Abstract
We recently described a Quebec family with an autosomal dominant bleeding disorder characterized by mildly reduced-low normal platelet counts, an epinephrine aggregation defect, multimerin deficiency, and proteolytic degradation of several, soluble α-granular proteins. Similar clinical features led us to investigate a second family with an unexplained, autosomal dominant bleeding disorder. The affected individuals had reduced to normal platelet counts, absent platelet aggregation with epinephrine, and multimerin deficiency. Their platelet α-granular proteins factor V, thrombospondin, von Willebrand factor, fibrinogen, fibronectin, osteonectin, and P-selectin were proteolyzed and comigrated with the degradation products found in patients from the other family. However, their platelet albumin, IgG, external membrane glycoproteins, CD63 (a lysosomal and dense granular protein), calpain, and plasma von Willebrand factor were normal, indicating restriction in the proteins proteolyzed. Electron microscopy studies indicated preserved α-granular ultrastructure, despite degradation of soluble and membrane α-granular proteins. Immunoelectron microscopy studies of the patients' platelets indicated that fibrinogen, von Willebrand factor, P-selectin, multimerin, and factor V were within α-granules, with normal to reduced labeling for these proteins. Pathologic proteolysis of α-granular contents, rather than a defect in targeting proteins to α-granules, may be the cause of the protein degradation in the Quebec platelet disorder.
CONGENITAL PLATELET disorders are rare but important causes of abnormal bleeding.1,2 Some inherited platelet disorders are associated with specific defects of platelet function and normal platelet numbers.1,2 Other platelet disorders, including gray platelet syndrome and Bernard-Soulier syndrome, are associated with both qualitative and quantitative platelet abnormalities.1-11 Many of the characterized inherited platelet disorders are due to genetic mutations that result in a deficiency or functional abnormality in a single glycoprotein (GP) or GP receptor complex.1,2 However, rare disorders, associated with abnormalities in multiple platelet GPs, have also been described.3-9,12 13
We recently reported the clinical and laboratory features of a novel, autosomal dominant bleeding disorder in a Quebec family that was associated with reduced to normal platelet counts, defective epinephrine aggregation, and multiple GP abnormalities.12 This disorder had been previously designated as factor V Quebec, because of their abnormal platelet factor V.14 We found that these patients were deficient in multimerin, a soluble, multimeric factor V binding protein found in platelet α-granules and in endothelium.12,15-21 However, we also found proteolytic degradation of their platelet α-granular proteins factor V, von Willebrand factor, fibrinogen, and thrombospondin. Similar findings, including degradation of platelet osteonectin, were reported by Janeway et al.13 The normal platelet GP Ib-IX,12 αIIbβ3 ,12 and PF413 in the Quebec patients indicated that some, but not all, of their platelet proteins were altered. The degraded α-granular proteins suggested a specific biochemical defect, because these abnormalities were not found in unrelated patients with disorders of platelet production and destruction.12 Curiously, although multiple platelet abnormalities were identified, their bleeding episodes have been unresponsive to platelet transfusions.12
The findings of unexplained, moderate-severe bleeding unresponsive to platelet transfusions led us investigate a second family with an autosomal dominant bleeding disorder. We report similar clinical and laboratory abnormalities and provide evidence that (1) there is restriction in the platelet proteins degraded and (2) both membrane and soluble α-granular proteins are proteolyzed in the Quebec platelet disorder. We also report electron microscopy findings that indicate that the pathologic proteolysis of platelet α-granular constituents in this disorder is not secondary to a defect in targeting proteins to α-granules.
MATERIALS AND METHODS
All studies were performed with institutional ethics approval and patient consent.
Platelet Aggregation Studies
Aggregation studies were performed in two of the affected individuals, using 6 and 40 μmol/L (final) epinephrine, 5 to 10 μmol/L adenine diphosphate (ADP), 1 to 2 μg/mL collagen, and 0.5 and 1.25 mg/mL ristocetin. Platelet-rich plasma (PRP) samples with platelet counts less than 250 × 109/L were compared with controls diluted to the same platelet count.12
Antibodies and GP Quantitations
Antibodies used for immunoblot, immunoprecipitation, and immunoelectron microscopy studies included monoclonal (JS-1) and polyclonal antisera against multimerin,15 monoclonal antithrombospondin (CH-1),15 monoclonal anti-GP αIIbβ3 (Raj-1),22 monoclonal anti-GP IX (Beb-1),22 polyclonal antihuman factor V (Alexis Biochemicals, San Diego, CA), polyclonal antihuman von Willebrand factor (Dako, Carpinteria, CA; electron microscopy [EM] studies from Dakopatts [Glostrup, Denmark]), polyclonal antihuman fibrinogen (Behring Diagnostics Inc, Westwood, MA; EM studies from Cappel Laboratories [Downington, PA]), polyclonal antihuman fibronectin (Organon Teknika Inc, Scarborough, Ontario, Canada), polyclonal anti–P-selectin (Cedarlane Laboratories, Hornsby, Ontario, Canada; EM studies were a gift from Dr Michael Berndt [Victoria, Australia]),23 monoclonal antihuman osteonectin (Haematologic Technologies, Essex, VT), monoclonal anti-CD63 (D545; gift from Dr Sara Israels [Winnipeg, Manitoba, Canada]),24 polyclonal antihuman albumin (Sigma, St Louis, MO), polyclonal antihuman IgG (heavy and light chains; Biocan Scientific Inc, Mississauga, Ontario, Canada), monoclonal anticalpain (gift from Dr John Elce, Kingston, Ontario, Canada),25 and goat antirabbit IgG coupled to 10 nmol/L colloidal gold (GARG10; Amersham, Les Ullys, France).
Platelet multimerin, factor V, thrombospondin, and fibrinogen were quantitated using enzyme-linked immunosorbent assays (ELISA) and pooled platelet lysate or purified plasma factor V as standards.12,26 Antibodies used for the factor V ELISA12,26 included commercial horse (Haematologic Technologies) and sheep (Affinity Biologicals, Hamilton, Ontario, Canada) polyclonal antihuman factor V,12 monoclonal antihuman factor V (HV-1 and 6A5),26 and affinity purified, rabbit antihuman factor V.27 Platelet β-thromboglobulin and von Willebrand factor were quantitated using a commercial radioimmunoassay (Amerlex, Johnson and Johnson Clinical Diagnostics, Rochester, NY) and ELISA (Diagnostica Stago, Asniéres, France).12
Preparation of Platelet Lysates
Platelets were collected in acid citrate dextrose (1:6 vol/vol) with protease inhibitors (2 μmol/L prostaglandin E1 , 50 μg/mL leupeptin, 1 mg/mL iodoacetamide, 0.25 mmol/L phenylmethyl sulfonyl fluoride [PMSF], and 1 μg/mL aprotonin). Washed platelet lysates (WPL) and solubilized PRP pellets (PRPL) were prepared as previously described.12
Studies of Biotin-Labeled Platelet Proteins
Platelets were washed twice using calcium-albumin free Tyrode's buffer (pH 6.2) or phosphate-buffered saline (PBS)-EDTA-theophylline (PBS, pH 6.7, containing 5 mmol/L EDTA and 1 mmol/L theophylline), resuspended in lysing buffer with inhibitors (20 mmol/L Tris, 120 mmol/L NaCl, pH 7.4, containing 5 mmol/L EDTA, 50 μg/mL leupeptin, 0.25 mmol/L PMSF, and 5 mmol/L N-ethylmaleimide), cooled on ice for 30 minutes, and then labeled with biotin.12,28 Aliquots of biotin-labeled resting platelets (0.2 × 109 platelets) were washed twice in quench buffer (50 mmol/L Tris-HCl, 10 mmol/L EDTA, 150 mmol/L NaCl, 20 mmol/L lysine, and 20 mmol/L glycine, pH 8.0), resuspended in lysis buffer containing protease inhibitors, and solubilized (1 × 109/mL) with 1% Triton X-100 or 1% Triton X-114.12,15,28 Triton X-114 aqueous (hydrophilic) and detergent (hydrophobic) fractions were prepared as described.15 Additional aliquots of biotin-labeled platelets were resuspended in lysing buffer (containing protease inhibitors) without detergent and separated into membrane and soluble protein fractions using three freeze-thaw cycles followed by ultracentrifugation (100,000g for 1 hour). The membrane pellet was washed twice in 2 mL of lysing buffer without detergent and then solubilized in buffer containing 1% Triton X-100. Biotin-labeled releasate from thrombin-stimulated platelets was prepared as described.12
Immunoblotting and Immunoprecipitation Analyses
Platelet lysates (5 to 50 μL of 1 × 109 platelets/mL) and biotin-labeled platelet proteins (10 μL) were separated using nonreduced and reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 4% to 8% gradient gels above a 15% gel, 8% to 15% gradient gels [osteonectin], or 5% to 15% gradient gels [CD63]) and nonreduced multimer gels16 and transblotted onto nitrocellulose membranes. Samples were analyzed using peroxidase-conjugated streptavidin (biotin-labeled samples)28 or by immunoblotting using peroxidase-conjugated secondary antibodies and chemiluminescent substrate.12 For analyses of platelet albumin, 1.5% gelatin was used as the blocking agent.
Electron Microscopy
Whole blood was collected into syringes containing an equal volume of 1% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.2, and mixed by gentle inversion. After 10 minutes (room temperature), the PRP was separated by centrifugation (300g for 5 minutes), and the platelets were harvested (820g for 10 minutes). The platelet pellet was fixed with 1% glutaraldehyde (in 0.1 mol/L phosphate buffer at 4°C for 1 hour), washed three times with 0.1 mol/L phosphate buffer, and transported in phosphate buffer containing 12.3% sucrose. Cells were embedded in glycol methacrylate. Thin sections were immunolabeled as previously described and examined under a Philips CM10 electron microscope.7 Fifty platelet sections, containing 5 to 10 α-granules per section, were examined for each condition and the number of gold particles per α-granule counted. Patients were compared with controls, which were processed in parallel.
RESULTS
Family History
The pedigree of the family is shown in Fig 1. The family is French-Canadian and the patients reside in the province of Quebec. No relations common to the family with the Quebec platelet disorder12 were identified despite extensive inquiries.
Family pedigree. Male (□) and female (○) family members with (▪, •) and without (□, ○) bleeding symptoms and the individuals studied () are shown. Six individuals who died at an early age of unknown causes are indicated (⋄).
Family pedigree. Male (□) and female (○) family members with (▪, •) and without (□, ○) bleeding symptoms and the individuals studied () are shown. Six individuals who died at an early age of unknown causes are indicated (⋄).
The index case, III-1, experienced frequent episodes of epistaxis in childhood. At 9 years of age, she broke an upper incisor tooth and bled for 1 month. The same year, she fell against the bar of her bicycle and developed an extensive hematoma of the vulva, complicated by a decrease in her hemoglobin level. Coagulation investigations indicated mild thrombocytopenia (80 to 120 × 109/L) and absent epinephrine aggregation (6 μmol/L); normal laboratory investigations included international normalized ratio (INR), activated partial thromboplastin time (aPTT), plasma factor V, factor VIII, fibrinogen, von Willebrand factor (antigen, ristocetin cofactor activity, multimers), and platelet morphology (light microscopy). Her bleeding times ranged from 3 to 20 minutes. An inherited platelet disorder was suspected, because similar laboratory abnormalities were found in her mother, II-4. The bleeding episode was treated with 2 U of whole blood and 78 U of platelets were transfused, with no apparent clinical response. At 15 years of age, she was placed on oral contraceptives for heavy menstrual periods, with good results. At 22 years of age, a spontaneous hemarthrosis of the right knee developed and resolved after 1 month. The same year, she underwent a therapeutic abortion at 2 months of pregnancy. Significant bleeding occurred, resulting in two dilatation and curettage procedures. Platelet transfusions were also administered, without evidence of clinical response, and bleeding continued for 1 month. Tranexamic acid (10-day course) was administered for subsequent surgical procedures (second therapeutic abortion and cosmetic nasal surgery). No bleeding problems were encountered with these procedures. Her platelet counts have been consistently abnormal, ranging from 80 to 120 × 109/L.
Her mother, II-4, also suffered from frequent episodes of epistaxis in childhood and bled for 15 days after dental extractions. She had four uncomplicated vaginal deliveries, without excessive bleeding. Excessive bleeding (duration, 3 to 4 weeks) occurred after two spontaneous abortions (at 2 months of pregnancy). After a laparoscopic tubal ligation, a large ecchymosis developed, without evidence of internal bleeding. She has had problems with frequent ecchymoses and gum bleeding when brushing her teeth. Coagulation investigations indicated mild thrombocytopenia (97 to 150 × 109/L); absent epinephrine aggregation (6 μmol/L); normal INR and aPTT, normal levels of plasma factor V, factor VIII, fibrinogen, and von Willebrand factor (antigen, ristocetin cofactor activity, and multimers); and normal platelet morphology (light microscopy). Her bleeding times were normal (3 minutes).
Other affected family members have had similar symptoms of mucocutaneous bleeding; delayed-onset (12 to 24 hours) bleeding after dental extractions, surgery, or trauma and, less commonly, hemarthroses.
Laboratory Investigations
Platelet counts.At the time of these investigations, the platelet counts of affected family members ranged from reduced to normal (3 patients studied; range, 80 to 156 × 109/L; normal mean platelet volumes). The platelet counts of the family members without bleeding symptoms were normal (2 studied; range, 242 to 298 × 109/L).
Platelet aggregation studies.Aggregation studies were performed on numerous occasions on patients II-4 and III-1. Normal aggregation was observed with ristocetin (patients, 88% aggregation with 1.25 mg/mL). Patients II-4 and III-1 had absent aggregation with both 6 and 40 μmol/L epinephrine. They also had consistently reduced aggregation (45% aggregation with 20% to 42% deaggregation) with 10 μmol/L ADP, compared with controls (82% to 83% aggregation with 0% to 5% deaggregation; PRP platelet counts, 116 to 250 × 109/L). The patients' platelet aggregation response to 1 to 2 μg/mL collagen was variable and ranged from 0% to 63% aggregation, compared with 88% aggregation seen in controls tested at the same platelet count (150 × 109/L).
GP analyses.Studies of the patients' biotin-labeled platelet proteins indicated abnormalities common to patients with the Quebec platelet disorder.12 Labeled α-granular proteins were detected in samples of patient and control surface-labeled, resting platelets that were washed in Tyrode's buffer (data not shown). Further studies were performed using platelets washed with EDTA-PBS-theophylline to minimize granule secretion and labeling of internal proteins (Fig 2). In these preparations, the patients' nonreduced/reduced platelet surface GP profile more closely resembled the control samples, with only minor differences evident (Fig 2, total lysates), and these findings were confirmed in studies of two members of the other family (family 1 patients F1 V-22 and F1 V-23; not shown). Similar to the patients from the other family,12 immunoprecipitates of the patients' membrane proteins GPs Ib-IX and αIIbβ3 were normal (not shown).
Studies of surface-labeled platelets and platelet releasate. Platelets were washed (PBS-EDTA-theophylline) and surface-labeled with biotin. In parallel experiments, the releasate from thrombin-activated platelets was labeled with biotin. Aliquots (10 μL) of the labeled releasates, solubilized surface-labeled platelets (total lysate), and the corresponding membrane fractions (solubilized, washed pellets from freeze-thaw lysates) of surface-labeled platelets were analyzed using nonreduced/reduced SDS-PAGE. Biotinylated proteins were detected using streptavidin-peroxidase and chemiluminescent substrate. Platelet GPs Ib, αIIbβ3 , α2β1 , and thrombospondin (normal thrombospondin []; degraded thrombospondin [➭]) are indicated. Samples from 2 patients and a control (C), processed in parallel, are shown.
Studies of surface-labeled platelets and platelet releasate. Platelets were washed (PBS-EDTA-theophylline) and surface-labeled with biotin. In parallel experiments, the releasate from thrombin-activated platelets was labeled with biotin. Aliquots (10 μL) of the labeled releasates, solubilized surface-labeled platelets (total lysate), and the corresponding membrane fractions (solubilized, washed pellets from freeze-thaw lysates) of surface-labeled platelets were analyzed using nonreduced/reduced SDS-PAGE. Biotinylated proteins were detected using streptavidin-peroxidase and chemiluminescent substrate. Platelet GPs Ib, αIIbβ3 , α2β1 , and thrombospondin (normal thrombospondin []; degraded thrombospondin [➭]) are indicated. Samples from 2 patients and a control (C), processed in parallel, are shown.
Additional aliquots of surface-labeled platelets were fractionated using ultracentrifugation (freeze-thaw lysates) and phase-partitioning (Triton X-114) lysates; the purpose of the fractionation studies was to distinguish labeled integral membrane from soluble proteins and hydrophobic from hydrophilic proteins. No abnormal bands were identified in nonreduced/reduced analyses of surface-labeled proteins in the patients' solubilized platelet membranes (Fig 2, membrane fraction; freeze-thaw lysates). Similarly, no abnormal, labeled proteins were found in the hydrophobic fraction (Triton X-114 lysates) of surface-labeled platelets (not shown). The minor differences identified in the patients' surface labeled platelet proteins compared with controls (Fig 2, total lysates; Triton X-100 solubilized platelets) corresponded with proteins that partitioned into the soluble (freeze-thaw lysates) and hydrophilic (Triton X-114 lysates) protein fractions (not shown). In contrast to their normal, platelet external membrane proteins, multiple abnormalities (including secreted degraded thrombospondin) were evident in the proteins released from the patients' thrombin-activated platelets (Fig 2, releasates). These findings suggested that the proteolysis might be restricted to internal stores of releasable proteins.
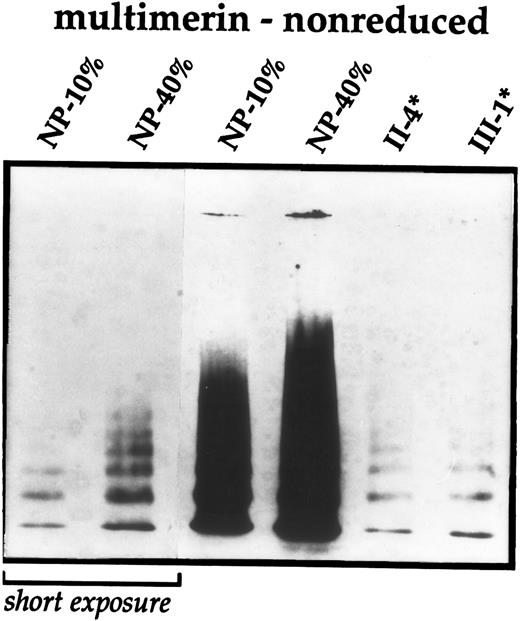
Further studies were performed to evaluate the patients' α-granular proteins. Similar to the Quebec platelet disorder,12 the patients (n = 3 studied) were deficient in their platelet stores of multimerin (range, <5% to 9% of the normal pool; normal range, 45% to 214%12). The multimerin levels in the family members without bleeding symptoms were normal (n = 2 evaluated; levels, 52% to 63% of the normal pool). Multimerin subunits and multimers, analyzed using both monoclonal and polyclonal antimultimerin, were of normal mobility in the patients and controls and no degradation products were detected in samples analyzed by nonreduced SDS-PAGE (not shown) or nonreduced multimer gels (Fig 3). The multimer pattern in the patients' samples resembled diluted samples of controls or normal pooled lysate, suggesting a quantitative deficiency.
Immunoblot analyses of platelet multimerin. Washed platelet lysates from several affected family members (*) were compared with dilutions of normal pooled platelet lysate (NP) and analyzed using nonreduced multimer gels and immunoblotting with monoclonal antimultimerin (JS-1). A short exposure of the multimers in the normal pooled lysate is shown for comparison. Similar multimerin abnormalities were found in patient II-13.
Immunoblot analyses of platelet multimerin. Washed platelet lysates from several affected family members (*) were compared with dilutions of normal pooled platelet lysate (NP) and analyzed using nonreduced multimer gels and immunoblotting with monoclonal antimultimerin (JS-1). A short exposure of the multimers in the normal pooled lysate is shown for comparison. Similar multimerin abnormalities were found in patient II-13.
Normal quantities of platelet fibrinogen (67% to 69% of the normal pool; normal range, 54% to 158%12), von Willebrand factor (60% to 85% of the normal pool; normal range, 40% to 148%12), thrombospondin (72% to 138% of the normal pool; normal range, 48% to 188%12), and β-thromboglobulin antigen (67% to 90% of the normal pool; range for 9 controls, 63% to 97% of the normal pool) were detected in the washed platelet lysates from patients II-4 and III-1.
Platelet factor V antigen levels were assessed by three different ELISA assays, using either horse and sheep polyclonal antibodies,12 rabbit polyclonal antibodies,26,27 or murine monoclonal antibodies (HV-1 and 6A5)26 directed against two epitopes in the human factor V C2 domain. The ELISA using two different polyclonal antisera (horse and sheep) for capture and detection12 indicated reduced levels of platelet factor V in patients II-4 and III-1 (20% of normal pool; normal range, 48% to 148%12). In contrast, higher levels of factor V antigen were detected using affinity-purified, rabbit antihuman factor V26,27 (52% to 58% of normal pool; normal pool, 6.5 μg/109 platelets). Normal levels of platelet factor V antigen were detected in patients II-4 and III-1 when the monoclonal antibodies were used for capture and detection of factor V antigen (178% to 193% of normal pool; normal pool, 13.6 μg/109 platelets). The amount of platelet factor V detected by these assays was noted to be significantly decreased by repeated cycles of freezing and thawing.
Immunoblot analyses were performed on five family members to determine if the patients' internal platelet proteins were proteolyzed. The patients' α-granular proteins factor V, thrombospondin, von Willebrand factor, fibrinogen (not shown), and osteonectin (Fig 4) were degraded and comigrated with the abnormal forms found in patients from the other family.12 No proteolytic degradation was found in samples from controls and unaffected family members. Although the patients' platelet von Willebrand factor was partially degraded, immunoblot analyses indicated that their plasma von Willebrand factor was normal (not shown).
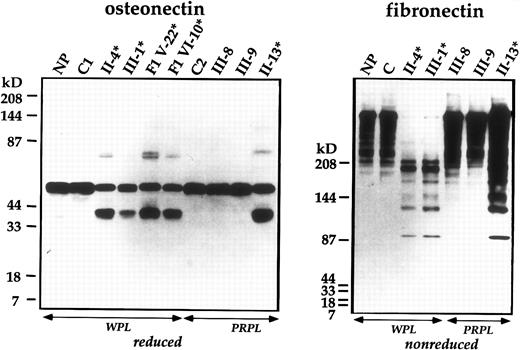
Immunoblot analyses of platelet osteonectin and fibronectin. Samples of WPL or PRPL from patients (*), unaffected family members, healthy controls (C), the normal pool (NP), and individuals from the first family (F1) were analyzed using SDS-PAGE and monoclonal (osteonectin) or polyclonal (fibronectin) antibodies.
Immunoblot analyses of platelet osteonectin and fibronectin. Samples of WPL or PRPL from patients (*), unaffected family members, healthy controls (C), the normal pool (NP), and individuals from the first family (F1) were analyzed using SDS-PAGE and monoclonal (osteonectin) or polyclonal (fibronectin) antibodies.
Investigations were performed to assess additional platelet proteins. Studies of platelet fibronectin,29 a soluble protein stored in α-granules, indicated that this protein was also degraded in the patients (Fig 4) and the same fibronectin degradation products were found in patients from the other family. In samples prepared by solubilizing PRP pellets, undegraded plasma fibronectin was also evident (Fig 4). The fibronectin degradation products were not found in unrelated patients with platelet production or consumption disorders (n = 5 tested; not shown). No abnormalities were found in immunoblot analyses of the patients' platelet IgG and albumin, indicating that not all α-granular proteins were degraded.
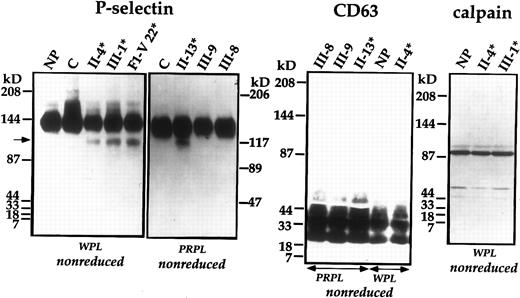
Because degraded proteins were secreted from the patients' thrombin-activated platelets, we considered that the proteolyzed proteins might be located within α-granules or within lysosomes. To determine if the α-granular membrane was proteolyzed, we evaluated P-selectin, an integral membrane GP found in α-granules and dense granules.9,30-33 Immunoblot analyses indicated that there was partial degradation of patients' platelet P-selectin, and an identical pattern of P-selectin degradation was found in patients from the other family (Fig 5).12 No abnormalities of P-selectin were found in the unaffected family members, controls (Fig 5), or unrelated patients with disorders of platelet production or consumption (not shown). Although the patients' internal, α-granular proteins were degraded, no abnormalities were detected in immunoblot analyses of their platelet CD63, a membrane protein found in dense granules and lysosomes24,33-36 (Fig 5). In addition, no abnormalities were found in their platelet calpain, a cytosolic cysteine protease25 37 (Fig 5).
Immunoblot analyses of platelet P-selectin, CD63, and calpain. Samples of WPL and PRPL from patients (*), unaffected family members, healthy controls (C), the normal pool (NP), and an individual from the first family (F1) were separated using nonreduced SDS-PAGE and analyzed by immunoblotting with polyclonal anti–P-selectin, monoclonal anti-CD63, and monoclonal anticalpain. The arrow indicates degraded P-selectin. Intact calpain heavy chain migrated slightly higher than the reported 80-85 kD molecular weight25,37 38 in the 4% to 8%/15% gradient gels.
Immunoblot analyses of platelet P-selectin, CD63, and calpain. Samples of WPL and PRPL from patients (*), unaffected family members, healthy controls (C), the normal pool (NP), and an individual from the first family (F1) were separated using nonreduced SDS-PAGE and analyzed by immunoblotting with polyclonal anti–P-selectin, monoclonal anti-CD63, and monoclonal anticalpain. The arrow indicates degraded P-selectin. Intact calpain heavy chain migrated slightly higher than the reported 80-85 kD molecular weight25,37 38 in the 4% to 8%/15% gradient gels.
Electron Microscopy Studies
Electron microscopy investigations were performed to determine if the degradation of α-granular proteins was associated with alterations in platelet morphology and to determine the intracellular location of several abnormal α-granular proteins. Two patients from both families (family 2, patients II-4 and III-1; and family 1, patients F1 V-22 and F1 V-23) and healthy controls (n = 2; samples processed in parallel with the patients) were studied.
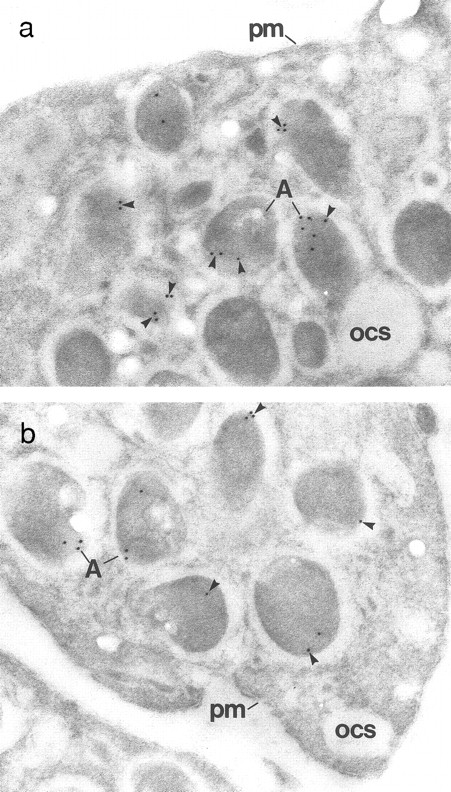
Compared with the control platelets, the patients' platelets contained similar numbers of α-granules. In addition, the ultrastructure within α-granules was well preserved in the patients' platelets (Fig 6). The number of von Willebrand factor-associated α-granular tubules/100 platelet profiles39 40 ranged from 3 to 9 in patients and from 12 to 13 in the two controls. Immunogold labeling experiments, using polyclonal (rabbit) antisera, indicated fibrinogen (Fig 6), von Willebrand factor (Fig 6), P-selectin, multimerin (Fig 7), and factor V were located within the α-granules of the patients' platelets and not in other structures. The patients' α-granular immunolabeling for von Willebrand ranged from reduced (III-1: 10% of control; Fig 6) to normal (3/4 patients). Similar results were also seen with fibrinogen, with normal labeling of platelets from 3 patients and significantly reduced labeling of platelets from 1 patient (III-1: 10% of control; Fig 6). The patients' α-granular P-selectin immunolabeling ranged from 50% to 100% of controls. All patients had reduced α-granular labeling for multimerin (Fig 7) and factor V. Control platelets contained immunogold label for multimerin in all sections, but only 64% to 84% of the patients' platelet sections contained multimerin label. Differences were also seen in the number of gold particles/α-granule labeled with antimultimerin: greater than 50% of the patients' labeled α-granules contained only 1 immunogold particle, whereas 2 or more gold particles seen in greater than 60% of the controls' labeled α-granules. The factor V immunolabeling studies indicated that greater than 65% of the patients' labeled α-granules contained 1 to 2 gold particles, in contrast to the greater than 5 gold particles found in greater than 50% of the controls' labeled α-granules.
Electron micrographs of α-granules (A) from control (a, c, and e) and patient (III-1; b, d, and f ) platelets. (a) The ultrastructure of normal α-granules is composed of a dense nucleoid and an eccentric zone in which the von Willebrand factor tubules (arrowheads) are located. (b) The patient's α-granule ultrastructure is well preserved and von Willebrand factor tubules (arrowheads) are present but are less abundant than in control platelets. (c) Immunolabeling for von Willebrand factor is found in normal α-granules in a zone of lower density (arrowheads). (d) In the patient's α-granules, immunolabeling for von Willebrand factor (arrowheads) is less abundant. (e) Fibrinogen immunolabeling (arrowheads) is prominent and is scattered throughout the matrix of normal α-granules. (f ) Immunolabeling for fibrinogen (arrowheads) is decreased in this patient's α-granules. (Original magnification × 60,000 for [a] through [f].)
Electron micrographs of α-granules (A) from control (a, c, and e) and patient (III-1; b, d, and f ) platelets. (a) The ultrastructure of normal α-granules is composed of a dense nucleoid and an eccentric zone in which the von Willebrand factor tubules (arrowheads) are located. (b) The patient's α-granule ultrastructure is well preserved and von Willebrand factor tubules (arrowheads) are present but are less abundant than in control platelets. (c) Immunolabeling for von Willebrand factor is found in normal α-granules in a zone of lower density (arrowheads). (d) In the patient's α-granules, immunolabeling for von Willebrand factor (arrowheads) is less abundant. (e) Fibrinogen immunolabeling (arrowheads) is prominent and is scattered throughout the matrix of normal α-granules. (f ) Immunolabeling for fibrinogen (arrowheads) is decreased in this patient's α-granules. (Original magnification × 60,000 for [a] through [f].)
Immunogold localization of multimerin in patient (III-1) and control platelets. (a) The characteristic, eccentric labeling of α-granular (A) multimerin17 (arrowheads) is seen in the control platelets. The plasma membrane (pm) and open cannalicular system (ocs) are not labeled. (b) The patient's platelets contain fewer immunogold particles per α-granule (A). However, the eccentric multimerin labeling pattern is preserved and no gold particles are found in structures other than α-granules. (Original magnification × 65,000 for both panels.)
Immunogold localization of multimerin in patient (III-1) and control platelets. (a) The characteristic, eccentric labeling of α-granular (A) multimerin17 (arrowheads) is seen in the control platelets. The plasma membrane (pm) and open cannalicular system (ocs) are not labeled. (b) The patient's platelets contain fewer immunogold particles per α-granule (A). However, the eccentric multimerin labeling pattern is preserved and no gold particles are found in structures other than α-granules. (Original magnification × 65,000 for both panels.)
DISCUSSION
Recent studies of a large Quebec family defined the phenotype of a novel, inherited platelet disorder.12,13 Similar clinical features led us to investigate a second Quebec family; studies of these patients are the subject of this report. The purpose of these investigations was to determine if the quantitative and qualitative platelet abnormalities, characteristic of the Quebec platelet disorder, were present and then to pursue several important, but unanswered questions. These questions included the following: (1) Does the pathologic proteolysis lead to degradation of only soluble platelet proteins? (2) Is the proteolysis restricted to α-granular proteins? (3) Is the proteolysis secondary to a defect in targeting these proteins to α-granules? Because patients with the Quebec platelet disorder do not respond to platelet transfusions,12 we also looked for evidence of a similar defect in their endothelial cells by studying their plasma von Willebrand factor.
We found similar clinical and laboratory abnormalities in patients from both families with the Quebec platelet disorder. These individuals suffer from an autosomal-dominant, moderate-severe bleeding disorder associated with reduced to normal platelet counts and abnormal epinephrine aggregation.12 In studies of the first family, we excluded a deficiency of platelet α2-adrenergic receptors as the cause of their epinephrine aggregation defect.12 In members of the second family, we found reduced aggregation with ADP and variable aggregation with collagen, abnormalities that we had observed in some patients from the other family.12 These findings suggest that the patients' aggregation defect, although most apparent with epinephrine, may not be restricted to α2-adrenergic receptor stimulation.
Patients from both families with the Quebec platelet disorder have characteristic and specific platelet GP abnormalities that are not found in unrelated patients with disorders of platelet production and consumption. These abnormalities include multimerin deficiency12 and proteolytic degradation of plasma-derived and megakaryocyte-synthesized soluble α-granular proteins, including factor V, fibrinogen, thrombospondin, von Willebrand factor, osteonectin,12,13 and fibronectin. Our studies of additional proteins indicate that there is restriction in the proteins proteolyzed in this disorder. We found no abnormalities in the patients' external membrane proteins when the studies were performed using conditions associated with minimal α-granular secretion. In contrast to their normal external membrane GPs, patients in both families had degraded platelet P-selectin, an internal, membrane protein found in α-and dense granules.9,30-33 However, CD63, an internal membrane protein found in dense granules and lysosomes, was not degraded.24 33-36 These data suggest that the pathologic proteolysis occurs in specific intracellular compartments. The feature common to all of the known degraded proteins is their storage within α-granules. P-selectin in the patients' dense granules might not be accessible to proteolytic degradation, and this could account for the incomplete proteolysis of their platelet P-selectin. However, several other α-granular proteins (eg, von Willebrand factor and osteonectin) were only partial proteolyzed, and some α-granular proteins (eg, albumin and IgG) were not degraded. These findings suggest that there are differences in the susceptibility of individual proteins to degradation. Because identical α-granular protein degradation products were found in patients from both families, we postulate that the same protease(s) is responsible for their platelet protein degradation.
Calpain is a ubiquitous, intracellular cysteine protease.37 In resting platelets, greater than 95% of the intracellular calpain exists as a proenzyme in the cytosol.25,37 After conversion to active calpain in intact platelets, a number of proteins are proteolyzed, including actin binding protein and talin (reviewed in Ariyoshi et al37 ). Calpain also cleaves a wide variety of protein substrates in vitro, including α-granular proteins.25,37 The monoclonal anticalpain25 used in our studies recognizes the intact heavy chain (catalytic subunit) of the proenzyme, the lower molecular weight (76 to 78 kD) heavy chain of active calpain, and smaller degradation products.25,37 38 We found similar quantities of intact calpain in patients and controls and no evidence of activated or degraded calpain in the patients' platelets. These findings suggest that this cytosolic enzyme is not altered in the Quebec platelet disorder. Further studies are needed to determine if the patients' α-granular proteins are degraded by calpain or if another enzyme(s) is responsible.
Although we found that many of the patients' α-granular proteins were degraded, these abnormalities were not associated with reduced numbers of α-granules or major alterations in α-granular ultrastructure. We had postulated that the proteolyzed α-granular proteins might be found in α-granules or in lysosomes because the patients' thrombin-activated platelets released degraded α-granular proteins. The electron microscopy studies indicated that multimerin, factor V, von Willebrand factor, fibrinogen, and P-selectin were stored in the patients' α-granules and not in an abnormal intracellular location. Quantitative abnormalities in the patients' α-granular tubules and their reduced α-granular multimerin and factor V immunolabeling confirmed pathology within their α-granular compartment. Variability and overlap in antigen levels in patients and controls and possible differences in the amounts of intact and degraded proteins in the patients may be the reason why the immunolabeling for P-selectin, von Willebrand factor, and fibrinogen was reduced in some but not all patients. In 1 patient, reduced α-granular von Willebrand factor and fibrinogen immunolabeling was found, but the levels of these proteins, measured by ELISA assays, were normal. The patients' platelet factor V levels ranged from reduced to normal in the different ELISA assays and were reduced when assessed by immunoelectron microscopy. We suspect that these discrepancies are due to the epitopes found in the intact and degraded proteins, their recognition by the different antisera, and the preservation of epitopes during sample preparation.
The mechanism of the multimerin deficiency in both families with the Quebec platelet disorder is uncertain. We were not able to show proteolysis of their platelet multimerin or a relative loss of high molecular weight multimers using both monoclonal and polyclonal antisera. We postulate that the patients' platelets could contain multimerin degradation products lacking epitopes recognized by our antisera. Other explanations for their multimerin deficiency have not been excluded, including an effect of their disorder on multimerin expression or an indirect effect of the proteolytic degradation on the ability to synthesize or store multimerin.
A feature of the Quebec platelet disorder (observed in both families) that is not yet understood is the poor response to platelet transfusions. This could be due to a number of factors, including an inhibitory effect of the released degraded α-granular proteins on hemostasis. We considered other explanations, including similar, pathologic proteolysis of endothelial cell proteins. Animal transplantation studies indicate that plasma von Willebrand factor is derived from endothelium,41,42 and a precedent for a selective abnormality affecting the platelet (but not endothelial) stores of von Willebrand factor has been established in gray platelet syndrome.43 We failed to detect proteolysis of plasma von Willebrand factor in patients with the Quebec platelet disorder, suggesting that pathologic degradation of von Willebrand factor occurs only in their platelets. However, because these investigations did not directly evaluate their endothelial von Willebrand factor or multimerin, further studies are needed to definitively exclude abnormalities in their endothelium.
The Quebec platelet disorder and gray platelet syndrome are the only known bleeding disorders associated with multiple abnormalities in platelet GPs.3-9,12,13 The genetic causes of these disorders are not yet known; however, a number of features suggest that unique molecular defects are responsible. Family studies of gray platelet syndrome suggest a recessive mode of inheritance,5 whereas the Quebec platelet disorder is inherited as an autosomal dominant trait.12-14 In gray platelet syndrome, the α-granules appear empty3-9; however, the α-granules in the Quebec platelet disorder are normal in appearance. The low levels of platelet fibrinogen, thrombospondin, von Willebrand factor, and β-thromboglobulin in gray platelet syndrome suggest a defect in targeting or storing these proteins in α-granules,3-9 whereas qualitative, α-granular protein abnormalities predominate in the Quebec platelet disorder.12,13 In contrast to the abnormal proteolysis of platelet P-selectin in the Quebec platelet disorder, P-selectin is not degraded in gray platelet syndrome.9 Although absent epinephrine aggregation is a consistent finding in the Quebec platelet disorder, this defect is not seen in gray platelet syndrome.3,4 12 Together, these findings define two distinct forms of α-granular disorders.
The cause of the bleeding disorder in the two Quebec families is not yet known. We were unable to identify relatives common to both families, suggesting that similar, but perhaps different, genetic mutations might be responsible. The province of Quebec has a population of 6,532,461 and 81% are French-Canadian in origin.44,45 These individuals are the descendants of a founding population of 8,527.46 Founder effects have been documented in other genetic disorders in French Canada, leading to a high prevalence of some genetic diseases that are rare in other populations (reviewed in Labuda et al47 ). Until the molecular-genetic defects in these families are known, we cannot exclude the possibility that these patients might be distant relatives.
These studies confirm the phenotype of an unusual, autosomal dominant platelet disorder associated with degradation of soluble and membrane constituents of α-granules. Although many α-granular proteins are proteolyzed, these proteins are found in their normal location, excluding a defect in α-granular protein targeting as the cause of the degradation. The degradation of P-selectin and of soluble plasma-derived and megakaryocyte-synthesized α-granular proteins suggest that proteolysis occurs within a shared compartment, perhaps within α-granules. The degradation of endocytosed and endogenously synthesized α-granular proteins also suggests that the proteolysis occurs late, after packaging and fusion of the exogenous and endogenous α-granular protein pools. The proteolysis could be secondary to an abnormal protease, the deficiency of a protease inhibitor, or the consequence of a protease(s) in an abnormal location. Protease mapping techniques may help identify the protease responsible for the patients' α-granular protein degradation. The molecular cause of the bleeding disorder in these families is under investigation.
ACKNOWLEDGMENT
We thank Dr J.G. Kelton, Dr D. Labuda, and Dr M. Labuda for their helpful discussions and M.A. Quinn-Allen, J.C. Moore, and J.W. Smith for their technical assistance. We are grateful to the members of both families for their participation in these studies.
Supported by a grant from the Heart and Stroke Foundation of Ontario and by grants from l'Association pour la Recherche sur le Cancer (ARC) and Fondation pour la Recherche Medicale. C.P.M.H. is a Research Scholar of the Heart and Stroke Foundation of Ontario and the recipient of an Ortho Biotech Inc/American Society of Hematology Scholar Award.
Address reprint requests to Catherine P.M. Hayward, MD, PhD, HSC Room 2N32, 1200 Main St W, Hamilton, Ontario, Canada L8N 3Z5.

![Fig. 2. Studies of surface-labeled platelets and platelet releasate. Platelets were washed (PBS-EDTA-theophylline) and surface-labeled with biotin. In parallel experiments, the releasate from thrombin-activated platelets was labeled with biotin. Aliquots (10 μL) of the labeled releasates, solubilized surface-labeled platelets (total lysate), and the corresponding membrane fractions (solubilized, washed pellets from freeze-thaw lysates) of surface-labeled platelets were analyzed using nonreduced/reduced SDS-PAGE. Biotinylated proteins were detected using streptavidin-peroxidase and chemiluminescent substrate. Platelet GPs Ib, αIIbβ3 , α2β1 , and thrombospondin (normal thrombospondin []; degraded thrombospondin [➭]) are indicated. Samples from 2 patients and a control (C), processed in parallel, are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1243/4/m_bl_0003f2.jpeg?Expires=1769268463&Signature=zZI7wChK3nIYJMtwOI8OKGcN6Tm7mqG0YP8VffNHYPzT5CAqbPWbVN9EXk3g3gH9CaRVjz96ZzUBshGNn3cij~yqKpFsC-awZ7UJI1F0wtljBLBw2I~rnRaeZPW6w6ZaklAJ8CfY~TaCYS0JpE7FkQ-kCwS1elN9ibGSEArLCBrzG0tn4p9PdfOFsqHY3rgyYlF0P3W6iv9ht~09uy3ypG34jLqq9I1ZvtcttVYRLZTiBUtp~J~-Pucg6V7c1M2ZCg2xkFzNC0Wir6wRVMcAUQIuPa88A~GCX34Qv8Cg3vSaTQ~tldsWNdPQbPUyAkO9wzhChhXPWTZPU4mnCEhhOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Electron micrographs of α-granules (A) from control (a, c, and e) and patient (III-1; b, d, and f ) platelets. (a) The ultrastructure of normal α-granules is composed of a dense nucleoid and an eccentric zone in which the von Willebrand factor tubules (arrowheads) are located. (b) The patient's α-granule ultrastructure is well preserved and von Willebrand factor tubules (arrowheads) are present but are less abundant than in control platelets. (c) Immunolabeling for von Willebrand factor is found in normal α-granules in a zone of lower density (arrowheads). (d) In the patient's α-granules, immunolabeling for von Willebrand factor (arrowheads) is less abundant. (e) Fibrinogen immunolabeling (arrowheads) is prominent and is scattered throughout the matrix of normal α-granules. (f ) Immunolabeling for fibrinogen (arrowheads) is decreased in this patient's α-granules. (Original magnification × 60,000 for [a] through [f].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1243/4/m_bl_0003f6.jpeg?Expires=1769268463&Signature=kfYvnfsH~0XMY3~3zn6SQ58GLegxVe-aVqjAkk14qHdLKNrvfyXNzv-SdqfS~RR72VXmsWqJSU0mQmt2CIpm4U3kJNmrLx38tHIVdqapdc9zRb1DlEyXdVPfM9G5xnbPRJR4VL-0Mzf~ugxv2665SAtvsQPMb4BNBGjH1Df9SI~l4ngWZA7HreccolKsHUMtWhm65b4utkrpqoDma0gaoaiUl1DnXE~4IrB-wiBazjqi4lc4LNsAtWr9vrTG-Nd8APwnrK5296epUpq0wfRcnuOQyDpkiXc3zISnXU5OqhbXrhBCJPA5GxUr4HjXDX7mDwjuG-nV0E~h9X-JJq8T3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal