Abstract
Thrombotic thrombocytopenic purpura (TTP) and sporadic hemolytic-uremic syndrome (HUS) are thrombotic microangiopathies that occur in the absence of an inflammatory response. Ultrastructural features of tissues involved in TTP/sporadic HUS suggest an apoptotic process. Consistent with these findings, we observed that TTP plasmas induce apoptosis in primary human endothelial cells (EC) of dermal microvascular but not umbilical vein origin (Laurence et al, Blood 87:3245, 1996). We now document the ability of plasmas from both TTP and sporadic HUS patients, but not from a patient with childhood/diarrhea-associated HUS, to induce apoptosis and expression of the apoptosis-associated molecule Fas (CD95) in restricted lineages of microvascular EC. EC of small vessel dermal, renal, and cerebral origin were susceptible to induction of Fas and an apoptotic cell death. In contrast, microvascular EC of pulmonary and hepatic origin, as well as EC of a large vessel, coronary artery, were resistant to both processes. This dichotomy parallels the in vivo pathology of TTP/sporadic HUS, with notable sparing of the pulmonary and hepatic microvasculature. Apoptotic EC also had some features of a procoagulant phenotype, including depressed production of prostaglandin I2 (prostacyclin). These phenomena support the pathophysiologic significance of microvascular EC apoptosis in TTP, extend it to a related disorder (sporadic HUS), and suggest consideration of apoptosis inhibitors in the experimental therapeutics of these syndromes.
THE FUNDAMENTAL LESION in thrombotic thrombocytopenic purpura (TTP) and adult/sporadic hemolytic-uremic syndrome (HUS) is thrombotic microangiopathy.1 Hyaline thrombi, composed of platelets and some fibrin, accompanied by localized endothelial cell (EC) proliferation and detachment, are found exclusively in the microvasculature of involved tissues, notably sparing the pulmonary and hepatic microvasculature.1 These thrombi subserve the clinical manifestations of both disorders.1
Although TTP is recognized classically by the pentad of thrombocytopenia, microangiopathic hemolytic anemia, fluctuating neurologic signs, renal abnormalities, and fever,2 and HUS by a triad of signs — the hematologic abnormalities of TTP plus renal disease2 — pathologic changes may overlap significantly. Sporadic HUS patients often have multiple organ involvement, similar to TTP, with dermal, ocular, and neurologic changes.1,3 In one study, cerebral microthrombi were found in one-half of 26 HUS patients at autopsy, with clinical neurologic manifestations in 15 of 44 cases and, reciprocally, renal dysfunction occurs in 40% to 80% of TTP cases.1
However, it is important to distinguish between two forms of HUS. The epidemic type, occurring primarily in children less than 5 years of age, is typically preceded by bloody diarrhea and has an excellent prognosis without plasma therapy.4,5 In contrast, sporadic forms of HUS occur in older children and adults, have a much poorer prognosis, are often relapsing, and respond to plasmapheresis with plasma exchange, as does TTP.1 Renal microangiopathy occurs in both forms of HUS, but distinct histologic patterns are found in the kidney.3 6
In diarrhea-associated HUS, fibrin thrombi predominate, larger vessel thrombosis with cortical necrosis may occur, and there is no EC proliferation.7 In sporadic HUS, as in TTP, platelet thrombi predominate, with EC activation, severe intimal proliferation leading to luminal stenosis, and a clear absence of inflammatory changes.4,7 The latter observations, together with EC detachment from renal and other affected microvasculature8 and their appearance in the periphery,9 are consistent with an apoptotic process. Indeed, a high frequency of apoptotic cells within glomerular capillary lumina, presumably of EC origin, has been described in adult HUS.10 These in vivo changes parallel our recent finding of TTP plasma-induced apoptosis among primary human dermal microvascular EC, but not large vessel umbilical vein EC, in vitro.11
We now document the ability of TTP and sporadic but not diarrhea-associated HUS plasmas to induce apoptosis in primary human microvascular EC of renal and cerebral as well as dermal origin, but not in similar cells of pulmonary or hepatic lineage. This reflects the pathology and distribution of thrombi in both disorders. These effects were paralleled by induction of the apoptosis-associated membrane receptor Fas (CD95) and by certain procoagulant features.
PATIENTS, MATERIALS, AND METHODS
Patients
Plasma samples were obtained from heparinized or EDTA-treated venous blood of 2 human immunodeficiency virus (HIV)-seronegative asymptomatic controls, 10 HIV-seronegative and 2 HIV-seropositive adult patients with active TTP, 1 HIV-seronegative adult with sporadic HUS, and a 2-year-old child with diarrhea-associated HUS and renal failure, whose disease responded to supportive measures. All of these individuals, except for 1 of the HIV+ patients, who had been treated by plasmapheresis, are different from those reported previously.11
TTP was diagnosed according to the following criteria: fever, defined as an unexplained oral temperature greater than 38.0°C; neurologic dysfunction, defined as any new abnormality on general medical neuropsychiatric exam; renal dysfunction, defined as a serum creatinine level of ≥1.2 mg/dL or greater than 150% of previous baseline; and thrombocytopenia, define as a platelet count less than 150,000/μL. Sporadic HUS was diagnosed by thrombocytopenia (platelet count <150,000/μL), renal dysfunction (serum creatinine ≥1.5 mg/dL), and the absence of clinical neurologic disease. All patients had microangiopathy on peripheral blood smear. Fever may or may not have been found on presentation.
EC Cultures
Two different primary human microvascular EC of dermal origin were purchased: CD36+ MVEC-1 (HMVEC 2753; Clonetics, San Diego, CA) and CD36− MVEC-2 (DHMVEC 30282; Cell Systems, Kirkland, WA). In addition, the following primary human microvascular cells were donated for this work by Cell Systems: HMVEC-LU (pulmonary), HMVEC-G (renal glomerular), HMVEC-B (cerebral), and HMVEC-H (hepatic). Large vessel coronary artery EC (HCAEC) were also donated by Cell Systems. EC were maintained in T-25 flasks (Falcon; Becton Dickinson Labware, Lincoln Park, NJ) in modified MCDB 131 medium containing recombinant human epidermal growth factor (rhEGF; 10 ng/mL), hydrocortisone (1 μg/mL), bovine brain extract, heparin (10 μg/mL), amphotericin B (50 ng/mL), gentamicin (50 μg/mL), 5% fetal bovine serum (FBS), and 5% pooled human serum and used in passages 2 through 8. Umbilical vein EC (UVEC) were obtained and prepared as previously described.11 All cells were positive for expression of von Willebrand's factor (vWF )/factor VIII antigen and had a doubling time of approximately 36 hours. Subcultures involved 5 to 10 minutes of exposure to 0.025% trypsin/0.01% EDTA or 0.1% collagenase, followed by washing with phosphate-buffered saline (PBS), pH 7.2.
To insure some uniformity of culture conditions, experiments were performed in apoptosis culture medium devoid of human serum (medium 199 plus 20% FBS, rhEGF, heparin, amphotericin B, and gentamicin) after 4 to 18 hours of preincubation of EC in that medium.
Apoptosis Assays
These assays were performed as previously described.11 12 EC were washed with PBS, assessed for viability by trypan blue dye exclusion, and then plated in macrowells at 0.1 to 0.15 × 106 viable cells/0.5 mL in apoptosis culture medium, alone or with dilutions of various plasmas. Cells were harvested 18 to 36 hours later, fixed in 70% cold ethanol, and incubated for 20 minutes at 4°C with propidium iodide (50 μg/mL) in the presence of RNaseA (300 U/mL), and 5 × 103 cells were analyzed in the cytofluorograph (EPICS Elite; Coulter, Hialeah, FL).
Apoptosis was recognized or quantitated in viable cells by two flow cytometric methods: detection of depressed forward scatter and increased side (right angle) scatter and computer-assisted DNA histogram analysis of propidium iodide-labeled cells with calculation of pre-G1 A0 peaks, defined by computer software (MCycle Av; Phoenix Flow Systems, San Diego, CA).11 The mathematical algorithm for analyzing DNA distributions involved a nonlinear least squares fit, by which S phase distribution of the cell cycle is described as a second degree polynomial, and G1 and G2 + M subpopulations as normally distributed.13 These assumptions have been confirmed by autoradiography.13 A0 is fit as a normally distributed curve of hypodiploid DNA just before the diploid DNA area.12 Most experiments involved at least two separate determinations, with A0 values for a given plasma sample and cell type of similar passage number and confluence in culture varying by ≤15%.
Features of apoptotic cells were also directly visualized. Staining of nucleic acid with 4′,6-diamidino-2-phenylindole (DAPI) can be used to discriminate among live, apoptotic, and necrotic cells.11,12 Two techniques, DNA histogram analysis and DAPI staining, were used to confirm the extent of apoptosis. This is prudent, because an assumption is made in computer-fitting of A0 peaks, ie, that the decrease in DNA stainability represents a change in DNA accessibility to propidium iodide, rather than a loss of cellular DNA, as is typical for necrosis. However, partial loss of DNA can occur during apoptosis.12
EC were preincubated with collagenase (0.025%) for 10 minutes at 37°C, washed, fixed with 70% ethanol, washed again, and then exposed to RNaseA (1,000 U/mL) for 20 minutes at 37°C. After washing, cells were resuspended in 0.1 mL PBS, were transferred onto glass slides by cytocentrifugation, and were fixed at room temperature in a 1:9 solution of glacial acetic acid and absolute ethanol. Slides were air-dried and 2 to 3 drops of a 1 μg/mL solution of DAPI (Molecular Probes, Inc, Eugene, OR) in 1 N NaOH were added, immediately followed by 2 to 3 drops of sulforhodamine 101 (1 μg/mL; Molecular Probes). The slides were kept at room temperature for 10 minutes and were washed with PBS, and the cells were photographed using a fluorescence microscope with a UG-1 filter.
Quantitation of Fas mRNA Expression
Fas is a 48-kD polypeptide belonging to a family of type I plasma membrane receptors that includes tumor necrosis factor (TNF )-I and TNF-II receptors.14,15 It has a central role in the regulation of apoptosis and programmed cell death in lymphoid cells, hepatocytes, epithelial cells, and possibly other cell types via interactions with its ligand, FasL.15,16 It has also been implicated in the pathogenesis of several viral infections, including HIV-associated apoptosis of CD4+ T lymphocytes,17 and in activation-induced18 and idiopathic19 programmed T-cell death.
Expression of Fas transcripts was evaluated by a quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) that we have developed.20 This assay uses a single set of oligonucleotide primers to amplify both the target gene, Fas, and a second fragment of different size that, in competing for the same primers, acts as an internal standard. Briefly, a PCR Mimic kit (Clontech, Palo Alto, CA) was used to construct Fas mimic, a nonhomologous internal fragment of 425 nucleotides having Fas-specific primer sequences at both ends of a fragment 124 nucleotides shorter than Fas. Fas mimic was amplified and column purified using techniques standard in our laboratory11 21 and then quantitated by absorbance spectroscopy at 260 nm. Fas primers capable of amplifying a segment from nucleotides 271 to 820 of Fas cDNA are Fas 1, 5′-CAAGTGACTGACATCAACTCC; and Fas 2, 5′-CCTTGGTTTTCCTTTCTGTGC. Fas mimic primers are FasM 1, 5′-CAAGTGACTGACATCAACTCCCAAGTTTCGTGAGCTGATTG (the last 20 nucleotides are a neutral sequence, not present in Fas); and FasM 2, 5′-CCTTGGTTTTCCTTTCTGTGCATTTGATTCTGGACCATGGC (the last 20 nucleotides are a neutral sequence).
Total cellular RNAs were isolated from 1 × 106 cells/sample by the TriZOL (GIBCO-BRL, Gaithersburg, MD) method.11 RNAs were treated with RNase-free DNase, and a constant amount (1 μg) of RNA was reverse transcribed into cDNA using 200 U of Moloney murine leukemia virus reverse transcriptase (GIBCO-BRL). cDNA aliquots of equal volume were then amplified by PCR, as described,11 along with 10-fold dilutions of the previously prepared Fas mimic cDNA (range, 2 to 2 × 10−3 attomoles or 500 to 0.5 fg). Reaction products were run on a 1.4% agarose gel, visualized by ethidium bromide staining under UV illumination, and photographed. Integrity of initial RNAs were checked by amplifying β-actin (sense primer, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA; antisense primer, 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG; Stratagene, La Jolla, CA). The expected sizes of the amplicons were Fas, 549 nucleotides; Fas mimic, 425; and β-actin, 661.
A range was identified in which Fas and Fas mimic bands were close in intensity, as assessed by direct visualization. A second PCR reaction was then run using constant volumes of cDNAs (10 μL) amplified with twofold to fourfold dilutions of Fas mimic, selected from the range defined by the initial PCR reaction. These products were then electrophoresed on agarose gels, the gels were scanned by a Scanman Easytouch scanner (Logitech, Freemont, CA), and the data were analyzed using Sigmagel software (Jandel Scientific, San Rafael, CA). Intensities of individual Fas and Fas mimic bands were plotted as a Fas:Fas mimic ratio versus input concentration (in femtograms) of Fas mimic cDNA. The point at which a ratio of 1 intersects the x-axis gives the concentration of cDNA in a given sample. Assuming that the efficiency of reverse transcription is equivalent for all reactions run in parallel on a given day (an assumption checked using duplicate samples), the number of Fas mRNA molecules can be calculated as femtograms per microgram of input RNA.20
Cdc2 Kinase Assay
Premature induction of a subset of protein kinases normally active only at mitosis correlates with apoptosis in many systems. One such kinase, Cdc2, is activated in the cytoplasm and requires nuclear localization to initiate both cytoplasmic and nuclear mitotic transformations.22 Its relative concentrations in cytoplasmic and nuclear fractions of plasma-treated EC were assessed. EC were treated with 2.5 mmol/L hydroxyurea (Sigma, St Louis, MO) for 6 hours at 37°C to arrest cells at S phase of the cell cycle and then incubated overnight with buffer or 1:5 to 1:100 dilutions of normal or TTP plasmas. EC were harvested with EDTA (0.01%), and 5 × 106 cells per condition were used to prepare cytoplasmic and nuclear extracts, using a modification of Dignam's procedure,23 as detailed elsewhere by our group.21 The protein content of all fractions was assessed using a MicroBCA Protein Assay Reagent kit (Pierce, Rockford, IL), and samples were stored at −70°C until use. Equal amounts of protein from each sample (∼50 μg) were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred overnight onto a polyvinylidene fluoride (PVDF) membrane (Millipore) using an LKB transfer apparatus. Half of the membrane was stained with Coomassie blue and half was used for immunoblotting with a mouse monoclonal antibody (MoAb) against Cdc2 (Oncogene Science, Uniondale, NY). Detection of immunoblots involved a horseradish peroxidase-labeled antimouse IgG Western kit (Kirkegaard & Perry Labs, Gaithersburg, MD). Blots were scanned using the Logitech Scanman, as described for quantitative PCR.
Antiapoptosis Reagents
A panel of MoAbs directed to Fas had been generated by immunizing mice with a purified fusion protein consisting of the extracellular domain of human Fas and the constant region of human IgG1.16 When added in solution, Fas MoAb M3 blocks Fas-mediated cell lysis, whereas Fas MoAb M31 binds to the antigen but has no agonistic or antagonistic properties.11 16
The trisodium salt of aurintricarboxylic acid (ATA; Sigma) was dissolved in PBS, pH 7.4, and used at final concentrations of 0.01 to 1 μmol/L. At these doses, ATA is a potent suppressor of apoptosis in many cell types, presumably related to its ability to block both proteases and Ca2+-dependent endonucleases.11 17
Assays for Procoagulant Activities
Prostaglandin I2 (PGI2 ; prostacyclin).The stable metabolite of PGI2 , 6-keto-prostaglandin F1α (6KPGF1α), was measured in culture supernatants by a competitive enzyme-linked immunoassay (ELISA) at 2 hours and 16 to 18 hours after initiation of cultures. Because prostaglandins are not stored in EC, these values represent total spontaneous PGI2 production during the incubation periods. Maximum production of 6KPGF1α from exogenous substrate was assessed at the 16- to 18-hour time point by exposing cultures to fresh medium containing 20 μmol/L sodium arachidonate for 15 minutes at 37°C and then collecting these supernatants.
Duplicate aliquots of culture supernatant, stored at −20°C until assayed, were incubated in microtiter plates (Nunc, Inc, Naperville, IL) coated with mouse antirabbit IgG in the presence of acetylcholinesterase-linked 6KPGF1α tracer (Cayman Chemical, Ann Arbor, MI) and a 10−5 dilution of anti-6KPGF1α rabbit polyclonal antibody. After overnight incubation at 25°C, plates were extensively washed and developed by incubation with Ellman's reagent [acetylthiocholine iodide in 5′-dithio-bis(2-nitrobenzoic acid)] to give a color product measurable at 405 nm. Experimental samples were quantitated by direct comparison to a standard curve with authentic unlabeled 6KPGF1α run in the same plate, using a DeltaSoft log-logit program. Intrassay variation was less than 10%, and cross-reactivity of the antiserum with other prostaglandins is less than 0.1%.
Annexin II.Membrane expression of annexin II, the EC receptor for plasminogen and tissue plasminogen activator (tPA),24 was analyzed by flow cytometry in EC cultures exposed for 16 to 18 hours to TTP or control plasmas. Cells were harvested, washed, and fixed in 2% paraformaldehyde. This was followed by incubation with preimmune mouse IgG or murine MoAbs antiannexin II IgG (100 μg/mL) or antiannexin I IgG (100 μg/mL; Zymed, Inc, Camarrero, CA) for 15 minutes at 4°C. After three washes, cells were incubated with fluorescein isothiocyanate-conjugated goat-antirabbit IgG (20 μg/mL for 15 minutes at 4°C), washed, and analyzed in the cytofluorograph.
Tissue factor.Tissue factor secreted into the culture medium was assessed by an Imubind Tissue Factor ELISA kit (American Diagnostics, Inc, Greenwich, CT), used according to the manufacturer's directions. This assay has a sensitivity of 10 pg/mL.
RESULTS
Apoptosis Data
We first examined the baseline DNA histogram and light scatter patterns of RNase-treated and propidium iodide stained primary human microvascular EC of five different lineages, as well as EC from a large vessel, coronary artery. Cells suspended in apoptosis culture medium were plated in uncoated macrowells and then exposed for 16 to 18 hours to control plasmas or plasmas derived from patients with TTP, adult/sporadic HUS, or childhood/diarrhea-associated HUS. Upon culture termination, loosely adherent EC cells were obtained by the removal of the supernatant, the addition of 2 mL PBS, and vigorous pipetting. Adherent EC were removed by treatment with 0.1% collagenase.
Microvascular EC from dermal, renal, and cerebral sources (Fig 1A), as well as EC from coronary artery (Fig 2) cultured in the presence of 1% normal plasma, showed similar cell-cycle profiles. Low percentages of cells were found in the hypodiploid pre-G1 area known as A0 (Figs 1A and 2), together with normal light scattergrams (data not shown). In this respect, they resembled control UVEC and dermal microvascular (MVEC-1 and -2) cells, as previously documented.11 No control plasma or serum samples gave an A0 value greater than 16%, and the majority gave values ≤10%.
DNA histograms of microvascular endothelial cells (MVEC) exposed to normal and TTP plasmas. Primary human microvascular EC from various tissues were cultured for 18 hours in human serum-free apoptosis culture medium in the presence of a 1:100 dilution of plasma from control donors and a 1:100 or 1:1,000 dilution of plasma from TTP patients. DNA histograms of ethanol-fixed, RNase-treated and propidium iodide-stained cells were obtained and analyzed by computer software, as described in the text. A0 peak values are indicated within each histogram, representing the more heavily shaded region just to the left of the large G1/S peak (see [B]). (A) Dermal, renal, and cerebral MVEC + normal plasma. (B) Dermal, renal, and cerebral MVEC + TTP plasmas.
DNA histograms of microvascular endothelial cells (MVEC) exposed to normal and TTP plasmas. Primary human microvascular EC from various tissues were cultured for 18 hours in human serum-free apoptosis culture medium in the presence of a 1:100 dilution of plasma from control donors and a 1:100 or 1:1,000 dilution of plasma from TTP patients. DNA histograms of ethanol-fixed, RNase-treated and propidium iodide-stained cells were obtained and analyzed by computer software, as described in the text. A0 peak values are indicated within each histogram, representing the more heavily shaded region just to the left of the large G1/S peak (see [B]). (A) Dermal, renal, and cerebral MVEC + normal plasma. (B) Dermal, renal, and cerebral MVEC + TTP plasmas.
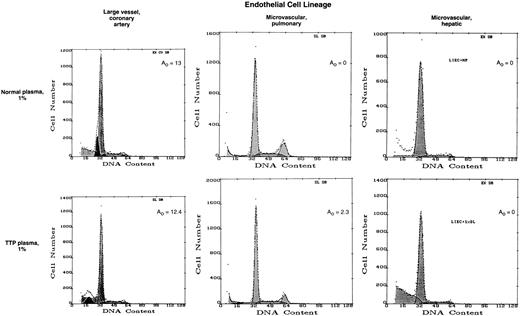
Effect of TTP plasma on coronary artery EC and MVEC of pulmonary and hepatic origin. Primary human EC were cultured and evaluated as described in the legend to Fig 1.
Effect of TTP plasma on coronary artery EC and MVEC of pulmonary and hepatic origin. Primary human EC were cultured and evaluated as described in the legend to Fig 1.
Exposure of microvascular EC of dermal, renal, and cerebral origin to 1:100 to 1:1,000 dilutions of plasma from either of 2 acute HIV-seronegative TTP patients or from 1 acute HIV+ TTP patient gave classic apoptotic patterns (Fig 1B and Table 1). Propidium iodide labeling showed prominent A0 regions, comprising almost 50% of the cell cycle in the DNA histogram of the dermal EC, with an A0 peak of approximately 34% in the presence of a 1:1,000 dilution of a particularly potent TTP plasma (Fig 1B). The majority of these treated cells also showed depressed forward light scatter, secondary to cell shrinkage, and either no change or some elevation in side scatter, due to chromatin condensation with increased granularity (data not shown). Differences in the degree of apoptosis in renal glomerular EC appeared to parallel the presence of clinical renal disease (Table 1), although enough samples from TTP/sporadic HUS patients with divergent clinical manifestations were not available to make a definitive statement in that regard.
Mean Specific Apoptosis Scores (%A0) for EC of Divergent Tissues Exposed to TTP Plasmas
| Tissue . | EC Sample . | No. of Experiments* . | Mean %A0 . | Range for %A0† . |
|---|---|---|---|---|
| Microvascular EC | ||||
| Lung | HMVEC-LU | 16 | 3.5 | 0-14.5 |
| Liver | HMVEC-H | 9 | 5.8 | 0-18.1 |
| Dermal | MVEC-1, -2 | 4 | 37.3 | 15.2-50.4 |
| Brain | HMVEC-B | 7 | 11.3 | 1.0-20.7 |
| Kidney | HMVEC-G | 5 (no renal disease)‡ | 9.2 | 0-15.5 |
| 3 (renal disease)‡ | 17.4 | 9.7-36.3 | ||
| Large vessel | ||||
| Coronary artery | HCAEC | 6 | 6.7 | 0-18.2 |
| Tissue . | EC Sample . | No. of Experiments* . | Mean %A0 . | Range for %A0† . |
|---|---|---|---|---|
| Microvascular EC | ||||
| Lung | HMVEC-LU | 16 | 3.5 | 0-14.5 |
| Liver | HMVEC-H | 9 | 5.8 | 0-18.1 |
| Dermal | MVEC-1, -2 | 4 | 37.3 | 15.2-50.4 |
| Brain | HMVEC-B | 7 | 11.3 | 1.0-20.7 |
| Kidney | HMVEC-G | 5 (no renal disease)‡ | 9.2 | 0-15.5 |
| 3 (renal disease)‡ | 17.4 | 9.7-36.3 | ||
| Large vessel | ||||
| Coronary artery | HCAEC | 6 | 6.7 | 0-18.2 |
All plasmas used at a 1% final concentration.
Control values, representing EC incubated for 18 hours in 1% normal plasma, were less than 10% and have been subtracted from these numbers.
A minimum of three different TTP plasma samples were used for each tissue type.
Pertains to plasmas obtained from acute TTP patients with and without clinical renal involvement.
All of our HIV-seronegative TTP (10 samples) and sporadic HUS (1 sample) plasmas and the 2 HIV+ TTP plasmas were then evaluated for the actual number of apoptotic cells by DAPI/sulforhodamine staining and direct visualization. Dermal MVEC-2 were used as targets for all plasmas. An example of a positive result is given for dermal EC in Fig 3. The mean number of apoptotic cells was 32.1%, with a range for different plasmas of 2.1% to 70.6%. Three TTP plasmas failed to show apoptosis greater than background levels. In this experiment, simultaneous control plasmas gave 3.5% (range, 6% to 10%) apoptotic cells, including one sample from a healthy donor with anti-UVEC antibodies. (It was also noted that the activity of plasma derived directly from venous blood was much greater than that of plasma derived from bags of citrated plasmapheresis material.)
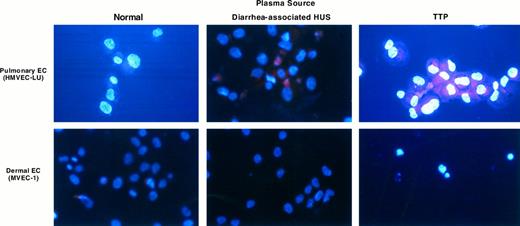
UV light photography of microvascular EC stained with DAPI/sulforhodamine. Microvascular EC of pulmonary and dermal lineages were exposed to a 1:100 dilution of normal plasma or plasma from individuals with childhood/diarrhea-associated HUS or TTP for 18 hours. Cells were harvested and stained with DAPI and sulforhodamine 101, as described in the text. Apoptotic cells show DNA in two compartments: intense blue staining of fragmented nuclear material, (or apoptotic bodies) and dispersed DNA outside of the nucleus, inferred from the uniformity of the blue or violet (red/blue) hue of the cytoplasm. In contrast, intact cells show red cytoplasmic staining, with uniformly blue nuclei.
UV light photography of microvascular EC stained with DAPI/sulforhodamine. Microvascular EC of pulmonary and dermal lineages were exposed to a 1:100 dilution of normal plasma or plasma from individuals with childhood/diarrhea-associated HUS or TTP for 18 hours. Cells were harvested and stained with DAPI and sulforhodamine 101, as described in the text. Apoptotic cells show DNA in two compartments: intense blue staining of fragmented nuclear material, (or apoptotic bodies) and dispersed DNA outside of the nucleus, inferred from the uniformity of the blue or violet (red/blue) hue of the cytoplasm. In contrast, intact cells show red cytoplasmic staining, with uniformly blue nuclei.
In contrast, large vessel coronary artery EC retained normal DNA histograms (Fig 2 and Table 1) and light scatter patterns (data not shown) upon exposure to TTP or sporadic HUS plasmas when tested in parallel with positive microvascular EC controls. This is consistent with our previous results with TTP plasma and UVEC, another large vessel but of venous origin.11
Two other lineages of primary human microvascular EC were also used in these experiments, pulmonary and hepatic. These studies were undertaken because the pulmonary microvasculature are never involved pathologically in TTP or sporadic HUS.1 Hepatic microthrombi may occur but, in contrast to the cerebral, dermal, coronary, ocular, or renal microvasculature, liver lesions are unusual and were completely absent from one large pathologic survey.25 Pulmonary thromboses are characteristic of childhood/diarrhea-associated HUS,26 as well as HUS syndromes associated with cyclosporin A and cancer chemotherapy.4,27 However, the lesions in these latter disorders are not representative of the platelet thrombi without inflammation seen in TTP/sporadic HUS, instead consisting of fibrin thrombi with entrapped leukocytes and erythrocytes, necrosis, and often inflammation.26
Plasmas from TTP patients were incapable of inducing apoptosis in pulmonary or hepatic microvascular EC (Fig 2 and Table 1). Increases in plasma concentration from 1:100 to 1:5 as well as prolonged incubation times (up to 72 hours) failed to show a difference in A0 values in either pulmonary or hepatic EC with control versus TTP/sporadic HUS plasmas. This dichotomy in EC lineage effects was supported by DAPI/sulforhodamine staining of dermal and pulmonary microvascular EC cultured for 18 hours with a 1:100 dilution of TTP plasma (Fig 3).
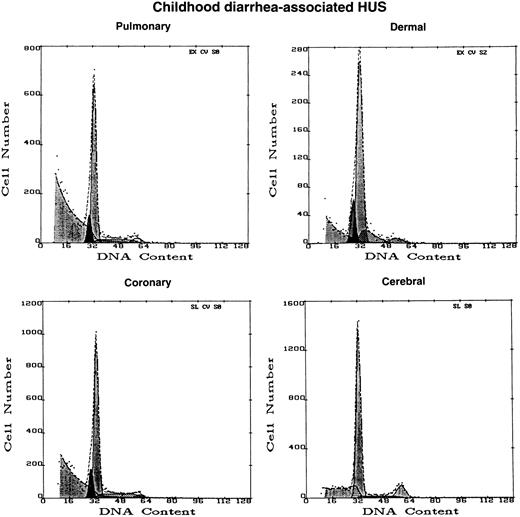
Plasma from the child with acute diarrhea-associated HUS did not induce apoptosis in either coronary artery or microvascular EC (Figs 3 and 4). However, this plasma did cause substantial cytotoxicity in certain EC cultures, with large amounts of cellular debris noted on flow cytometry (Fig 4), consistent with the work of other groups using large vessel EC.7
Effect of diarrhea-associated HUS plasma on EC of different tissue origin. DNA histograms were obtained with microvascular EC of dermal, cerebral, and pulmonary origin, as well as large vessel EC derived from coronary artery, after 16 hours of exposure to 1% plasma from a 2-year-old infant with acute, diarrhea-associated HUS. A0 values greater than background were not obtained from any sample. However, please note the large peak of cellular debris, partially cut-off, to the extreme left of the pulmonary MVEC and coronary artery histograms, as compared with the small or absent peaks for such cells exposed to normal and TTP plasmas shown in Fig 2.
Effect of diarrhea-associated HUS plasma on EC of different tissue origin. DNA histograms were obtained with microvascular EC of dermal, cerebral, and pulmonary origin, as well as large vessel EC derived from coronary artery, after 16 hours of exposure to 1% plasma from a 2-year-old infant with acute, diarrhea-associated HUS. A0 values greater than background were not obtained from any sample. However, please note the large peak of cellular debris, partially cut-off, to the extreme left of the pulmonary MVEC and coronary artery histograms, as compared with the small or absent peaks for such cells exposed to normal and TTP plasmas shown in Fig 2.
Fas Expression
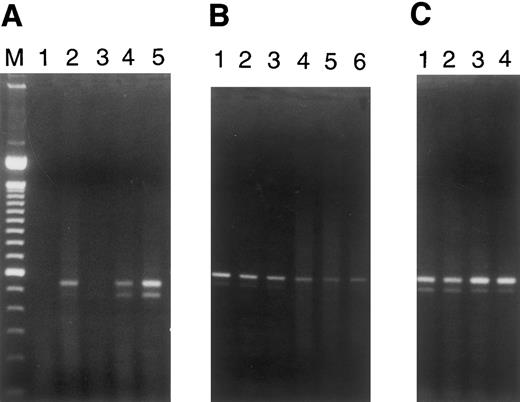
Fas transcripts were induced by plasma from patients with TTP/sporadic HUS in microvascular EC of dermal, renal, and cerebral origin, but not in pulmonary or hepatic microvascular EC (Fig 5A and B). This paralleled the ability of these plasmas to mediate apoptosis exclusively in the former group of EC (Table 1). Baseline Fas mRNA expression was observed in both large vessel EC, umbilical vein and coronary artery, but was not elevated by exposure of cells to TTP plasma (Fig 5C).
RT-PCR for Fas (CD95) expression in EC. PCR analysis of cDNA reverse transcribed from RNAs obtained from microvascular EC of various tissues and from large vessel umbilical vein and coronary artery EC cultured for 18 hours in the presence of various plasmas (1:100 dilutions) was performed. Simultaneous RT-PCR for β-actin, using aliquots from these same samples, served as a control for integrity of the starting materials and gave equivalent signals for all samples (data not shown). The two bands for Fas recognized in some lanes represent the membrane-bound and secreted forms of this molecule.17 (A) Lane 1, dermal MVEC-2 + normal plasma; lane 2, dermal MVEC-2 + TTP-1; lane 3, renal HMVEC-G + normal plasma; lane 4, renal HMVEC-G + TTP-1; lane 5, renal HMVEC-G + TTP-2. (B) Lane 1, pulmonary HMVEC-LU + normal plasma; lane 2, pulmonary HMVEC-LU + TTP-1; lane 3, pulmonary HMVEC-LU + TTP-2; lane 4, hepatic HMVEC-H + normal plasma; lane 5, hepatic HMVEC-H + TTP-1; lane 6, hepatic HMVEC-H + TTP-3. (C) Lane 1, UVEC + normal plasma; lane 2, UVEC + TTP-1; lane 3, coronary HCAEC + normal plasma; lane 4, coronary HCAEC + TTP-1.
RT-PCR for Fas (CD95) expression in EC. PCR analysis of cDNA reverse transcribed from RNAs obtained from microvascular EC of various tissues and from large vessel umbilical vein and coronary artery EC cultured for 18 hours in the presence of various plasmas (1:100 dilutions) was performed. Simultaneous RT-PCR for β-actin, using aliquots from these same samples, served as a control for integrity of the starting materials and gave equivalent signals for all samples (data not shown). The two bands for Fas recognized in some lanes represent the membrane-bound and secreted forms of this molecule.17 (A) Lane 1, dermal MVEC-2 + normal plasma; lane 2, dermal MVEC-2 + TTP-1; lane 3, renal HMVEC-G + normal plasma; lane 4, renal HMVEC-G + TTP-1; lane 5, renal HMVEC-G + TTP-2. (B) Lane 1, pulmonary HMVEC-LU + normal plasma; lane 2, pulmonary HMVEC-LU + TTP-1; lane 3, pulmonary HMVEC-LU + TTP-2; lane 4, hepatic HMVEC-H + normal plasma; lane 5, hepatic HMVEC-H + TTP-1; lane 6, hepatic HMVEC-H + TTP-3. (C) Lane 1, UVEC + normal plasma; lane 2, UVEC + TTP-1; lane 3, coronary HCAEC + normal plasma; lane 4, coronary HCAEC + TTP-1.
The differential induction of apoptosis in renal microvascular EC, paralleling clinical nephrotoxicity, also appeared to relate to upregulation of Fas mRNAs. This was best shown by competitive quantitative RT-PCR. Plasmas from the 3 of 5 TTP patients without nephrotoxicity we evaluated upregulated Fas mRNA by 1.5-fold over control plasmas in glomerular EC (Table 2). In contrast, plasma from the sporadic acute HUS patient and a TTP patient with prominent renal disease induced Fas mRNA by threefold (Table 2). The degree of Fas mRNA alterations for several other EC exposed to TTP versus normal plasma is noted in Table 2 as well.
Quantitation of Fas mRNA Expression in Microvascular EC of Varying Lineages and Changes Induced by TTP/Sporadic HUS Plasmas
| EC lineage | Dermal | Renal | Cerebral | Hepatic | Pulmonary |
| Cell line designation | MVEC-2 | HMVEC-G | HMVEC-B | HMVEC-H | HMVEC-LU |
| Baseline Fas mRNA, fg/μg RNA* | 17.7 | 3.9 | 18.8 | 37.7 | 12.1 |
| 1% TTP plasma, without clinical renal dysfunction, fold ΔFas mRNA | 1.2↑ | 1.5↑ | 1.1↑ | 0.8↓ | 0.8↓ |
| 1% TTP plasma, clinical renal disease, fold ΔFas mRNA | ↔ | 2.9↑ | ND | ND | ND |
| 1% adult/sporadic HUS plasma, fold ΔFas mRNA | 1.4↑ | 3.0↑ | ND | ND | ↔ |
| EC lineage | Dermal | Renal | Cerebral | Hepatic | Pulmonary |
| Cell line designation | MVEC-2 | HMVEC-G | HMVEC-B | HMVEC-H | HMVEC-LU |
| Baseline Fas mRNA, fg/μg RNA* | 17.7 | 3.9 | 18.8 | 37.7 | 12.1 |
| 1% TTP plasma, without clinical renal dysfunction, fold ΔFas mRNA | 1.2↑ | 1.5↑ | 1.1↑ | 0.8↓ | 0.8↓ |
| 1% TTP plasma, clinical renal disease, fold ΔFas mRNA | ↔ | 2.9↑ | ND | ND | ND |
| 1% adult/sporadic HUS plasma, fold ΔFas mRNA | 1.4↑ | 3.0↑ | ND | ND | ↔ |
Concentration of Fas-specific mRNAs (in femtograms per microgram of RNA) in microvascular EC exposed to a 1:100 dilution of HIV-seronegative normal plasma for 16 hours was assessed by a competitive RT-PCR reaction we had developed. Fold changes in the presence of various experimental plasmas, also used at 1:100 dilutions, were similarly assessed.
Abbreviation: ND, not done.
Mean of two to four determinations.
Inhibition of TTP/HUS Plasma-Associated EC Apoptosis and Fas Expression
Induction of Fas transcripts by components of TTP and HUS plasmas could simply reflect an activated cell phenotype and be irrelevant or only peripherally related to the apoptosis observed. With this concern, we had initially reported11 that soluble anti-Fas MoAb M3, but not MoAb M31, partially suppressed TTP plasma-mediated EC apoptosis. We have confirmed this using two additional microvascular EC lines, renal and cerebral. Inhibition either of A0 values or the percentage of apoptotic cells by DAPI staining remained incomplete (<50%; data not shown). In contrast, aurintricarboxylic acid, a general inhibitor of apoptosis, completely blocked EC apoptosis related to TTP or sporadic HUS plasmas in all EC tested (data not shown), similar to our earlier report with dermal EC.11
Effect of TTP Plasma on PGI2 Production by Microvascular EC of Dermal and Pulmonary Lineage
| Incubation . | Normal Plasma . | TTP-2 Plasma . | TTP-3 Plasma . | |||
|---|---|---|---|---|---|---|
| . | Dermal . | Pulmonary . | Dermal . | Pulmonary . | Dermal . | Pulmonary . |
| Baseline (2 h) | 535 | 448 | 562 | 448 | 651 | 345 |
| 16 h | 2,417 (4.5) | 906 (2.0) | 1,797 (3.2) | 762 (1.7) | 1,697 (2.6) | 695 (2.0) |
| 16 h, stimulated | 1,743 (3.3) | 10,756 (24.0) | 1,904 (3.4) | 8,197 (18.3) | 1,718 (2.6) | 5,509 (16.0) |
| Incubation . | Normal Plasma . | TTP-2 Plasma . | TTP-3 Plasma . | |||
|---|---|---|---|---|---|---|
| . | Dermal . | Pulmonary . | Dermal . | Pulmonary . | Dermal . | Pulmonary . |
| Baseline (2 h) | 535 | 448 | 562 | 448 | 651 | 345 |
| 16 h | 2,417 (4.5) | 906 (2.0) | 1,797 (3.2) | 762 (1.7) | 1,697 (2.6) | 695 (2.0) |
| 16 h, stimulated | 1,743 (3.3) | 10,756 (24.0) | 1,904 (3.4) | 8,197 (18.3) | 1,718 (2.6) | 5,509 (16.0) |
Dermal (MVEC-2) or pulmonary (HMVEC-LU) microvascular EC were plated as 1 × 105 cells in 0.5 mL apoptosis medium and exposed to a 1:100 dilution of plasma from an HIV-seronegative normal donor or HIV-seronegative patients with active TTP (TTP-2 and -3). One-hundred–microliter aliquots of culture supernatant were taken at 2 and 16 hours of culture. The remaining culture medium was then discarded, 0.5 mL fresh apoptosis medium plus sodium arachidonate was added, and 100-μL samples were taken after 15 minutes of incubation at 37°C. PGI2 levels, in picograms per milliliter, were measured as described in the text. The fold increase is in parentheses.
Cdc2 Activation
No change in relative levels of Cdc2, detected by immunoblotting, in cytoplasmic versus nuclear fractions was induced in hydroxyurea-treated UVEC by normal or TTP plasmas, with a nuclear:cytoplasmic ratio of 1:1. Exposure of dermal MVEC-1 to normal plasma under identical conditions similarly resulted in a nuclear:cytoplasmic Cdc2 kinase ratio near unity (1:1.05). In marked contrast, exposure of dermal MVEC-1 to a 1% dilution of TTP plasma led to a 50% increase in levels of this kinase in nuclear fractions (ratio, 1.5:1), in association with an A0 peak of 30%. Hydroxyurea treatment itself had no ability to induce apoptosis in these cells and did not significantly alter absolute A0 levels.
Procoagulant Features of EC
Three phenomena were examined: PGI2 production, annexin II expression, and tissue factor production. PGI2 secretion after 2 hours of exposure of dermal or pulmonary microvascular EC to TTP or normal plasmas was similar, in the range of 350 to 650 pg/mL (Table 3). In pulmonary EC, these levels increased approximately twofold at 16 hours and 16- to 24-fold upon sodium arachidonate stimulation at 16 hours, in the presence of normal or TTP plasmas (Table 3). In contrast, PGI2 increased 4.5-fold in unstimulated dermal EC in the presence of normal plasmas at 16 hours, but only 2.6-fold in the presence of TTP plasma. After sodium arachidonate stimulation, PGI2 production remained low in the dermal EC, with no additional elevation over baseline, in the presence of normal or TTP plasmas (Table 3). These studies were repeated with four different TTP plasmas. The distinction between dermal and pulmonary microvascular EC in terms of PGI2 production stimulated by sodium arachidonate was seen in all experiments.
Levels of tissue factor were unchanged in the culture supernatants of any EC exposed to 1% to 5% TTP or HUS plasma.
Annexin I was used as a control for changes in annexin II plasminogen/tPA receptor expression in dermal and pulmonary microvascular EC. Annexin I was not detected in baseline EC of either lineage and was not induced after exposure to TTP plasmas. However, annexin II was detectable by flow cytometry on dermal MVEC-1 and pulmonary microvascular cells. Levels of expression remained constant on pulmonary EC exposed to normal or TTP plasmas for 16 to 36 hours, in the range of 2% to 8%. This experiment was repeated four times. Annexin II expression by dermal EC was more variable. It was downregulated by TTP plasmas in three of four experiments with, eg, 7.0% expression in the presence of normal plasma but 2.3% ± 0.4% expression after exposure to three different TTP plasmas. However, there was no correlation between the degree of apoptosis induced by a particular TTP plasma and the level of change in annexin II expression.
DISCUSSION
EC injury is a fundamental event in TTP and sporadic HUS.1,7 Data presented here advance our original postulate that microvascular EC apoptosis is of pathophysiologic importance in TTP. They extend this mechanism to encompass a related syndrome, adult/sporadic HUS, and differentiate this disorder from childhood/diarrhea-associated HUS. They are consistent with an ultrastructural study documenting apoptotic bodies, ostensibly derived from EC, within the glomerular capillary lumina of an adult with HUS responsive to plasma exchange.10 These experiments also offer a possible explanation for the striking difference in the clinical pathology of TTP/sporadic HUS versus endemic/diarrhea and chemotherapy-associated forms of HUS. These differences are seen even though some toxins involved in diarrhea-associated HUS, such as verocytotoxin-1, may cause microvascular EC apoptosis in vitro.28
Significant overlap between TTP and sporadic HUS, in terms of distribution of microthrombi and clinical manifestations, had been recognized since the first descriptions of these diseases. However, tissue-specific pathologic restrictions do exist. Dermal microvessels are usually involved in all cases of TTP1 and in sporadic HUS.3 In one review,1 some 70% of 271 cases of TTP had significant renal involvement characteristic of all types of HUS. Conversely, apart from the kidneys, the microvasculature of numerous organs is affected in sporadic HUS, including cerebral microthrombi in 50% of autopsied patients1; cardiomyopathy2; infarction and perforation of the bowel secondary to intestinal microvascular disease2; ocular microvascular changes, unrelated to hypertension and occasionally leading to blindness29; and microthromboses in the pancreas and adrenal glands.30
In marked contrast, two organs are absolutely or relatively spared in TTP/sporadic HUS, the lung and liver, respectively.25,31 This is not due to some intrinsic resistance of pulmonary and hepatic microvasculature to apoptosis, which can be induced in these cells by other mechanisms,32 and neither are these tissues spared in other types of HUS. For example, the pulmonary microvasculature is the major site of thrombosis in both childhood/diarrhea-associated and chemotherapy-linked HUS.26 But these latter syndromes lack the pathologic changes of EC activation, proliferation, and detachment in the absence of an inflammatory response that are classic for TTP/sporadic HUS. They are also poorly responsive, if at all, to therapeutic maneuvers with plasma that are usually effective in TTP/sporadic HUS.3,4,27 Indeed, the presence of both necrosis and inflammation in many types of HUS other than the sporadic cases26 is reminiscent of injury by EC toxins. This is consistent with the necrotic cell death we observed after incubation of large vessel and microvascular EC with childhood HUS plasma, a phenomenon reported by others using large vessel EC.7 Such necrosis did not mask an accelerated apoptosis in these cells, because examination of childhood HUS plasma-treated cultures at earlier time points after plasma exposure did not show apoptosis above baseline values (data not shown).
Our findings of a lower level of PGI2 production in TTP/sporadic HUS plasma-mediated apoptotic EC and very low levels of induced PGI2 in cells susceptible to apoptosis, parallel certain in vivo findings in these syndromes. EC synthesize many substances involved in coagulation and fibrinolysis, including vWF, tissue factor, thrombomodulin, tPA, plasminogen activator inhibitor, PGI2 , and nitric oxide. Alterations in their levels have been reported in TTP/HUS and may correlate with disease.1,33 TTP plasma also suppresses the synthesis of PGI2 in a normal vessel wall.1 Failure to increase production of PGI2 by activated EC susceptible to apoptosis may be of pathophysiologic importance, because PGI2 normally suppresses platelet aggregation induced by cytokines, shear stress, etc.
The consequence of alteration of annexin II binding sites on EC in vivo is less clear. Although changes were seen with dermal and not pulmonary EC, our results did not correlate with the degree of apoptosis. EC bind components of the fibrinolytic system, including plasminogen and tPA, via annexin II.24 By translocating annexin II to its external surface, the EC may actively modulate plasmin generation, blocking formation of a fibrin clot.34 Its relative loss could encourage clot formation, although fibrin is only a small component of the TTP clot.
Further pursuit of possible procoagulant features in our model for EC apoptosis is of interest in light of a report that apoptotic UVEC shed procoagulant membrane blebs.35 Such large vessel EC are not involved in TTP/HUS, however, rendering UVEC a poor model for these syndromes. Large vessel EC are known to respond differently from microvascular EC to a variety of injuries.36 Demonstration of changes in microvascular EC associated with procoagulant activity after induction of apoptosis may be important in defining interactions among platelets, EC, and fibrin in the ontogeny of TTP/HUS microthrombi.
Finally, the ability of TTP/sporadic HUS plasmas to induce Fas in vitro among those lineages of microvascular EC that parallel the in vivo organ distribution of platelet thrombi is of some interest. It is consistent with the appearance of Fas on other cell types that undergo activation-related apoptosis.15,16,18 Evidence for microvascular EC activation in vivo, including increased vacuolization and aggregation of rounded cells,1 is characteristic of the early stages of Fas-linked apoptosis. EC mitochondrial swelling is a prominent feature of TTP1 and is also a dominant feature of apoptotic cells in vitro.37 This led us to hypothesize that Fas cross-linking, perhaps via soluble Fas ligand in TTP plasmas, was etiologically involved.11 This postulate is consistent with the lineage-restricted pattern of Fas induction in EC (Fig 5) and with the TTP-plasma induced nuclear translocation of Cdc2.
However, there are problems with this hypothesis. We could only partially inhibit EC apoptosis by soluble anti-Fas MoAb11 (data not shown). The lack of TTP plasma-induced apoptosis in some microvascular EC that had increased Fas upon exposure to diarrhea-associated HUS plasma and in those UV and coronary artery EC that did express baseline Fas11 is also unexplained. This could relate to a requirement for certain threshold levels of Fas expression, consistent with the differential susceptibility of normal versus HIV-infected CD4+ T cells, both of which express Fas and FasL, but at different levels, to Fas cross-linking.17 Also, high levels of baseline Fas expression paradoxically suppress Fas-mediated apoptosis in some systems, ostensibly via a receptor/messenger imbalance counteracted by protein synthesis inhibitors.15 There are also qualitative differences in how different cells perceive signals through Fas, regardless of the degree of Fas expression.38 In any event, we are also pursuing other molecules, related to the Fas/TNF receptor family, that exist in soluble form for release into plasma and can be expressed in microvascular EC.39
We persist in concentrating on this pathway, given our results showing promotion of cytoplasmic to nuclear translocation of Cdc2 on exposure of microvascular EC to TTP plasmas. Cdc2 and Cdk2, the two catalytic subunits associated with cyclin A, are activated after exposure to Fas-like apoptosis-inducing agents tested in two large surveys.22,40 Bcl-2 can block Cdc2-associated apoptosis by preventing transport of this kinase into the nucleus,40 but EC appear to lack this control pathway.41 Signaling interactions between Fas and a Fas-related molecule, partially blocked by anti-Fas antibody, are conceivable, given the ability of two distinct members of the TNF family, p55 TNF-R and Fas, to cross-talk via a single cytoplasmic receptor.42
The agents responsible for initiating TTP and sporadic HUS remain unknown. Both disorders have been linked to HIV-1 infection, with a recent report of a striking excess of TTP/adult HUS cases in the setting of HIV disease: 1.4% of a cohort of 1,070 consecutively diagnosed acquired immunodeficiency syndrome cases43 versus 0.1 per 100,000 in the general population. (However, this difference was much greater than reported from previous retrospective surveys.44 ) Several cell lineages undergo accelerated apoptosis in vivo in HIV disease, including lymph node CD4+ and CD8+ T lymphocytes and B cells,45 epithelial cells,46 renal tubular cells,47 and neurons and endothelial cells in the central nervous system.48 Microvascular EC may be yet another cell lineage susceptible to apoptotic signals induced by infectious agents such as HIV. But, even in the absence of a known etiology, manipulation of apoptosis may be a new modality for the treatment of TTP/sporadic HUS. This hope is predicated on the fact that a family of proteases form a common core pathway for apoptotic death of many divergent cells.49 The ability to suppress the apoptotic process in vitro with ATA, a cysteine protease inhibitor that, given its ability to block platelet-EC interactions,50 51 has been used in the experimental therapeutics of TTP, suggests that inhibitors of EC apoptosis should be considered in the design of new treatments for thrombotic microangiopathies.
ACKNOWLEDGMENT
We thank C. MacLow and G. Lam for technical support and Dr J. Moake (Baylor College of Medicine, Houston, TX) and Drs B. Gordon and J. Bussell (Cornell University Medical College) for providing plasma samples. Dr D. Lynch (Immunex, Seattle, WA) provided the anti-Fas MoAbs. We also thank Dr A. Asch (Cornell) for helpful discussions.
Supported in part by National Institutes of Health Grants No. HL55646, AI41327, and DE11348 to J.L.; HL42493 and HL46403 to K.A.H.; and HL18828.
Address reprint requests to Jeffrey Laurence, MD, Cornell University Medical College, 411 E 69th St, New York, NY 10021.

![Fig. 1. DNA histograms of microvascular endothelial cells (MVEC) exposed to normal and TTP plasmas. Primary human microvascular EC from various tissues were cultured for 18 hours in human serum-free apoptosis culture medium in the presence of a 1:100 dilution of plasma from control donors and a 1:100 or 1:1,000 dilution of plasma from TTP patients. DNA histograms of ethanol-fixed, RNase-treated and propidium iodide-stained cells were obtained and analyzed by computer software, as described in the text. A0 peak values are indicated within each histogram, representing the more heavily shaded region just to the left of the large G1/S peak (see [B]). (A) Dermal, renal, and cerebral MVEC + normal plasma. (B) Dermal, renal, and cerebral MVEC + TTP plasmas.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1224/4/m_bl_0024f1a.jpeg?Expires=1769141486&Signature=W2VNYXElSjC-C7emcBl38tucxgCzt3wc8UHSrdgIZAfaBAe8sCi-AhDn0SJPLyytgafPo7nxp-o433pm0sF~BtBOzXCV8Nmay4lRF~pTHj9EtRaDcy5M~8THKz9EflpddeP0yMI-EB6uDG9GNpWOZdJk7SgsCqj07kWz1awqeYNYg~RzrQWIJ19txQ~Vljo~tSh5hDgAMg5U03zhHOTbT-Ao2RM-2Lpek1us1ZTq-~sqzlhtynm2KXcVyGLtqP5XXWMbodhCjYhmvTJK-WcnvsJkfXm~K6wKkZnxvhXdSU6vUV9Mti0yDD1Jk64Rnl~WZGQvJiOKbchk2x-FfiUsRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. DNA histograms of microvascular endothelial cells (MVEC) exposed to normal and TTP plasmas. Primary human microvascular EC from various tissues were cultured for 18 hours in human serum-free apoptosis culture medium in the presence of a 1:100 dilution of plasma from control donors and a 1:100 or 1:1,000 dilution of plasma from TTP patients. DNA histograms of ethanol-fixed, RNase-treated and propidium iodide-stained cells were obtained and analyzed by computer software, as described in the text. A0 peak values are indicated within each histogram, representing the more heavily shaded region just to the left of the large G1/S peak (see [B]). (A) Dermal, renal, and cerebral MVEC + normal plasma. (B) Dermal, renal, and cerebral MVEC + TTP plasmas.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1224/4/m_bl_0024f1b.jpeg?Expires=1769141486&Signature=t6nO5WyHroNrdljID9kdkdSA6kngJqfP~Fcaio8wJbMv1-OYyqD3CTmaX-Gfa-kyPchcAcCMNXi65AJ0GUeBfWvDVFIwTGzn-w9DXZeUUm8OGEB7NZzD7SSgbE86wynpDSLSnYyFMrvRmfjnYp8MbW2FN1vCnXuS4wkblYkaFmZlkZmOAg-uTDAasEX-qxfAjsvEptIWYq48iDGb8ohiVtl~uv7Cuy3Pws61UEa7GJzE40bU~tZWV0VdaaLVX7UUvs~Z5DAiC0BptGZd4plA~RcOcu2YpwRYGUq0wU8lwIebyBQhwQ9iDTRJdS~hJMPajRM9vWLmAJOdQXSJytsf5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal