Abstract
Activation of platelets by collagen is mediated through a tyrosine kinase-dependent pathway that is associated with phosphorylation of the Fc receptor γ chain, the tyrosine kinase syk, and phospholipase Cγ2 (PLCγ2). We recently described a collagen-related triple-helical peptide (CRP) with the sequence GCP*(GPP*)GCP*G (single letter amino acid code: P* = hydroxyproline; Morton et al, Biochem J 306:337, 1995). The cross-linked peptide is a potent stimulus of platelet activation but, unlike collagen, does not support α2β1-mediated, Mg2+-dependent adhesion, suggesting that its action is independent of the integrin α2β1 . This finding suggests the existence of a platelet receptor other than α2β1 that underlies activation. In the present study, we show that CRP stimulates tyrosine phosphorylation of the same pattern of proteins in platelets as collagen, including syk and PLCγ2. Protein tyrosine phosphorylation induced by CRP is not altered in the absence of Mg2+ or the presence of monoclonal antibodies (MoAbs) to the integrin α2β1 (MoAb 6F1 and MoAb 13), conditions that prevent the interaction of collagen with the integrin. In contrast, phosphorylation of syk and PLCγ2 by collagen is partially reduced by MoAb 6F1 and MoAb 13 or by removal of Mg2+. This may reflect a direct role of α2β1 in collagen-induced signaling events or an indirect role in which the integrin facilitates the binding of collagen to its signaling receptor. The results show an α2β1-independent pathway of platelet activation by CRP that involves phosphorylation of syk and PLCγ2. This pathway appears to contribute to platelet activation by collagen.
THE EXTRACELLULAR matrix protein collagen has a fundamental role in hemostasis. It provides a site for adhesion of platelets to the extracellular matrix serving as a platform for the developing hemostatic plug. It also stimulates platelet aggregation and secretion, including the release of mediators that induce further activation of platelets, eg, thromboxanes and adenosine diphosphate (ADP).
The adhesion of platelets to collagen involves Mg2+-dependent and Mg2+-independent events. Mg2+-dependent adhesion is mediated predominantly through the integrin α2β1 (also known as glycoprotein [GP] Ia/IIa) and is inhibited by antibodies raised against the integrin such as monoclonal antibody (MoAb) 6F1 and MoAb 13.1-6 The degree of Mg2+-dependent adhesion varies considerably with experimental conditions and is of particular importance under flow.1 6-8
Activation of platelets by collagen is mediated through a tyrosine kinase-dependent pathway that involves tyrosine phosphorylation of the Fc receptor γ chain, syk, and PLCγ2.9-15 We propose that these proteins form part of a signaling cascade analogous to that used by immune receptors, ie, T- and B-cell receptor complexes and Fc receptors.12 13 Activation of this family of receptors leads to tyrosine phosphorylation of an immuno-receptor tyrosine-based activation motif (ITAM; also called ARAM or TAM), which has the sequence YXXL/IX8-12YXXL/I (single letter amino acid code in which X is any amino acid), which is found within at least one of the polypeptide chains in the receptor complex. Phosphorylation of the conserved tyrosine residues in the ITAM provides a site of interaction for tandem SH2 domains in the tyrosine kinases syk or zap-70. This leads to tyrosine phosphorylation of further substrates, including PLCγ2.
The relationship between Mg2+-dependent adhesion of platelets to collagen and their activation by collagen is poorly defined. In particular, it is unclear whether tyrosine phosphorylation of the above proteins is mediated by binding of collagen to the integrin α2β1 or through a separate cell surface receptor. There is considerable evidence for the participation of other surface molecules in platelet activation by collagen.3,8 In particular, several candidate receptors for collagen have been proposed based on purification studies, use of antibodies, and identification of individuals with bleeding disorders. The list of collagen receptors other than α2β1 includes CD36 (also known as glycoprotein [GP] IV16), GPVI,17,18 and uncharacterized proteins of 62 to 65 kD.19 20
Recently, the synthesis of two simple collagen-like peptides, collagen-related triple-helical peptide (CRP) and GKP*(GPP*)10GKP*G (single letter amino acid code: P* = hydroxyproline), was described.21 The peptides contain the repeat sequence, GPP*, to facilitate adoption of the stable triple-helical structure found in collagen and are cross-linked through cysteine or lysine residues because quaternary structure is required in collagen-platelet reactivity. Both peptides are potent stimulants of platelet activation, whereas their non–cross-linked counterparts inhibit aggregation induced by the two cross-linked peptides and by collagen.21 This finding shows the importance of their quaternary structure for aggregation and indicates that the peptides have the same structural requirements as collagen in mediating activation. This implies also that the non–cross-linked peptides act as antagonists at a receptor common to both collagen and the cross-linked peptides, further emphasizing their similarity in action. However, adhesion to the cross-linked peptides is independent of Mg2+ and not inhibited by antibodies to the integrin α2β1 . This suggests that the cross-linked peptides are unable to bind to α2β1 and implies that activation is mediated through an alternative cell surface receptor. This finding is consistent with the two-step, two-site model for activation of platelets by collagen proposed by Morton et al22 and Santoro et al23 based on studies with cyanogen bromide fragments of collagen or acetylated collagen. The model predicts the presence of distinct determinants in the collagen molecule recognizing α2β1 and an uncharacterized cell surface receptor.
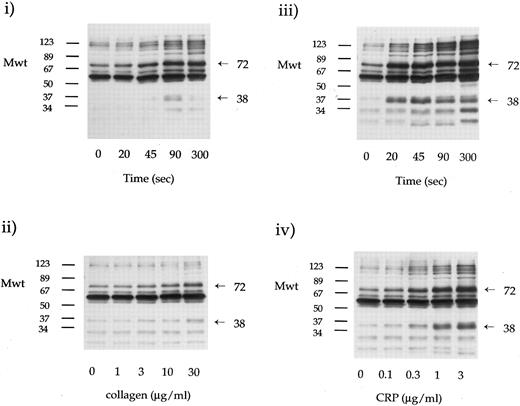
Dose-response and time course studies of collagen and CRP stimulation of tyrosine phosphorylation. Platelets (4 × 108/mL) were stimulated with collagen or CRP as indicated. Whole cell lysates were resolved on 10% SDS-PAGE and immunoblotted using antiphosphotyrosine MoAb 4G10. Time-matched ECL exposures of platelets stimulated by collagen (i and ii) and CRP (iii and iv) are shown. The time course of tyrosine phosphorylation stimulated by collagen (30 μg/mL) and CRP (3 μg/mL) are shown in (i) and (iii), respectively. The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by collagen and CRP are shown in (ii) and (iv), respectively. The time course and dose-response relationships were determined on separate donors. Similar results were observed on 10 donors.
Dose-response and time course studies of collagen and CRP stimulation of tyrosine phosphorylation. Platelets (4 × 108/mL) were stimulated with collagen or CRP as indicated. Whole cell lysates were resolved on 10% SDS-PAGE and immunoblotted using antiphosphotyrosine MoAb 4G10. Time-matched ECL exposures of platelets stimulated by collagen (i and ii) and CRP (iii and iv) are shown. The time course of tyrosine phosphorylation stimulated by collagen (30 μg/mL) and CRP (3 μg/mL) are shown in (i) and (iii), respectively. The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by collagen and CRP are shown in (ii) and (iv), respectively. The time course and dose-response relationships were determined on separate donors. Similar results were observed on 10 donors.
CRP stimulates tyrosine phosphorylation of syk and PLCγ2. syk and PLCγ2 were immunoprecipitated from lysates of platelets stimulated by CRP or collagen and immunoblotted for phosphotyrosine as described in the Materials and Methods. (A) Tyrosine phosphorylation of syk (i and ii) and PLCγ2 (iii and iv) in CRP-stimulated platelets. The time course of tyrosine phosphorylation stimulated by CRP (3 μg/mL) is shown in (i) and (iii). The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by CRP is shown in (ii) and (iv). The location of syk, PLCγ2, and the heavy chain of the immunoprecipitating antibody (IgG) are shown. An unknown band of 75 kD (indicated by the arrow in [i]) was present in syk immunoprecipitates. Three prominent uncharacterized proteins of approximately 47, 78, and 120 kD were present in PLCγ2 immunoprecipitates and are indicated by arrows in (iii) and (iv). Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions (not shown). (B) Tyrosine phosphorylation of syk and PLCγ2 in collagen-stimulated platelets. Platelets were stimulated with collagen for 90 seconds. The antiphosphotyrosine blots of syk and PLCγ2 immunoprecipitates shown in the upper part of each figure were stripped and reprobed for syk and PLCγ2 as described in the Materials and Methods and are shown in the lower part of each figure. Results are representative of three experiments.
CRP stimulates tyrosine phosphorylation of syk and PLCγ2. syk and PLCγ2 were immunoprecipitated from lysates of platelets stimulated by CRP or collagen and immunoblotted for phosphotyrosine as described in the Materials and Methods. (A) Tyrosine phosphorylation of syk (i and ii) and PLCγ2 (iii and iv) in CRP-stimulated platelets. The time course of tyrosine phosphorylation stimulated by CRP (3 μg/mL) is shown in (i) and (iii). The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by CRP is shown in (ii) and (iv). The location of syk, PLCγ2, and the heavy chain of the immunoprecipitating antibody (IgG) are shown. An unknown band of 75 kD (indicated by the arrow in [i]) was present in syk immunoprecipitates. Three prominent uncharacterized proteins of approximately 47, 78, and 120 kD were present in PLCγ2 immunoprecipitates and are indicated by arrows in (iii) and (iv). Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions (not shown). (B) Tyrosine phosphorylation of syk and PLCγ2 in collagen-stimulated platelets. Platelets were stimulated with collagen for 90 seconds. The antiphosphotyrosine blots of syk and PLCγ2 immunoprecipitates shown in the upper part of each figure were stripped and reprobed for syk and PLCγ2 as described in the Materials and Methods and are shown in the lower part of each figure. Results are representative of three experiments.
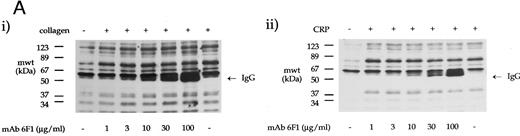
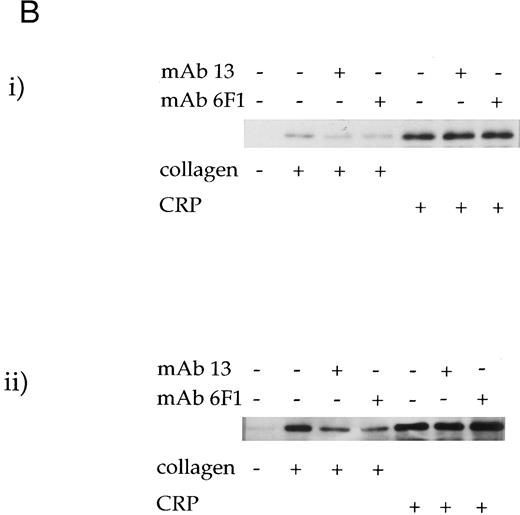
The effect of antibodies to the integrin α2β1 on tyrosine phosphorylation. (A) Platelets were stimulated with (i) collagen (30 μg/mL) or (ii) CRP (3 μg/mL) for 90 seconds in the presence of MoAb 6F1 (1 to 100 μg/mL) and subsequently analyzed by immunoblotting using the antiphosphotyrosine MoAb 4G10 as described in the Materials and Methods. MoAb 6F1 was administered 5 minutes before the agonist. ECL exposures from collagen-stimulated platelets were exposed for a longer period than that from CRP-stimulated cells, as indicated by the greater number of bands in the basal lane. The location of the heavy chain of MoAb 6F1 (IgG) is indicated by the arrow. (B) Tyrosine phosphorylation of syk and PLCγ2 in the presence of antibodies to the integrin α2β1 . Samples were immunoprecipitated using antibodies to (i) syk and (ii) PLCγ2 as described in the Materials and Methods before immunoblotting with MoAb 4G10. Samples were incubated with MoAb 6F1 (30 μg/mL) or MoAb 13 (5 μg/mL) for 5 minutes before the addition of agonist. Results are representative of two experiments.
The effect of antibodies to the integrin α2β1 on tyrosine phosphorylation. (A) Platelets were stimulated with (i) collagen (30 μg/mL) or (ii) CRP (3 μg/mL) for 90 seconds in the presence of MoAb 6F1 (1 to 100 μg/mL) and subsequently analyzed by immunoblotting using the antiphosphotyrosine MoAb 4G10 as described in the Materials and Methods. MoAb 6F1 was administered 5 minutes before the agonist. ECL exposures from collagen-stimulated platelets were exposed for a longer period than that from CRP-stimulated cells, as indicated by the greater number of bands in the basal lane. The location of the heavy chain of MoAb 6F1 (IgG) is indicated by the arrow. (B) Tyrosine phosphorylation of syk and PLCγ2 in the presence of antibodies to the integrin α2β1 . Samples were immunoprecipitated using antibodies to (i) syk and (ii) PLCγ2 as described in the Materials and Methods before immunoblotting with MoAb 4G10. Samples were incubated with MoAb 6F1 (30 μg/mL) or MoAb 13 (5 μg/mL) for 5 minutes before the addition of agonist. Results are representative of two experiments.
Effect of the removal of divalent cations on collagen- and CRP-stimulated tyrosine phosphorylation. Platelets were prepared and resuspended in Tyrode's-HEPES buffer without MgCl2 in the presence of 1 mmol/L EDTA and 1 mmol/L EGTA. Where indicated, MgCl2 (3 mmol/L) was added 5 minutes before stimulation. Tyrosine phosphorylation of (i) syk and (ii) PLCγ2 after stimulation by collagen (30 μg/mL) or CRP (3 μg/mL) for 90 seconds was measured using MoAb 4G10 as described in Fig 2. Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions. (The apparent reduction in tyrosine phosphorylation of syk by CRP in the presence of Mg2+ was due to a reduction of sample, as indicated by reprobing for syk [see also part (iii)]). (iii) The concentration response relationship for tyrosine phosphorylation of syk by CRP. Tyrosine phosphorylation was measured in syk immunoprecipitates using MoAb 4G10 as described in Fig 2. Results are representative of two to four similar experiments. A weakly tyrosine phosphorylated protein band that runs just above syk was detected in some but not all experiments (compare parts [i] and [iii]).
Effect of the removal of divalent cations on collagen- and CRP-stimulated tyrosine phosphorylation. Platelets were prepared and resuspended in Tyrode's-HEPES buffer without MgCl2 in the presence of 1 mmol/L EDTA and 1 mmol/L EGTA. Where indicated, MgCl2 (3 mmol/L) was added 5 minutes before stimulation. Tyrosine phosphorylation of (i) syk and (ii) PLCγ2 after stimulation by collagen (30 μg/mL) or CRP (3 μg/mL) for 90 seconds was measured using MoAb 4G10 as described in Fig 2. Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions. (The apparent reduction in tyrosine phosphorylation of syk by CRP in the presence of Mg2+ was due to a reduction of sample, as indicated by reprobing for syk [see also part (iii)]). (iii) The concentration response relationship for tyrosine phosphorylation of syk by CRP. Tyrosine phosphorylation was measured in syk immunoprecipitates using MoAb 4G10 as described in Fig 2. Results are representative of two to four similar experiments. A weakly tyrosine phosphorylated protein band that runs just above syk was detected in some but not all experiments (compare parts [i] and [iii]).
Tyrosine kinase inhibitors block CRP-induced aggregation and secretion of [3H]5-HT. (A) The effect of tyrosine kinase inhibitors on platelet aggregation induced by CRP (3 μg/mL). Platelets were resuspended in modified Tyrode's-HEPES buffer in the absence of EGTA and indomethacin and then preincubated with (i) vehicle, (ii) 3 μmol/L staurosporine (1 minute), or (iii) 100 μmol/L tyrphostin ST271 (5 minutes) before the addition of CRP (indicated by the arrowhead). The aggregation results are from one experiment that is representative of three others. (B) CRP-stimulated secretion of [3H]5-HT. (i) Platelets were stimulated with CRP (0.1 to 5 μg/mL) for 90 seconds and [3H]5-HT was analyzed as described in the Materials and Methods. (ii) Platelets were preincubated with 3 μmol/L staurosporine (stauro) or 100 μmol/L ST271 for 1 and 5 minutes, respectively, before the addition of CRP (1 μg/mL). (C) CRP-stimulated tyrosine phosphorylation is inhibited by staurosporine. The upper part shows whole cell tyrosine phosphorylation induced by CRP (3 μg/mL) in the presence of staurosporine (3 μmol/L) and the lower part shows phosphorylation of the tyrosine kinase syk under the same conditions. Results are the means ± SE of between two and four experiments.
Tyrosine kinase inhibitors block CRP-induced aggregation and secretion of [3H]5-HT. (A) The effect of tyrosine kinase inhibitors on platelet aggregation induced by CRP (3 μg/mL). Platelets were resuspended in modified Tyrode's-HEPES buffer in the absence of EGTA and indomethacin and then preincubated with (i) vehicle, (ii) 3 μmol/L staurosporine (1 minute), or (iii) 100 μmol/L tyrphostin ST271 (5 minutes) before the addition of CRP (indicated by the arrowhead). The aggregation results are from one experiment that is representative of three others. (B) CRP-stimulated secretion of [3H]5-HT. (i) Platelets were stimulated with CRP (0.1 to 5 μg/mL) for 90 seconds and [3H]5-HT was analyzed as described in the Materials and Methods. (ii) Platelets were preincubated with 3 μmol/L staurosporine (stauro) or 100 μmol/L ST271 for 1 and 5 minutes, respectively, before the addition of CRP (1 μg/mL). (C) CRP-stimulated tyrosine phosphorylation is inhibited by staurosporine. The upper part shows whole cell tyrosine phosphorylation induced by CRP (3 μg/mL) in the presence of staurosporine (3 μmol/L) and the lower part shows phosphorylation of the tyrosine kinase syk under the same conditions. Results are the means ± SE of between two and four experiments.
In the present study, we have compared the ability of the synthetic peptide, CRP, and collagen to stimulate tyrosine phosphorylation in platelets. The results show the existence of a cell surface receptor other than α2β1 that is coupled to phosphorylation of cellular proteins including syk and PLCγ2 in collagen and CRP-stimulated platelets.
MATERIALS AND METHODS
Materials.The antiphosphotyrosine MoAb, 4G10, was purchased from Upstate Biotechnology Inc (TCS Biologicals Ltd, Buckinghamshire, UK). An anti-syk rabbit polyclonal antibody syk (LR) raised against a polyhistidine fusion protein containing amino acids 257-352 of syk was purchased from Santa Cruz (Santa Cruz, CA). An anti-syk MoAb 101 raised against histidine-tagged human syk 144-249 was from Wako Chemicals (Tokyo, Japan). Rabbit anti-PLCγ2 antiserum was generated as described.24 A suspension of type I collagen fibers from equine tendon was obtained as Horm collagen from Nycomed (Munich, Germany). CRP was synthesized and cross-linked as previously described.21 The mouse MoAb 6F1 recognizing the integrin α2-subunit was kindly donated by Dr B.S. Coller (Mount Sinai School of Medicine, New York, NY). The rat MoAb 13 recognizing the human integrin β1-subunit was purchased from Becton Dickinson (Northampton, UK). All other reagents were from previously described sources.13 25
Preparation of human platelets.Human blood was taken from drug-free volunteers on the day of the experiment using acidic citrate dextrose (ACD; 120 mmol/L sodium citrate, 110 mmol/L glucose, 80 mmol/L citric acid) as anticoagulant. Platelet-rich plasma was obtained by centrifugation at 200g for 20 minutes and incubated with 10 μCi [3H]5-hydroxytryptamine (5-HT) for 60 minutes as required. Platelets were isolated by centrifugation at 1,000g for 10 minutes in the presence of prostacyclin (0.1 μg/mL) and resuspended in 25 mL of a modified Tyrodes-HEPES buffer (134 mmol/L NaCl, 0.34 mmol/L Na2HPO4 , 2.9 mmol/L KCl, 12 mmol/L NaHCO3 , 20 mmol/L HEPES, 5 mmol/L glucose, 1 mmol/L MgCl2 , pH 7.3) and 3 mL of ACD in the presence of prostacyclin (0.1 μg/mL). Platelets were centrifuged at 1,000g for 10 minutes and resuspended at a concentration of 4 × 108 cells/mL in Tyrodes-HEPES buffer containing EGTA (1 mmol/L) and indomethacin (10 μmol/L), unless indicated. MgCl2 was omitted and 1 mmol/L EDTA was added to the Tyrodes-HEPES buffer where stimulation in the absence of MgCl2 was required. Experiments were performed at 37°C in an aggregometer (Chrono-Log Corp, Havertown, PA) with continuous stirring (1,200 rpm). [3H]5-HT release was measured as previously described.25
Immunoblotting studies.Reactions were stopped by adding an equal volume of Laemmli buffer (4% sodium dodecyl sulfate [SDS], 10% mercaptoethanol, 20% glycerol, 50 mmol/L Tris, pH 6.8) and samples were boiled for 10 minutes.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 10%) and transferred to polyvinylidene difluoride (PVDF ) membranes by semidry transfer. Membranes were incubated for 30 minutes at room temperature with 10% (wt/vol) bovine serum albumin (BSA) dissolved in TBS-T (20 mmol/L Tris, 137 mmol/L NaCl, 0.1% Tween-20, pH 7.6). Primary and secondary antibodies were diluted in TBS-T containing 2% (wt/vol) BSA. For antiphosphotyrosine immunoblots, membranes were incubated with MoAb 4G10 (dilution 1:1,000) for 60 minutes. Membranes were washed for 120 minutes in TBS-T and incubated for 60 minutes with horseradish peroxidase-conjugated sheep antimouse Ig. Membranes were washed in TBS-T and treated with ECL reagents before exposure to Hyperfilm-ECL (Amersham, Bucks, UK). In immunoprecipitation studies, membranes were first probed for antiphosphotyrosine as described above and then stripped by washing in TBS-T containing 2% SDS and 5% mercaptoethanol at 80°C for 40 minutes. The membranes were washed and probed with the anti-syk MoAb 101 or anti-PLCγ2 (both antibodies diluted 1:1,000). After washing, membranes were incubated with horseradish peroxidase-conjugated sheep antimouse Ig (1:10,000) or horseradish peroxidase-conjugated donkey antirabbit Ig (1:20,000), respectively.
Immunoprecipitation studies.Platelets (4 × 108 cells/mL) were lysed and incubated for 30 minutes on ice with an equal volume of ice-cold lysis buffer (final concentration, 1% Nonidet P-40, 1 mmol/L EDTA, 1 mmol/L EGTA, 2.5 mmol/L Na3VO4 , 1 mmol/L phenylmethylsulphonyl fluoride, 150 mmol/L NaCl, 10 mmol/L Tris, 5 μg/mL leupeptin, 5 μg/mL aprotinin, 0.5 μg/mL pepstatin A, pH 7.5). Insoluble debris was removed by centrifugation at 13,000g for 5 minutes and the lysate was precleared with 25 μL of protein A-Sepharose CL 4B (50% wt/vol suspension in TBS-T) for 60 minutes at 4°C. Syk was immunoprecipitated from 100 μL lysate using 4 μg of the polyclonal antibody syk (LR). PLCγ2 was immunoprecipitated from 900 μL lysate using 5 μL of anti-PLCγ2 antiserum. After rotation at 4°C for 60 minutes, 25 μL of a 50% (wt/vol) suspension of protein A-Sepharose CL 4B was added and mixing continued for 60 minutes. The Sepharose pellet was washed once with lysis buffer and twice with TBS-T before the addition of 20 μL Laemmli buffer before boiling for 10 minutes. Protein were separated by SDS-PAGE (10%) and transferred to PVDF.
Densitometric analysis.Densitometric analysis of ECL exposures was performed using Adobe Photoshop or Intelligent Quantifier (BioImage, Crewe, UK). Multiple exposures of each Western blot were taken so as to ensure that the signal fell within range.
RESULTS
Collagen and CRP induce tyrosine phosphorylation of a common set of proteins.The ability of collagen and CRP to stimulate tyrosine phosphorylation in platelets was compared using the antiphosphotyrosine MoAb 4G10. Time course and dose-response relationships are shown in Fig 1. CRP induced a pattern of tyrosine phosphorylation similar to that observed in collagen-stimulated platelets but with greater intensity and less variation between individuals. CRP was approximately 30 times more potent than collagen on a weight-by-weight basis, with threshold and maximal effects at 0.1 and 1 to 3 μg/mL, respectively (the molecular weight of collagen monomers is approximately 30 times greater than that of CRP monomers).
Densitometric analysis of the antiphosphotyrosine immunoblots showed that collagen and CRP increased the level of tyrosine phosphorylation of the same set of protein bands, but with quantitative and kinetic differences (data not shown). Both stimuli induced rapid and marked phosphorylation of a 72-kD band that comigrates with the tyrosine kinase syk (Fig 1). On the other hand, CRP induced a more rapid and marked increase in tyrosine phosphorylation of a 38-kD band (Fig 1). Densitometry showed that this 38-kD band was the only detectable band whose level of tyrosine phosphorylation had begun to decline by 300 seconds in both collagen- and CRP-stimulated platelets.
Measurement of tyrosine phosphorylation of syk and PLCγ2 was performed by immunoprecipitation followed by immunoblotting with MoAb 4G10 (Fig 2). Reprobing with an antibody to syk and PLCγ2 was used to measure the amount of immunoprecipitated protein, as shown in Fig 2B. CRP and collagen stimulated tyrosine phosphorylation of syk and PLCγ2 with time course and dose-response relationships similar to those for whole cell tyrosine phosphorylation (Figs 1 and 2; data not shown for collagen). The magnitude of the response to a maximally effective concentration of CRP was consistently greater than that to collagen. After stimulation, three uncharacterized proteins of 47, 78, and 115 kD coimmunoprecipitated with PLCγ2. An unidentified protein of 75 kD coimmunoprecipitated with syk in some but not all experiments, eg, see Fig 2A.
Role of the integrin α2β1 .Two antibodies that inhibit adhesion of platelets to collagen via α2β1 were used to investigate the role of the integrin in collagen- and CRP-stimulated tyrosine phosphorylation. MoAb 6F1, which recognizes the α2-subunit and inhibits Mg2+-dependent adhesion of platelets to collagen at a concentration of 1 μg/mL,4,6 caused little reduction in tyrosine phosphorylation induced by collagen or CRP over the concentration range of 1 to 100 μg/mL (Fig 3A). Similar results were observed with 10 μg/mL MoAb 13, which is raised against the β1 subunit and inhibits Mg2+-dependent adhesion at a concentration of 5 μg/mL6 (data not shown). Consistent with this, neither antibody had a significant effect on phosphorylation of syk and PLCγ2 induced by CRP (Fig 3B). However, both antibodies reduced phosphorylation of syk and PLCγ2 stimulated by collagen, with a more pronounced inhibitory effect against PLCγ2 (ranging from 30% to 55% as measured by densitometry; n = 3). Asazuma et al15 have recently reported a similar partial inhibition of phosphorylation of syk in collagen-stimulated platelets in the presence of an MoAb to the integrin α2β1 .
The importance of Mg2+ in the action of collagen and CRP was investigated by omission of the cation and addition of EDTA (1 mmol/L) to chelate residual ions. MgCl2 (3 mmol/L) was added 5 minutes before stimulation as required. Phosphorylation of syk and PLCγ2 by collagen (30 μg/mL) was substantially reduced in the absence of Mg2+, whereas this had little effect on phosphorylation of either protein by CRP (1 μg/mL; Fig 4i and ii). Similarly, omission of Mg2+ had no apparent effect on the concentration response relationship for phosphorylation of syk (Fig 4iii) and PLCγ2 (data not shown) by CRP.
Secretion of [3H]5-HT and aggregation.CRP stimulates aggregation and [3H]5-HT secretion with a similar time course and concentration response relationship to that for tyrosine phosphorylation (Fig 5A). A similar result is seen in the presence of the ADP scavenger, apyrase (not shown). Stimulation of aggregation and secretion induced by CRP is inhibited in the presence of the nonselective inhibitor of serine-threonine and tyrosine kinases, staurosporine, or by the tyrphostin tyrosine kinase inhibitor, ST271 (Fig 5B). Staurosporine inhibited completely the increase in whole cell tyrosine phosphorylation and tyrosine phosphorylation of syk induced by CRP (Fig 5C). A concentration of ST271 (100 μmol/L) that inhibits activation of phospholipase C by collagen by more than 90% also substantially inhibited the increase in tyrosine phosphorylation of these proteins (not shown).
We have reported previously that CRP monomer inhibits aggregation by collagen fibers by approximately 50%.21 CRP monomer (100 μg/mL) stimulated whole cell tyrosine phosphorylation and phosphorylation of syk but to a lesser extent than that induced by CRP (not shown). Importantly, when the two stimuli were administered together, the level of tyrosine phosphorylation was similar to that induced by the CRP monomer but smaller than that to CRP (not shown). These observations provide evidence for interaction at a common site and that the CRP monomer is a partial agonist at this site. The partial agonist activity of the CRP monomer may be due to spontaneous formation of aggregates.21
DISCUSSION
Considerable controversy surrounds the identity of the receptor underlying platelet activation by collagen. It is well established that the integrin α2β1 is required for normal platelet-collagen interaction, but there is substantial evidence for participation of additional cell surface receptors in the action of collagen, several of which are uncharacterized (see above). A full understanding of the role of each receptor protein in collagen signaling requires the development of selective reagents.
CRP, a relatively simple, triple-helical, cross-linked peptide, is a powerful stimulus of platelet aggregation and secretion but does not support Mg2+-dependent adhesion to the integrin α2β1 .21 This finding suggests that CRP lacks the critical structural determinant required for the interaction between collagen and the integrin. The relatively simple structure of CRP makes it likely that its triple helical nature is an essential part of the structural component required for binding to its receptor.
The results of the present study suggest that the receptor recognized by CRP is also recognized by collagen. Collagen and CRP induce a similar pattern of protein tyrosine phosphorylation in platelets, which includes the tyrosine kinase syk and PLCγ2. The increase in tyrosine phosphorylation by CRP is not altered in the presence of antibodies to α2β1 or by removal of Mg2+ ions, confirming that its action is independent of α2β1 . In contrast, the response to collagen is reduced under these conditions, although tyrosine phosphorylation of cellular proteins including syk and PLCγ2 is still present. This finding suggests that tyrosine phosphorylation induced by collagen is partially dependent on the integrin α2β1 but that it can also be initiated independently of the integrin. The increase in tyrosine phosphorylation by CRP appears to be critical for aggregation and secretion as these two responses are blocked by the kinase inhibitors staurosporine and the tyrphostin ST271.
The role of α2β1 in collagen-induced increases in tyrosine phosphorylation and platelet activation may be direct or indirect. It has been speculated that α2β1 serves as a major adhesion receptor for collagen under conditions of flow where a strong interaction is essential to attract rapidly flowing platelets.7 This would bring the collagen molecule into close vicinity with other cell surface proteins including a signaling receptor, as suggested in the two-step, two-site model for activation of platelets by collagen proposed by Morton et al22 and Santoro et al.23 The integrin α2β1 may also have a direct role in the stimulation of tyrosine phosphorylation and downstream events. For example, monomeric collagen (ie, helical collagen, which lacks quaternary structure) stimulates activation-dependent adhesion of platelets through an α2β1-dependent pathway6 (it is not known whether this is mediated through a tyrosine kinase-dependent pathway) and activatory antibodies to α2β1 induce tyrosine phosphorylation of multiple proteins, including syk, in Jurkat T cells26 and THP-1 monocytes.27 It is of special interest to investigate the effect of α2β1 activation on tyrosine phosphorylation in platelets.
In summary, this study has provided additional support for a pathway of platelet activation by collagen that is independent of α2β1 . The demonstration that activation of platelets by CRP has several features in common with signaling by collagen makes this an important tool in the dissection of collagen-induced signaling events that are independent of α2β1 .
ACKNOWLEDGMENT
We are grateful to Dr B. Coller for the MoAb 6F1 and to Drs Y.H. Lee, P.-G. Suh, and S.H. Ryu for the polyclonal antisera to PLCγ2. M.J.B. and L.F.M. are MRC external staff.
Supported by the Wellcome Trust, British Heart Foundation and Medical Research Council. S.P.W. is a Royal Society Research Fellow. M.A. is in receipt of a British Heart Foundation studentship.
Address reprint requests to Steve P. Watson, PhD, University Department of Pharmacology, Mansfield Road, Oxford OX1 3QT, UK.

![Fig. 2. CRP stimulates tyrosine phosphorylation of syk and PLCγ2. syk and PLCγ2 were immunoprecipitated from lysates of platelets stimulated by CRP or collagen and immunoblotted for phosphotyrosine as described in the Materials and Methods. (A) Tyrosine phosphorylation of syk (i and ii) and PLCγ2 (iii and iv) in CRP-stimulated platelets. The time course of tyrosine phosphorylation stimulated by CRP (3 μg/mL) is shown in (i) and (iii). The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by CRP is shown in (ii) and (iv). The location of syk, PLCγ2, and the heavy chain of the immunoprecipitating antibody (IgG) are shown. An unknown band of 75 kD (indicated by the arrow in [i]) was present in syk immunoprecipitates. Three prominent uncharacterized proteins of approximately 47, 78, and 120 kD were present in PLCγ2 immunoprecipitates and are indicated by arrows in (iii) and (iv). Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions (not shown). (B) Tyrosine phosphorylation of syk and PLCγ2 in collagen-stimulated platelets. Platelets were stimulated with collagen for 90 seconds. The antiphosphotyrosine blots of syk and PLCγ2 immunoprecipitates shown in the upper part of each figure were stripped and reprobed for syk and PLCγ2 as described in the Materials and Methods and are shown in the lower part of each figure. Results are representative of three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f2a.jpeg?Expires=1769147827&Signature=K~ZIlEZYLFXTl29Wg3pqNkPi-GmhBEn8~Ja0jpZmEVO7YWl0roERs4ejqUcLXknUdXggS5awWQHrhon77EIM61OmiLKB~vdCnU9oycKV2379VpVWxDex6tqTgvpHFp14uvdFMwJiBT9sxwo6UVYXMb0ZCbwmQrbLrQJSRV-go~Z3uCW98HF6et7TW6DMq8dTZ~NnK5BIQWFNalgBVY3PWEpyE0G8K3OQxDUGs5lyUfon-H8oMVzfJ0FMEqRGkstdMDnCLSsNVjuBgDlo1VUPMull659JI6N4wWwRWotA12KHRVBVCg5OLkW0SeLu5F77Gis-2DHedqC3phYmP-DN6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. CRP stimulates tyrosine phosphorylation of syk and PLCγ2. syk and PLCγ2 were immunoprecipitated from lysates of platelets stimulated by CRP or collagen and immunoblotted for phosphotyrosine as described in the Materials and Methods. (A) Tyrosine phosphorylation of syk (i and ii) and PLCγ2 (iii and iv) in CRP-stimulated platelets. The time course of tyrosine phosphorylation stimulated by CRP (3 μg/mL) is shown in (i) and (iii). The concentration response relationship (90 seconds of incubation) for tyrosine phosphorylation by CRP is shown in (ii) and (iv). The location of syk, PLCγ2, and the heavy chain of the immunoprecipitating antibody (IgG) are shown. An unknown band of 75 kD (indicated by the arrow in [i]) was present in syk immunoprecipitates. Three prominent uncharacterized proteins of approximately 47, 78, and 120 kD were present in PLCγ2 immunoprecipitates and are indicated by arrows in (iii) and (iv). Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions (not shown). (B) Tyrosine phosphorylation of syk and PLCγ2 in collagen-stimulated platelets. Platelets were stimulated with collagen for 90 seconds. The antiphosphotyrosine blots of syk and PLCγ2 immunoprecipitates shown in the upper part of each figure were stripped and reprobed for syk and PLCγ2 as described in the Materials and Methods and are shown in the lower part of each figure. Results are representative of three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f2b.jpeg?Expires=1769147827&Signature=LTnbLnXt0Q1F8Ru47GUWjIN85WVseYYyAwHC-1z6F-Z3RqvNj93VYZjGe3PTydAc32Byv4ZNCEIndL88aZmPSMNGPkYCzi8FizBLvlqHEvl4aOdxXCHSx0etHoCENesRx2Lf4Sq2gci9bxYlYUvUkY2HMBg~8XGdjHzI4DekvWeiOzRr5HlDGQONoyfh2Kjvx0yGU0FylsqNqzPNwu9SAhZUaTFDj6hJIKkCk13C0cRjNMFWjVNtUmw-XKOCaNjr77c0t8bfbl9l~IfqWaWHkvwZC6DtQAYD4oE9xiVfgcxezLs1DjaatyHiETU3AcDp3O2m608CAFVWIMNQfOeD8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of the removal of divalent cations on collagen- and CRP-stimulated tyrosine phosphorylation. Platelets were prepared and resuspended in Tyrode's-HEPES buffer without MgCl2 in the presence of 1 mmol/L EDTA and 1 mmol/L EGTA. Where indicated, MgCl2 (3 mmol/L) was added 5 minutes before stimulation. Tyrosine phosphorylation of (i) syk and (ii) PLCγ2 after stimulation by collagen (30 μg/mL) or CRP (3 μg/mL) for 90 seconds was measured using MoAb 4G10 as described in Fig 2. Reprobing for syk and PLCγ2 showed that similar levels of each protein were precipitated under all conditions. (The apparent reduction in tyrosine phosphorylation of syk by CRP in the presence of Mg2+ was due to a reduction of sample, as indicated by reprobing for syk [see also part (iii)]). (iii) The concentration response relationship for tyrosine phosphorylation of syk by CRP. Tyrosine phosphorylation was measured in syk immunoprecipitates using MoAb 4G10 as described in Fig 2. Results are representative of two to four similar experiments. A weakly tyrosine phosphorylated protein band that runs just above syk was detected in some but not all experiments (compare parts [i] and [iii]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f4.jpeg?Expires=1769147827&Signature=3d1TMC~52SJV3-s29U8jr-gzT2zGvI7rQLdYE~j9Kl3k0zTmOfCsK2kuTvqloy5qAGzTUPqTO940MrGkCKla4mDjdVmB1MCUg7W5C1-3PVvizLzaH142gdQZTxnb~InKahqO~UZ6XzNKoXKfyStheDF0QzLTKHGZKrvRU9FeE8AhYdp8uG-vF2v~jv1ZkE7HBd5EJwiIbr5dsqZw-yDeDKnV3edFrrShH0IP0xfKTY3Tb12sW0phcVAQb3XmbTkQJ33-edPvxaEAVcTMA0VNxOEe~dJYlIyCrl0Q9Gxmhq9B8QmfQkO7jb0QAqkwoejFTBwPBG9iO4vBAO-rxLnCRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Tyrosine kinase inhibitors block CRP-induced aggregation and secretion of [3H]5-HT. (A) The effect of tyrosine kinase inhibitors on platelet aggregation induced by CRP (3 μg/mL). Platelets were resuspended in modified Tyrode's-HEPES buffer in the absence of EGTA and indomethacin and then preincubated with (i) vehicle, (ii) 3 μmol/L staurosporine (1 minute), or (iii) 100 μmol/L tyrphostin ST271 (5 minutes) before the addition of CRP (indicated by the arrowhead). The aggregation results are from one experiment that is representative of three others. (B) CRP-stimulated secretion of [3H]5-HT. (i) Platelets were stimulated with CRP (0.1 to 5 μg/mL) for 90 seconds and [3H]5-HT was analyzed as described in the Materials and Methods. (ii) Platelets were preincubated with 3 μmol/L staurosporine (stauro) or 100 μmol/L ST271 for 1 and 5 minutes, respectively, before the addition of CRP (1 μg/mL). (C) CRP-stimulated tyrosine phosphorylation is inhibited by staurosporine. The upper part shows whole cell tyrosine phosphorylation induced by CRP (3 μg/mL) in the presence of staurosporine (3 μmol/L) and the lower part shows phosphorylation of the tyrosine kinase syk under the same conditions. Results are the means ± SE of between two and four experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f5a.jpeg?Expires=1769147827&Signature=U-N6MmbJkd~Hy8lHeCxGNBMBx52sVMB-YKuQcRsYHxK8yXlqy8nptkTVLXebFsGB~k9IMiIylFT-EWkiwfWwVhJ5jkKjq17mW6H2mvx03O7u5Sud3LLxtG2Elotb8aO5BXlfG0XQaWQTFuYw1dnbFCyjfzk96Er7tsDZHfGPVyFsYQZD2zvkovl54q84H5jhbmlpJqz5mvCPmViODqmbpxbtjp01y~8iW6xfAm4JGMsElydk5oKi7O6M8hMEVZK~UsOuktjoFWVI~6CgzYkSkYNc7hLH0qjoCJ31HLISf3LUrwsBPeoeEdbCmZjUm95rtSj9J4hC7cAjJrR0q7AN-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Tyrosine kinase inhibitors block CRP-induced aggregation and secretion of [3H]5-HT. (A) The effect of tyrosine kinase inhibitors on platelet aggregation induced by CRP (3 μg/mL). Platelets were resuspended in modified Tyrode's-HEPES buffer in the absence of EGTA and indomethacin and then preincubated with (i) vehicle, (ii) 3 μmol/L staurosporine (1 minute), or (iii) 100 μmol/L tyrphostin ST271 (5 minutes) before the addition of CRP (indicated by the arrowhead). The aggregation results are from one experiment that is representative of three others. (B) CRP-stimulated secretion of [3H]5-HT. (i) Platelets were stimulated with CRP (0.1 to 5 μg/mL) for 90 seconds and [3H]5-HT was analyzed as described in the Materials and Methods. (ii) Platelets were preincubated with 3 μmol/L staurosporine (stauro) or 100 μmol/L ST271 for 1 and 5 minutes, respectively, before the addition of CRP (1 μg/mL). (C) CRP-stimulated tyrosine phosphorylation is inhibited by staurosporine. The upper part shows whole cell tyrosine phosphorylation induced by CRP (3 μg/mL) in the presence of staurosporine (3 μmol/L) and the lower part shows phosphorylation of the tyrosine kinase syk under the same conditions. Results are the means ± SE of between two and four experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f5c.jpeg?Expires=1769147827&Signature=APiEMyg3qTsy3SzdE2QiOAYxyjcScFkoq7iwTo~uW4rTbl8uhshYNLmu6mYRi7~QwMvvaZq7qRrcUUyICu-eMbOTtMYlr0xumdnqcLVeU0EERrRVZpet-r9IcB~l3q7C89sWvOrd~ANOR1zfKJpcQdYrasJFaVPCi6Lj9FJyHW0bM3tfxfHQ2fcFB4NHKM7hSqWbrAAgYV7ybRQYBSCMyaTWRmdjbXMvk0GOt5P8iGFH6Zk-PpusR6BrdqZHTm4Tm1--e8nZlXN5xe9DiQbETwEbSVD3r~VNNik6F4d-i45Pk~w93pVMUrOWWnaAgwVKaQqGj7D9C3~WwvdEPJZPlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Tyrosine kinase inhibitors block CRP-induced aggregation and secretion of [3H]5-HT. (A) The effect of tyrosine kinase inhibitors on platelet aggregation induced by CRP (3 μg/mL). Platelets were resuspended in modified Tyrode's-HEPES buffer in the absence of EGTA and indomethacin and then preincubated with (i) vehicle, (ii) 3 μmol/L staurosporine (1 minute), or (iii) 100 μmol/L tyrphostin ST271 (5 minutes) before the addition of CRP (indicated by the arrowhead). The aggregation results are from one experiment that is representative of three others. (B) CRP-stimulated secretion of [3H]5-HT. (i) Platelets were stimulated with CRP (0.1 to 5 μg/mL) for 90 seconds and [3H]5-HT was analyzed as described in the Materials and Methods. (ii) Platelets were preincubated with 3 μmol/L staurosporine (stauro) or 100 μmol/L ST271 for 1 and 5 minutes, respectively, before the addition of CRP (1 μg/mL). (C) CRP-stimulated tyrosine phosphorylation is inhibited by staurosporine. The upper part shows whole cell tyrosine phosphorylation induced by CRP (3 μg/mL) in the presence of staurosporine (3 μmol/L) and the lower part shows phosphorylation of the tyrosine kinase syk under the same conditions. Results are the means ± SE of between two and four experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1235/4/m_bl_0022f5b.jpeg?Expires=1769147827&Signature=PROc4m-ud1-INGko6aZYK9qW6veMlHYsgeU-CJV1itUAOiLBl5yV-8HTlWGXk4k0-wiDXCvk-F1jiuayj9moMAAs~aIT-u6xBIURGxeu2YMReOmN9xKc5FRl6OI8BcoVOO~atLnDevgRMruOmjGBhjLLaeTdzwbc7MOC~CGjNFK3JjY4hHrVGC9X5yHwKolpWtIAUw~Kaajwm1aI~qGEmUD1hDfrCUan4uChG18~HrSe13PDwlXbO6I-Gf6dorXGTrDcssrNP-LpqgNqQh8gjzUTF3oqJfpHAkG-LglRN-xoMn8naC-BS5HlDqxE34XNd7B3oY9v8U2WU5dpIXFvBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal