched irrelevant control antibodies yielded similar results (not shown). After a single wash, cells were incubated with an antimouse IgG-FITC–conjugated antibody for 45 minutes on ice. Cells were analyzed after a final wash. PBMC viability was determined by costaining with propidium iodide (PI; 0.02 μg/mL; Sigma). Only PI-negative cells were analyzed.
Detection of intracellular antigens by flow cytometry was performed according to a previously published protocol.16 Briefly, PBMCs were incubated for 4 hours in the presence of monensin (1 μmol/L) and then fixed for 10 minutes in ice-cold paraformaldehyde (4% in PBS). After blocking with 0.1% saponin, 10% human AB serum, 0.01 mol/L HEPES buffer, and 100 μg/mL goat IgG for 10 minutes, cells were incubated with an anti–TGF-β MoAb (5 μg/mL diluted in saponin buffer for permeabilization) for 45 minutes at room temperature and subsequently washed in saponin buffer. The remaining experimental procedure was identical to that of regular flow cytometry (see above).
Enzyme-linked immunosorbent assays (ELISA).TNF-α in the supernatant of either untreated, irradiated, or LPS-treated PBMCs was determined by the ELISA sandwich technique. Briefly, 96-well plates were coated with the anti–TNF-α MoAb MAK199 and subsequently (hyper)incubated with the relevant cell culture supernatants or titrated TNF-α standards, respectively. Development was performed with a biotinylated anti–TNF-α MoAb (MAK195) and a peroxidase conjugate according to a standard protocol. The ELISA for the detection of TGF-β2 in the supernatants of PBMCs and ECs was performed exactly according to the manufacturer's instructions.
Cell cycle analysis.Cell cycle analysis was performed by analyzing DNA-synthesis in relation to DNA-content, as described.17 Briefly, ECs were cocultured for 18 hours with either PBMCs or cell-free supernatants of these cells. Bromodeoxyuridine (BrdU; Amersham-Kit 1:1,000; Amersham, Arlington Heights, IL) was added to the medium 4 hours before the incubation was complete. Subsequently, PBMCs were removed, and the ECs were washed and trypsinized, followed by fixation in 70% EtOH for 30 minutes at −20°C. Cells were then resuspended in 0.2 mg/mL pepsin in 2 N HCl and incubated for 30 minutes at room temperature. Neutralization was performed with 0.1 mol/L Na2B4O7 . After washing, cells were resuspended in PBS/0.5% Tween-20/2% fetal calf serum (buffer) containing an anti–BrdU-FITC — conjugated antibody (Boehringer Mannheim, Mannheim, Germany) and incubated for 30 minutes. After a final wash, the ECs were resuspended in buffer containing RNAse A (0.5 mg/mL; Boehringer Mannheim) and 50 μg/mL PI (Sigma) and incubated for at least 30 minutes at room temperature. BrdU incorporation as a parameter of DNA synthesis was measured by flow cytometric analysis (see above) and compared with DNA content, as determined by PI incorporation. The resulting dot and/or contour plots were gated to define the G0 /G1 -, S-, and G2 -phases.
Statistical analysis.The significance of differences between experimental values was assessed by means of the Student's t-test.
RESULTS
Preactivated PBMCs induce apoptosis in ECs through the action of transmembrane TNF-α: protective role of IL-10.To investigate the influence of PBMCs on EC viability, PBMCs were isolated from the peripheral blood of healthy human blood donors and were left either untreated or treated with LPS (10 ng/mL) and/or exposed to IR (4Gy). Four grays was chosen as the clinically relevant dose based on usual pretransplant conditioning schedules before BMT, which use 3 × 4 Gy TBI.1,9 The applied LPS concentration approximates the serum levels observed in various cases of endotoxemia.18 After 4 hours of incubation, PBMCs were washed, coincubated with ECs for 48 hours, and subsequently assayed for the presence of apoptotic cells via DAPI stain analysis (see the Materials and Methods). The results of this experiment are shown in Fig 1A. Endothelial monocultures (EC) showed no signs of apoptosis. In contrast, IR- and LPS-treated PBMCs in fact caused programmed cell death in ECs, whereas untreated PBMCs did not. A combined treatment with IR and LPS did not significantly change the number of apoptotic cells (data not shown) and was therefore omitted in all of the following experiments. Costaining for factor VIII-related antigen expression by indirect immunofluorescence (not shown) showed the purity of EC cultures after PBMCs had been removed by vigorous washing.
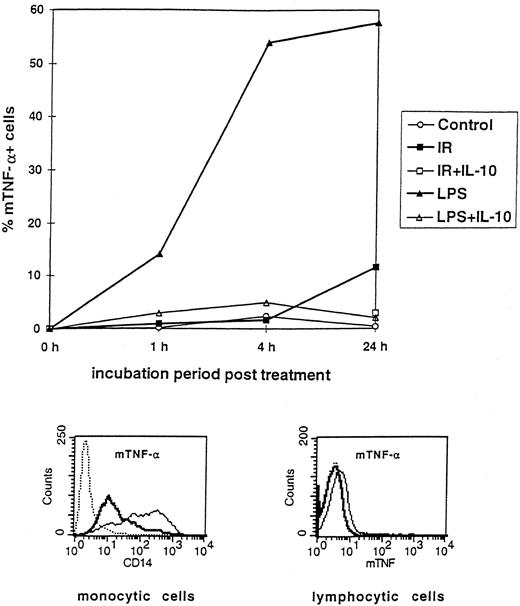
Preactivated PBMCs induce apoptosis in ECs. Quantitative microscopic analysis of DAPI-stained ECs after 48 hours of coculture. Cells were microscopically analyzed by counting apoptotic and nonapoptotic cells (n = 10 microscopic fields including an average of 70 cells per field). (A) PBMCs were either untreated (PBMC), irradiated (PBMC-IR; 4 Gy), or incubated with bacterial endotoxin (PBMC-LPS; 10 ng/mL) for 4 hours. (B) Human IL-10 (0.1 μg/mL) and MAK 195 (anti-TNF-α MoAb; 20 μg/mL) inhibit the apoptosis-inducing effects of either irradiated or LPS-treated PBMCs on ECs. Results are given as the percentage of apoptotic cells (± standard deviation [SD]). These results are representatives of at least four independent experiments. *,**P < .001 of apoptosis of control ECs versus ECs coincubated with preactivated PBMCs and control ECs versus ECs cocultured with preactivated PBMCs in the presence of IL-10 or anti–TNF-α MoAb MAK195.
Preactivated PBMCs induce apoptosis in ECs. Quantitative microscopic analysis of DAPI-stained ECs after 48 hours of coculture. Cells were microscopically analyzed by counting apoptotic and nonapoptotic cells (n = 10 microscopic fields including an average of 70 cells per field). (A) PBMCs were either untreated (PBMC), irradiated (PBMC-IR; 4 Gy), or incubated with bacterial endotoxin (PBMC-LPS; 10 ng/mL) for 4 hours. (B) Human IL-10 (0.1 μg/mL) and MAK 195 (anti-TNF-α MoAb; 20 μg/mL) inhibit the apoptosis-inducing effects of either irradiated or LPS-treated PBMCs on ECs. Results are given as the percentage of apoptotic cells (± standard deviation [SD]). These results are representatives of at least four independent experiments. *,**P < .001 of apoptosis of control ECs versus ECs coincubated with preactivated PBMCs and control ECs versus ECs cocultured with preactivated PBMCs in the presence of IL-10 or anti–TNF-α MoAb MAK195.
To assess the role of mTNF-α in the induction of EC apoptosis, as has been shown for EC monocultures and microvascular endothelial cells in previous studies,3 PBMCs were preactivated as before and cocultured with ECs in the presence or absence of inhibitory anti–TNF-α MoAb MAK195 (Fig 1B). In the same experiment, the ability of the antagonistic cytokine IL-10 to interfere with PBMC-triggered apoptosis was investigated. Figure 1B shows that MAK195 fully blocked apoptosis induced by both types of preactivated PBMCs, thereby suggesting crucial involvement of mTNF-α in EC damage. IL-10 was also capable of inhibiting EC programmed cell death to control levels, probably by interfering with the mTNF-α expression on PBMCs.
It is interesting to note that ECs that had themselves been irradiated were sensitized for apoptosis by coculture with mTNF-α–bearing cells to an extent similar to that seen with untreated ECs (data not shown).
To support these observations with an alternative apoptosis assay, PBMCs were preactivated as already described and cocultured with ECs for the same period of time. Subsequently, EC apoptosis was assessed by flow cytometry, detecting the increase in side scatter intensity as a parameter of programmed cell death. The results, ie, that IR- and LPS-treated PBMCs caused EC apoptosis, whereas untreated PBMCs did not (data not shown), were nearly identical to those obtained with DAPI stain analysis.
PBMCs express mTNF-α in response to LPS and IR, which can be inhibited by IL-10.To verify the suggestion mTNF-α's role in EC apoptosis as mediated by LPS- or IR-treated PBMCs, kinetic surface analysis of TNF-α expression was performed with PBMCs that were either untreated, irradiated (IR, 4 Gy), or treated with LPS and then incubated in the presence or absence of IL-10 (0.1 μg/mL). As evident from Fig 2, LPS (10 ng/mL) induced mTNF-α expression on PBMCs as early as 1 hour after treatment. It is important to note that only the CD14+ monocytic subpopulation expressed mTNF-α, as shown by costaining with an anti-CD14 MoAb. CD14+ and CD14− cells were separately gated and subsequently assayed for TNF-α expression (Fig 4, histogram inserts). Four hours after LPS treatment, more than 50% of the CD14+ PBMCs expressed mTNF-α, which persisted up to 24 hours after LPS challenge. Coincubation of LPS-treated PBMCs with IL-10 completely abrogated mTNF-α expression, suggesting that the antiapoptotic action of IL-10 was due to inhibition of mTNF-α expression on CD14+ PBMCs. IR treatment of monocytic cells lead to a lesser but significant upregulation of mTNF-α only after 24 hours of incubation, suggesting a different mechanism of induction. Because mTNF-α induction by IR was weaker despite comparable apoptotic results, one can conclude that the overall amount of mTNF-α is not as important as its induction. However, IL-10 proved to antagonize IR-mediated mTNF-α expression as effectively as after LPS induction (Fig 2).
Kinetic flow cytometric analysis showing the effect of IR and LPS on mTNF-α expression on PBMCs and the protective role of IL-10. PBMCs were either untreated (Control), irradiated (IR), or incubated with LPS in the presence or absence of IL-10. Results are shown as the percentage of mTNF-α+ cells, with staining for mTNF-α at 1, 4, and 24 hours after treatment as described in the Materials and Methods. Results representative of three independent experiments are shown. (Histogram inserts) Only the monocytic CD14+ subpopulation expresses mTNF-α. Flow cytometric analysis with a costaining of PBMCs with anti–TNF-α and anti-CD14 MoAbs. Dotted line, isotype-matched control antibody; solid thin line, staining with anti-CD14; solid thick line, staining with anti–TNF-α.
Kinetic flow cytometric analysis showing the effect of IR and LPS on mTNF-α expression on PBMCs and the protective role of IL-10. PBMCs were either untreated (Control), irradiated (IR), or incubated with LPS in the presence or absence of IL-10. Results are shown as the percentage of mTNF-α+ cells, with staining for mTNF-α at 1, 4, and 24 hours after treatment as described in the Materials and Methods. Results representative of three independent experiments are shown. (Histogram inserts) Only the monocytic CD14+ subpopulation expresses mTNF-α. Flow cytometric analysis with a costaining of PBMCs with anti–TNF-α and anti-CD14 MoAbs. Dotted line, isotype-matched control antibody; solid thin line, staining with anti-CD14; solid thick line, staining with anti–TNF-α.
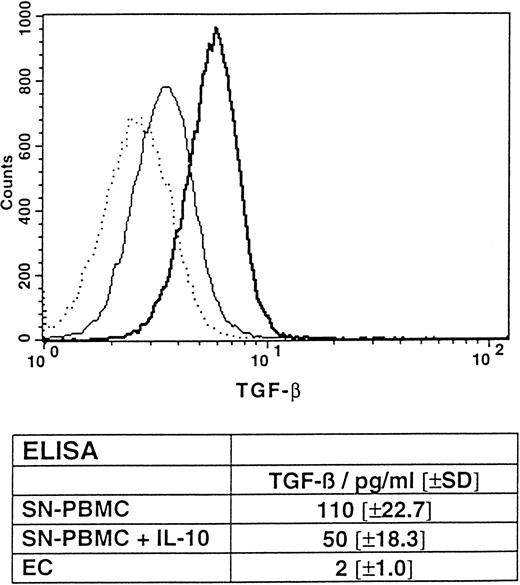
Intracellular flow cytometric analysis showing the constitutive expression of TGF-β by PBMCs, with partial protection by IL-10. PBMCs were freshly prepared and incubated for 4 hours in the presence (thin line) or absence (thick line) of IL-10. Subsequently, flow cytometry was performed as described in the Materials and Methods. The nil control is represented by a dotted line. (Table insert) ELISA, detecting TGF-β2 in the supernatants of overnight cultured, untreated PBMCs in the presence or absence of IL-10 (0.1 μg/mL) and ECs. Results are given in mean concentrations in picograms per milliliter (±SD) of triplicates. P < .05 for the mean TGF-β concentration of PBMCs coincubated with IL-10 versus PBMCs alone.
Intracellular flow cytometric analysis showing the constitutive expression of TGF-β by PBMCs, with partial protection by IL-10. PBMCs were freshly prepared and incubated for 4 hours in the presence (thin line) or absence (thick line) of IL-10. Subsequently, flow cytometry was performed as described in the Materials and Methods. The nil control is represented by a dotted line. (Table insert) ELISA, detecting TGF-β2 in the supernatants of overnight cultured, untreated PBMCs in the presence or absence of IL-10 (0.1 μg/mL) and ECs. Results are given in mean concentrations in picograms per milliliter (±SD) of triplicates. P < .05 for the mean TGF-β concentration of PBMCs coincubated with IL-10 versus PBMCs alone.
The observation that anti–TNF-α MoAb MAK195 completely inhibited PBMC-mediated programmed cell death of ECs argues against an alloantigen-restricted mechanism like that of a cytotoxic T-lymphocyte response. In other experimental settings we actually observed alloantigeneic responses against ECs, but independently of TNF-α (Eissner et al, unpublished observations). Additionally, we could reproduce the PBMC results with the monocytic cell line Mono Mac 6 (data not shown), clearly indicating that only mTNF-α–bearing cells account for endothelial apoptosis.
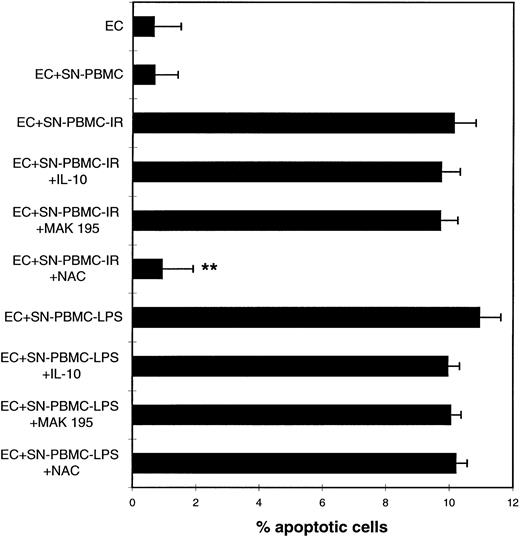
Cell-free supernatants of IR- or LPS-treated PBMCs trigger endothelial programmed cell death independent of TNF-α.We next sought to determine whether preactivated PBMCs could mediate programmed cell death by means other than mTNF-α. Therefore, PBMCs were treated as described for the experiments in Fig 1. Cell-free supernatants of these cells were collected 4 hours after treatment and coincubated with ECs for another 48 hours. Figure 3 clearly shows that cell-free supernatants of either IR- or LPS-treated PBMCs induced apoptosis in ECs to a similar extent as in the coculture model, whereas supernatants of untreated PBMCs did not interfere with EC viability. To investigate whether cytokines and TNF-α in particular were involved in this cell death signalling, PBMCs were additionally pretreated with either anti–TNF-α or IL-10. Both reagents remained in the culture medium throughout the entire experimental procedure. As was expected from previous observations,3 the anti–TNF-α MoAb MAK195 did not interfere with EC apoptosis mediated by either supernatant, suggesting that soluble TNF-α did not participate (Fig 3), although it was present at extremely high levels in the supernatant after LPS treatment (up to 3,258 ng/mL after 24 hours of incubation, Table 1). IR treatment led to low but detectable levels of soluble TNF-α in the PBMC supernatant (Table 1). IL-10 also failed to prevent apoptosis in both types of PBMC supernatants (Fig 3). Because IR is well known to induce reactive oxygen species (ROS) in various cells, including leukocytes,19 N-acetylcystein (NAC) was used as an antioxidant in the apoptosis assays. In fact, NAC (3 mmol/L) could inhibit EC death mediated by supernatants of IR-treated PBMCs, but not that of LPS-treated PBMCs, suggesting that distinctly different apoptotic mechanisms result from the two types of treatments.
Cell-free supernatants of preactivated PBMCs cause programmed cell death in ECs. NAC inhibits the IR-induced effects. HUVECs were coincubated with supernatants of either untreated PBMCs (SN-PBMC) or irradiated (SN-PBMC-IR) or LPS-treated PBMCs (SN-PBMC-LPS), respectively, in the presence or absence of IL-10, MAK 195, or NAC (3 mmol/L). Quantitative microscopic analysis of DAPI-stained cells. **P < .001 of apoptosis of SN-PBMC-IR in the presence of NAC versus SN-PBMC-IR alone. These results are representative of four independent experiments. For experimental details, see the legend to Fig 1.
Cell-free supernatants of preactivated PBMCs cause programmed cell death in ECs. NAC inhibits the IR-induced effects. HUVECs were coincubated with supernatants of either untreated PBMCs (SN-PBMC) or irradiated (SN-PBMC-IR) or LPS-treated PBMCs (SN-PBMC-LPS), respectively, in the presence or absence of IL-10, MAK 195, or NAC (3 mmol/L). Quantitative microscopic analysis of DAPI-stained cells. **P < .001 of apoptosis of SN-PBMC-IR in the presence of NAC versus SN-PBMC-IR alone. These results are representative of four independent experiments. For experimental details, see the legend to Fig 1.
PBMCs Express Soluble TNF in Response to LPS and IR
| Treatment . | TNF-α (pg/mL) . | ||
|---|---|---|---|
| . | 2 h . | 24 h . | 48 h . |
| Control | <4 | <4 | <4 |
| IR | 8 | 31 | 15 |
| LPS | 1,402 | 3,258 | 1,870 |
| Treatment . | TNF-α (pg/mL) . | ||
|---|---|---|---|
| . | 2 h . | 24 h . | 48 h . |
| Control | <4 | <4 | <4 |
| IR | 8 | 31 | 15 |
| LPS | 1,402 | 3,258 | 1,870 |
Supernatants of PBMCs either untreated (Control), irradiated (IR), or incubated in the presence of LPS were analyzed 2, 24, and 48 hours after treatment for soluble TNF-α (in picrograms per milliliter) by the ELISA sandwich technique as described in the Materials and Methods. These results are representative of three independent experiments.
PBMCs and PBMC supernatants cause cell cycle arrest of HUVECs in G0/1 by the constitutive expression of TGF-β: protective role of IL-10.Because EC proliferation is a prerequisite for repair mechanisms after cell damage, it was important to investigate whether PBMCs interfere with the proliferative capacity of cultured ECs. Therefore, cell cycle analyses by flow cytometry (see the Materials and Methods) were performed with untreated ECs, ECs cocultured with PBMCs, or ECs exposed to cell-free supernatants of PBMCs. Interestingly, even untreated PBMCs and supernatants of these cells caused cell cycle arrest of the ECs in the G0/1 phase (Table 2). Untreated ECs showed a classical cell cycle distribution of proliferating cells (22.5% ± 11.6% of the cells in the S-phase), whereas the coculture with PBMCs led to an almost complete halt in DNA synthesis. IR treatment (Table 2) and LPS treatment (not shown) did not significantly change this effect. This finding strongly indicates the antiproliferative capacity of PBMCs to be due to a constitutive factor in the supernatant of the blood cells. It has been previously reported that TGF-β can downregulate EC proliferation20 and induce cell cycle arrest in G0/1 .21 Therefore, the potential presence of TGF-β in PBMC preparations was determined by intracellular flow cytometry. Figure 4 shows that, in fact, PBMCs constitutively expressed TGF-β to a significant extent. In accordance with the data obtained from the cell cycle analyses, treatment of PBMCs with either IR or LPS did not significantly change constitutive TGF-β expression (data not shown). Interestingly, IL-10 was found to reduce TGF-β expression by approximately 50% (Fig 4). It should be noted that only the lymphocytic, but not the monocytic subopulation of the PBMC preparation showed a significant TGF-β expression, as determined by costaining with an anti-CD3 MoAb (not shown). Alternatively, the presence of TGF-β in the supernatants of PBMCs could be shown by a commercially available ELISA kit. As depicted in the table insert of Fig 4, untreated PBMCs (SN-PBMC) expressed 110 ± 22.7 pg/mL TGF-β constitutively. IL-10 was found to downregulate the level of TGF-β expression by 54% (50 ± 18.3 pg/mL, P < .05). In contrast, human ECs showed no significant expression of this cytokine, suggesting that PBMCs produced the entire amount of TGF-β (Fig 4, table insert).
PBMCs and SN of PBMCs Induce Cell Cycle Arrest of ECs in G0/1 Independent of Any Preactivation
| Treatment . | % of Cells* (±SD) in . | ||
|---|---|---|---|
| . | G0/1-Phase . | S-Phase . | G2-Phase . |
| EC | 68 (±11.8) | 22.5 (±11.6) | 7.5 (±2.4) |
| EC+SN-PBMC | 82 (±4.9) | 3 (±2.4)† | 12.3 (±4.3) |
| EC+SN-PBMC-IR | 78.3 (±4.2) | 5 (±3)† | 14 (±4.3) |
| EC+PBMC | 81.3 (±1.2) | 6 (±2.6)† | 10.7 (±1.5) |
| EC+PBMC-IR | 81 (±5) | 4.7 (±2.1)† | 11 (±5.3) |
| Treatment . | % of Cells* (±SD) in . | ||
|---|---|---|---|
| . | G0/1-Phase . | S-Phase . | G2-Phase . |
| EC | 68 (±11.8) | 22.5 (±11.6) | 7.5 (±2.4) |
| EC+SN-PBMC | 82 (±4.9) | 3 (±2.4)† | 12.3 (±4.3) |
| EC+SN-PBMC-IR | 78.3 (±4.2) | 5 (±3)† | 14 (±4.3) |
| EC+PBMC | 81.3 (±1.2) | 6 (±2.6)† | 10.7 (±1.5) |
| EC+PBMC-IR | 81 (±5) | 4.7 (±2.1)† | 11 (±5.3) |
Cell-cycle analysis was performed as described in the Materials and Methods. Results are given as the percentage of cells in the respective cell cycle phase (±SD).
Mean of at least three experiments.
P < .05 of BrdU+ (S-phase) control ECs versus BrdU+ ECs after incubation with (SN−) PBMCs treated as given.
To further investigate the role of TGF-β in the PBMC-mediated G0/1 arrest of ECs, another cell cycle analysis, with untreated and SN-PBMC–treated ECs in the presence or absence of an inhibitory anti–TGF-β antibody, was performed. Additionally, we asked whether IL-10 was not only protective in terms of antiapoptotic signalling, but if it could also interfere with the cell cycle arrest. Table 3 clearly shows that the anti–TGF-β MoAb as well as IL-10 completely rescued ECs from PBMC-caused G0/1 arrest. As a positive control, we found that an equal mixture of recombinant TGF-β1 and β2 (1 ng/mL) arrested ECs in a manner similar to that mediated by PBMCs (data not shown).
IL-10 and an Anti–TGF-β MoAb Rescue ECs From PBMC-Caused Cell Cycle Arrest
| Treatment . | % of Cells3-150 (±SD) in . | ||
|---|---|---|---|
| . | G0/1-Phase . | S-Phase . | G2-Phase . |
| EC | 65.8 (±11.3) | 22.6 (±10) | 9.6 (±5.1) |
| EC+SN-PBMC | 77.2 (±8.4) | 4.8 (±3.4) | 15 (±5.4) |
| EC+SN-PBMC +IL-10 | 61.3 (±6.7) | 16.8 (±5.6)3-151 | 18.5 (±1.7) |
| EC+ SN-PBMC+anti – TGF-β | 62.3 (±2.2) | 16.3 (±12.4) | 16.7 (±12.3) |
| Treatment . | % of Cells3-150 (±SD) in . | ||
|---|---|---|---|
| . | G0/1-Phase . | S-Phase . | G2-Phase . |
| EC | 65.8 (±11.3) | 22.6 (±10) | 9.6 (±5.1) |
| EC+SN-PBMC | 77.2 (±8.4) | 4.8 (±3.4) | 15 (±5.4) |
| EC+SN-PBMC +IL-10 | 61.3 (±6.7) | 16.8 (±5.6)3-151 | 18.5 (±1.7) |
| EC+ SN-PBMC+anti – TGF-β | 62.3 (±2.2) | 16.3 (±12.4) | 16.7 (±12.3) |
ECs were either untreated (ECs) or incubated with cell-free supernatant of PBMCs (SN-PBMC) in the presence or absence of IL-10 (0.1 μg/mL) and the anti–TGF-β MoAb AB-101-NA.
Mean of at least three experiments.
P < .05 of proliferating cells after treatment with SN-PBMC in the presence of inhibitors versus SN-PBMC alone.
In summary, these data suggest that PBMCs induce cell cycle arrest of ECs in the G0/1 phase by constitutively expressing TGF-β and that this effect can be effectively eliminated by the antagonistic cytokine IL-10. IL-10 is likely to interfere in this setting by blocking TGF-β secretion from PBMCs.
DISCUSSION
Previous work from our group3 prompted us to examine the influence of PBMCs on the viability of ECs in vitro. In the present report, we show that preactivated PBMCs induce programmed cell death in ECs. The most striking findings are summarized in Fig 5. PBMCs, either irradiated or treated with LPS before coculture with ECs, cause EC apoptosis through the action of mTNF-α, because anti–TNF-α MoAb MAK 195 and IL-10 effectively block coculture-mediated cell damage. Importantly, only the monocytic subpopulation of PBMCs actually express mTNF-α, as shown by PBMCs costaining with the monocytic marker CD14. Further evidence that the monocytes were responsible for the mTNF-α–triggered apoptosis was provided by experiments using the differentiated monocytic cell line Mono Mac 6.22 Although these cells exhibited different kinetics of mTNF-α expression in response to LPS, they induced endothelial apoptosis to the same extent as did PBMCs (data not shown).
Pathophysiologic model showing the influence of preactivated PBMCs on human ECs. LPS- or IR-treated PBMCs drive ECs into programmed cell death via distinct signalling pathways and may prevent vascular repair by causing an EC cycle arrest in G0/1 . IL-10 as an antagonistic cytokine can protect against apoptosis as well as against cell cycle arrest and may be of potential therapeutic value.
Pathophysiologic model showing the influence of preactivated PBMCs on human ECs. LPS- or IR-treated PBMCs drive ECs into programmed cell death via distinct signalling pathways and may prevent vascular repair by causing an EC cycle arrest in G0/1 . IL-10 as an antagonistic cytokine can protect against apoptosis as well as against cell cycle arrest and may be of potential therapeutic value.
mTNF-α is known to mediate cellular cytotoxicity in various experimental settings3,23,24 distinct from soluble TNF-α. However, the intracellular death signals downstream from the TNF-α receptor(s) that account for this differential regulation remain enigmatic. Nevertheless, TNF-α becomes increasingly important in the pathogenesis of various inflammatory diseases9,25 and therefore may be a suitable target for an antiinflammatory therapy.10
In addition to the coculture-mediated apoptosis of ECs, cell-free supernatants (SN) of either IR- or LPS-treated PBMCs can trigger programmed cell death independently of TNF-α. Because EC death by SN of IR-treated PBMCs can be blocked by antioxidants (NAC), ROS may be involved in this process. ROS are known to occur in response to IR and can induce programmed cell death in a variety of cells.19 Because of the extremely short half life of ROS, they are unlikely to be involved in the apoptotic machinery but rather may participate in a different signal transduction cascade. It is interesting to note that we found NAC also capable of blocking coculture-mediated apoptosis by PBMC-IR (data not shown), which suggests that ROS may also participate in the induction of IR-triggered mTNF expression. It remains to be elucidated what compound in the SN of LPS-treated PBMCs can induce apoptosis. Because NAC did not block this path to programmed cell death, it is obviously distinct from soluble factors induced by IR.
Also unanswered remains the question as to why anti–TNF-α MoAb MAK195 and IL-10 fully blocked coculture-mediated cell death despite the presence of those soluble factors. Perhaps there are three (or more) distinct apoptotic mechanisms, one mediated by mTNF-α and two other by as yet unidentified, soluble mediators (in the case of IR, probably ROS). Inhibitor studies suggest that these separate mechanisms are restrictively triggered, ie, once mTNF-α is engaged, negatively regulating cofactors may block the other apoptotic signalling pathways. Alternatively, the mTNF-α–TNF receptor interaction may initiate a negative feedback mechanism in PBMCs, thereby disrupting the secretion of apoptosis-inducing soluble factors. However, this would require that mTNF-α not only serves as ligand, but also as a receptor capable of transducing intracellular signals in PBMCs. Such retrograde signalling was recently described for T cells,26 where it was shown that TNF-α–specific antibodies can enhance CD3-mediated T-cell activation by cross-linking membrane-integrated TNF-α. We currently are investigating whether one of these presumptions applies for ECs.
Importantly, PBMCs not only induce EC apoptosis, but cell cycle arrest in G0/1 (Fig 5) as well. This can be effectively blocked by anti–TGF-β MoAb and IL-10, suggesting that TGF-β, which is constitutively expressed even by untreated PBMCs, accounts for this antiproliferative signal. This finding is consistent with previously published data, which show TGF-β20 to inhibit EC proliferation and wound repair by inducing a cell cycle arrest in G0/1 .21 IL-10 was able to downregulate TGF-β synthesis in PBMCs, probably contributing to the cell cycle rescue. Negative regulation of TGF-β by IL-10 was previously described in bone marrow cultures.27 The mode of TGF-β regulation by IL-10 suggests that there is a certain threshold of cytokine expression required for inducing cell cycle arrest. Our studies cannot strictly exclude that the source of TGF-β may be contaminating platelets in the PBMCs preparations. However, preliminary cell cycle analyses with SN of PBMCs partially depleted of platelets still induced cell cycle arrest in ECs, and the SN of platelets isolated from peripheral blood did not arrest ECs in G0/1 . In addition, we failed to detect TGF-β in preparations of nonpermeabilized PBMCs, which virtually excludes the possibility that TGF-β may be derived from platelets attached to the surface of PBMCs (not shown).
In vivo studies have shown local organ irradiation to induce EC proliferation instead of arrest about 3 weeks after treatment.28 Two major differences between this in vivo model and our in vitro system may account for this. First of all, fivefold higher radiation doses were used for the in vivo experiments. Secondly, the additional inflammatory stimuli associated with transplantation were absent in the organ irradiation model. Therefore, the two systems are not necessarily contradictory.
Another interesting question is whether TGF-β, in addition to inducing cell cycle arrest in ECs, also participates in apoptosis, as was shown for transformed fibroblasts.29 However, our observation that untreated PBMCs and their SN did not induce EC apoptosis, despite the constitutive expression of TGF-β, and that a neutralizing anti–TGF-β MoAb could not block programmed cell death by preactivated PBMCs (not shown) argues against a contribution of of this growth factor to cell death signalling.
In conclusion, we propose a pathophysiologic model (Fig 5) in which PBMCs induce endothelial apoptosis and/or concurrent cell cycle arrest in proliferating ECs. Thus, PBMCs may hinder the repair mechanisms of endothelial linings. This may contribute to our understanding of the severe endothelial complications occurring after radiotherpy and after the TBI used for conditioning in BMT, as well as after the onset of graft-versus-host disease.
Previously, IL-10 has been shown in vitro to antagonize proinflammatory cytokines,30 including the prevention of apoptosis.31 In vivo IL-10 protects LPS-challenged animals against lethal endotoxemia.32,33 Evidence is accumulating that IL-10 can protect ECs against various cytokine-mediated dysfunctions and activation processes. 3,34,35 It is is unlikely that the antagonistic effect of IL-10 is derived from endogenous PBMC sources instead of the therapeutically appplied exogenous IL-10. LPS-stimulated monocytes actually produce IL-10, but with a late onset, as compared with the action of TNF-α and other proinflammatory cytokines.36 However, it is possible that endogenous IL-10 contributes to a sustained protective effect of IL-10 in an autoregulatory fashion.
The potential of IL-10 to prevent apoptosis and antagonize cell cycle arrest in EC warrants its further investigation as a therapeutic agent in various inflammatory disorders, including radiation- and endotoxin-induced EC damage.
ACKNOWLEDGMENT
The authors thank Dr W. Kolch for helpful suggestions and Dr W. Kolch and C. Hall for critically reviewing the manuscript.
Supported in part by Grants No. Ho1142/1-2 and Schu777/2-2 from the Deutsche Forschungsgemeinschaft (DFG).
Address reprint requests to Günther Eissner, PhD, GSF-Institute für Clinical Molecular Biology and Tumor Genetics, Marchioninistr. 25, D-81377 Munich, Germany.

![Fig. 1. Preactivated PBMCs induce apoptosis in ECs. Quantitative microscopic analysis of DAPI-stained ECs after 48 hours of coculture. Cells were microscopically analyzed by counting apoptotic and nonapoptotic cells (n = 10 microscopic fields including an average of 70 cells per field). (A) PBMCs were either untreated (PBMC), irradiated (PBMC-IR; 4 Gy), or incubated with bacterial endotoxin (PBMC-LPS; 10 ng/mL) for 4 hours. (B) Human IL-10 (0.1 μg/mL) and MAK 195 (anti-TNF-α MoAb; 20 μg/mL) inhibit the apoptosis-inducing effects of either irradiated or LPS-treated PBMCs on ECs. Results are given as the percentage of apoptotic cells (± standard deviation [SD]). These results are representatives of at least four independent experiments. *,**P < .001 of apoptosis of control ECs versus ECs coincubated with preactivated PBMCs and control ECs versus ECs cocultured with preactivated PBMCs in the presence of IL-10 or anti–TNF-α MoAb MAK195.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.1931/3/m_bl_0026f1.jpeg?Expires=1768176420&Signature=MIH7QyCYag3nZyHfbAdmsR-Af0ISfP05nKuk~PSm8BwZjtCVVt1Nbi6jr-3n1pQV3uglxWvPxnVI1Ry7PY8Rk~BOfHn5y7jqkS5QT1UNqQ4Im7fsgipqwI68wYTg61hx~7yM-T2Ej1cQ4p4wjbVj5qOF7gfxmlMA2L52yaZVBZjoTCGqk~uQLLl5NZjpue7SlQ8MVD1fGBv6TgQKsILkuxJMMVTRu1pGtVl5YOsykCj5ycDod0DeGpG~sXwga0M1QQubau3nklnf98e75r7oFVbl2~e0pnOARuj6M5PFG5jnAawdH4xwrgV2umFNfTy~Jr66nUf98vXEfvPIkqYkBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal