Abstract
The purposes of the research reported here were first to explore a murine model for human placental and umbilical cord blood transplantation and second to evaluate the engraftment ability of ex vivo cultured hematopoietic cells. Murine near-term fetal and neonatal peripheral blood (FNPB) cells, genetically marked with the human multiple drug resistance transgene (MDR1) were used for syngeneic transplants into sublethally irradiated adult mice. Donor cells were transplanted either fresh and untreated, or after ex vivo culture in the presence of the hematopoietic growth factors recombinant murine stem cell factor, recombinant human interleukin-3 (rHu IL-3), and rHu IL-6, in a liquid culture system. To evaluate, count, and characterize FNPB progenitor cell-derived colonies, neonatal mouse mononuclear cells were cultured directly in methylcellulose with growth factors. To assess their ex vivo expansion ability, FNPB mononuclear cells were first cultured in liquid medium for 3 to 8 days and then transferred to semisolid assay plates. Evaluation of the cell counts after liquid culture showed a 1.4- to 11.6-fold increase, and the numbers of colonies observed in methylcellulose were similar to those produced by fresh FNPB cells. Donor-type engraftment was demonstrated by polymerase chain reaction (PCR) amplification of the human MDR1 transgene in the peripheral blood of all surviving animals (5 of 7 recipients of the fresh, and 3 of 8 recipients of the ex vivo–cultured cells) 2 to 4 months after transplantation. The proportion of donor leukocytes in the peripheral blood of the recipients (chimerism) was evaluated using fluorescence in situ hybridization (FISH) analysis 4 to 6 months after transplantation and ranged from 2% to 26%. In addition, bone marrow cultures were obtained from two recipient animals: one had received fresh-untreated cells and was evaluated 8 months after transplant, the other had received ex vivo cultured cells and was tested 14 months after grafting. The derived hematopoietic colonies were tested by PCR and the transgene was detected, conclusively proving long-term engraftment of donor cells. These results indicate that FNPB transplants can be successfully performed in sublethally irradiated mice with and without ex vivo culture. Long-term donor-type engraftment with sustained chimerism has been demonstrated. Thus, murine neonatal blood grafts can be used as an animal model for cord blood transplantation for gene therapy studies where complete myeloablation is not desirable and partial replacement of defective marrow may be sufficient. Furthermore, the possibility of numerically expanding hematopoietic progenitor cells contained in neonatal blood without affecting their engraftment ability could facilitate use of cord blood grafts in adult recipients.
HUMAN PLACENTAL/UMBILICAL cord blood (PCB), as a source of hematopoietic progenitor cells for bone marrow (BM) reconstitution, has been shown to yield successful transplants from siblings,1,2 and more recently, from unrelated donors.3,4 In comparison with BM, the collection of PCB is easier and the transmission of certain infectious agents, particularly cytomegalovirus, to recipients, is much less common.5 Most importantly, PCB grafts may result in decreased incidence of graft-versus-host disease (GVHD), even in partially mismatched cases.3,4 Some of us are currently involved in a study evaluating the feasibility of large-scale use of PCB for unrelated donor transplantation.6 While diverse aspects of the clinical use of PCB are being investigated, research in animals could accelerate progress in this field. For example, it is important to determine whether it is the lower alloresponsiveness of PCB cells that allows major histocompatibility complex (MHC)-disparate transplants to engraft without excessive GVHD and, if so, to define the level of MHC-incompatibility that may be tolerated. Similarly, given the higher rates of gene transfer into these cells,7 genetic engineering of PCB-derived stem cells is being actively pursued, and an animal model would facilitate testing and accelerate the definition of optimal experimental conditions. Murine and rat models for several genetic diseases (severe combined immune deficiency, thalassemia, mucopolysaccharidoses) are available, which would allow a more rapid evaluation of gene therapy with transduced fetal and neonatal blood stem cells.
The mouse is probably the most thoroughly explored model: it has been used in studies of the immunogenetics of soft tissue and bone marrow transplantation (BMT) for several decades. Preparative radiation regimens, BM cell doses, posttransplant recovery, and clinical characteristics of GVHD are all well established. Relatively simple hematopoietic stem/progenitor cell assays of murine BM-derived colonies in semisolid media are widely available8,9 and short-term suspension cultures of BM cells in the presence of hematopoietic growth factors have been recently shown to expand the committed progenitor cell population. The combination of c-kit, interleukin-3 (IL-3), and IL-6 in these cultures resulted in the greatest expansion, as measured by transplantation results: while 105 ex vivo expanded adult BM cells achieved radioprotection, no less than 106 unmodified cells were needed for BM restoration of lethally irradiated animals.10,11 Accelerated recovery of peripheral blood cell counts was observed with the ex vivo expanded donor cells, and their long-term repopulating ability appeared to be preserved in serial transplantation studies.11
The goal of our study was to develop a murine transplantation model with particular applications to the treatment of genetic diseases. We report that murine fetal and neonatal peripheral blood (FNPB) cells engraft when transplanted into sublethally irradiated adult recipients and lead to stable, long-term chimerism. Using the mouse as a model for human PCB transplantation, appears warranted for conditions in which partial BM replacement may suffice.
MATERIALS AND METHODS
Syngeneic Transplants of FNPB Cells Into Adult Mice
Donors.Adult (6 to 12 weeks) female FVB mice, purchased from Jackson Laboratories (Bar Harbor, ME), were maintained in the Center for Laboratory Animal Science at the Mt. Sinai School of Medicine. They were mated with one transgenic male FVB mouse, homozygous for the human MDR1 cDNA.12,13 This male was from a line rendered transgenic by methods described previously in detail.14 The offspring, hemizygous for the transgene, were used as donors of the fetal and neonatal peripheral blood cells.
Collection of FNPB.On gestational days 18 to19, pregnant females were killed by cervical dislocation. The skin was washed with ethanol, the uterus was opened, and each fetus was extracted with its separate, intact amniotic sac and placenta. Peripheral blood, collected directly from the neck vessels of the fetuses, was aspirated into sterile, heparinized Pasteur pipettes. Fetal blood was pooled from all pups of each litter; collected volumes ranged from 250 μL to 500 μL/litter (four to eight neonates). Nucleated cell counts, obtained using a Coulter ZBI counter (Coulter Electronics, Inc, Hialeah, FL) ranged between 3 and 6 × 106/mL. The presence of the transgene MDR1 cDNA was confirmed by Southern blot analysis of placental DNA. In some experiments, newborn mice (<24 hours old) were used, instead of near-term fetuses. Blood was collected using the same technique.
Transplantation.Donor cells (carrying the MDR1 transgene) were transplanted either as fresh-untreated blood (within 4 hours of collection), or after ex vivo culture with hematopoietic growth factors. A volume of 150 to 200 μL of blood, providing a nucleated cell dose of 0.5 to 1 × 106 cells was either injected into one of the tail veins of each recipient animal or cultured (see below), and then all derived cells were administered to a single recipient.
Recipients.Adult female FVB mice (Jackson Laboratories) were sublethally irradiated (750 cGy, single dose radiation from a 137Cs source at a dose rate of 82 rads/min) and transplanted within 4 hours. Irradiated recipients were maintained in a laminar flow environment at the Animal Facility of the New York Blood Center; they were given sterilized food and sterile, acidified water. Irradiated, but not transplanted, mice were used as controls. All mice were examined daily for the first 30 days and twice weekly thereafter. The time of death was noted. Necropsies were performed in two animals that received ex vivo cultured cells to investigate the distribution of engrafted cells in the tissues.
Hematopoietic Progenitor Cell Assays of FNPB Cells
Mononuclear cells were obtained from murine FNPB by centrifugation at 450g for 30 minutes at 18°C on Ficoll-Hypaque (Acurate Chemical and Scientific Corporation, Westbury, NY; density 1.077, osmolality 265/mL).
Liquid cultures.Liquid cultures were as previously described.15 Briefly, medium consisted of 10% fetal bovine serum (Armour Pharmaceutic Intergen Co, Purchase, NY) in Iscove's Medium (IMDM; GIBCO, Life Technologies, Inc, Grand Island, NY) with the following growth factors: recombinant murine stem cell factor (rMu SCF) (20 ng/mL; kindly provided by Immunex, Seattle, WA), recombinant human (rHu) IL-3 (50 U/mL; R & D Systems, Minneapolis, MN), and rHu IL-6 (20 ng/mL; kindly provided by Immunex). All cultures contained 0.1 U/mL penicillin and 0.1 μg/mL streptomycin (GIBCO). Tissue culture flasks (Falcon, 25 cm2; Becton Dickinson, Lincoln Park, NJ) containing 10 mL of medium were inoculated with mononuclear cells obtained from 300 to 500 μL of blood. The cultures were incubated up to 8 days at 37°C, 4% CO2 and high humidity. Cells were recovered from liquid cultures by centrifugation at 450g for 10 minutes at 18°C. The recovered cells were used either for transplantation or for methylcellulose culture.
Methylcellulose progenitor cell assays.Mononuclear cells from FNPB, or cells previously cultured in liquid media, were plated in methylcellulose as previously described.16 Briefly, medium consisted of 0.8% methylcellulose (Fisher Scientific Co, Pittsburgh, PA) in IMDM, 30% fetal bovine serum (Armour Pharmaceutic Intergen Co), 1% bovine serum albumin (Sigma Chemical Co, St Louis, MO), 10-4 M β-mercaptoethanol (Sigma Chemical Co), rHu erythropoietin (Epo, 2 U/mL, kindly provided by RW Johnson Pharmaceutical Research Institute, Raritan, NJ), rHu granulocyte-macrophage colony-stimulating factor (GM-CSF; 1 ng/mL, R & D Systems), rHu IL-3 (50 U/mL), and rMu SCF (20 ng/mL). The cultures were incubated at 37°C, 4% CO2 and high humidity for up to 45 days and were examined twice weekly for the development of progenitor cell-derived colonies.
Analysis of Transplanted Mice
Polymerase chain reaction (PCR) amplification of peripheral blood leukocytes.All animals were tested at least 60 days after transplantation (range, 62 to 134 days). Peripheral blood (50 to 75 μL) was collected from the sectioned lateral veins of the tail of each recipient into microcapillary tubes. Buffy coats (2.5 to 7.5 × 105 leukocytes) were dispersed into 100 μL of DNA extraction buffer: 50 mmol/L KCl, 10 mmol/L Tris HCl pH 8.0, 2.2 mmol/L MgCl2 , 0.1 mg/mL gelatin, 0.45% Nonidet P40, 0.45% Tween 20, and 10 μg proteinase K for 2 hours at 55°C. DNA isolation was performed by sequential phenol/chloroform extraction and ethanol precipitation. DNA concentration was estimated with a commercial kit (DNA DipStick; Invitrogen, San Diego, CA). Transgene detection was accomplished by PCR amplification of DNA with primers specific for the human MDR1 cDNA: sense primer (residues 2596 to 2615), 5′ CCATCATTGCAATAGCAGG 3′, and antisense primer (residues 2733 to 2752), 5′ GTTCAAACTTCTGCTCCTGC 3′.17 PCR amplification of approximately 100 ng of template DNA was performed using Perkin-Elmer's PCR kit (Perkin-Elmer, Branchburg, NJ) following manufacturer's instructions. Final concentrations in the reaction were: 100 pmol/L specific primers, 2.5 mmol/L MgCl2 , 200 μmol/L dNTPs, 1× PCR buffer, and 2.5 U Taq DNA polymerase. The final volume of the PCR reaction was adjusted to 100 μL. PCR was performed for 40 cycles: 95°C × 5 minutes, then 94°C × 1 minute, 60°C × 1 minute, and 72°C × 2 minutes in a Perkin-Elmer thermal cycler. Reactions were set up using aerosol-proof tips and standard PCR precautions to avoid cross-contamination.18 Seventy microliters of the PCR products were subjected to gel electrophoresis in 1.8% agarose containing 0.5 ng/mL ethidium bromide to allow visualization of the amplified products and transferred to nylon membranes. Southern blots were hybridized with a 32P-labeled human MDR cDNA probe,14 washed and incubated with an autoradiographic plate overnight. Positive controls were obtained by appropriate dilution of transgenic placental DNA; negative controls were from normal mouse spleen DNA. Molecular sizes were estimated from a commercial 100-bp ladder (GIBCO).
Fluorescence In Situ Hybridization (FISH) Analysis of Peripheral Blood Leukocyte
At 129 to 196 days after transplantation, 50 to 100 μL of peripheral blood was collected from the transplant recipients. The buffy coat was separated, cytospin preparations were made, and the slides were processed according to previously published FISH methods,19 20 modified as follows. Red blood cells were lysed in 1:1 (vol/vol) acetic acid: methanol, for 4 minutes and fixed in 1:3 (vol/vol) acetic acid: methanol. After dehydration in 75%, 80%, and 100% ethanol, the DNA on the slides was denatured in 70% Formamide/2x SSC for 5 minutes at 75°C. A full-length human MDR1 cDNA probe was labeled by nick translation with deoxyuridine triphosphate (dUTP)-digoxigenin (Boehringer Mannheim, Indianapolis, IN). The probe (20 ng/μL) was diluted in Hybrisol VI (Oncor, Gaitherburg, MD) and the mixture was denatured for 5 minutes at 70°C. Approximately 100 ng of the probe mixture were placed on each slide and in situ hybridization was performed for 16 hours at 37°C. The signal was detected using a fluorescein isothiocyanate (FITC) conjugated antidigoxigenin antibody (Boehringer Mannheim). Interphase nuclei were counter-stained with 0.2 ng/μL DAPI (Sigma) for 5 minutes. Between 130 and 500 cells from each animal were scored and images of positive cells were generated with a Cytovision System (Applied Imaging, Pittsburgh, PA).
PCR Analysis of BM-Derived Colonies
BM-derived colonies from two long-term transplant survivors were evaluated for the presence of the transgene. The first animal, recipient of fresh, untreated cells was tested 8 months after transplant, the other had received cells after ex vivo culture and was evaluated 14 months after transplant.
Murine Near-Term Fetal and Neonatal Peripheral Blood Hematopoietic Progenitor Cell-Derived Colonies in Methylcellulose Cultures
| Cells . | Type of Colony . | Day(s) of Peak Growth* . | Colonies/1 × 105 Cells . | No. of Cultures‡ . | |
|---|---|---|---|---|---|
| . | . | . | Mean + SD† . | Range† . | . |
| Fresh FNPB mononuclear cells | CFU-E | 2-7 | 78 + 73 | 7-166 | 4/6 |
| BFU-E | 6-12 | 8 + 8 | 2-19 | 5/6 | |
| CFU-GM | 10-11 | 3 + 2 | 2-5 | 2/6 | |
| HPP-CFC | 19-45 | 2 + 1 | 1-3 | 4/6 | |
| Ex vivo cultured FNPB cells | CFU-E | 6-7 | 105 + 76 | 52-159 | 2/4 |
| BFU-E | 7-10 | 15 + 21 | 2-47 | 4/4 | |
| CFU-GM | 10-11 | 3 + 2 | 1-4 | 2/4 | |
| HPP-CFC | 21-31 | 1 + 1 | 1-2 | 2/2 | |
| Cells . | Type of Colony . | Day(s) of Peak Growth* . | Colonies/1 × 105 Cells . | No. of Cultures‡ . | |
|---|---|---|---|---|---|
| . | . | . | Mean + SD† . | Range† . | . |
| Fresh FNPB mononuclear cells | CFU-E | 2-7 | 78 + 73 | 7-166 | 4/6 |
| BFU-E | 6-12 | 8 + 8 | 2-19 | 5/6 | |
| CFU-GM | 10-11 | 3 + 2 | 2-5 | 2/6 | |
| HPP-CFC | 19-45 | 2 + 1 | 1-3 | 4/6 | |
| Ex vivo cultured FNPB cells | CFU-E | 6-7 | 105 + 76 | 52-159 | 2/4 |
| BFU-E | 7-10 | 15 + 21 | 2-47 | 4/4 | |
| CFU-GM | 10-11 | 3 + 2 | 1-4 | 2/4 | |
| HPP-CFC | 21-31 | 1 + 1 | 1-2 | 2/2 | |
Counted from the first day in any culture medium.
At the time of peak growth.
Number of cultures in which colonies were observed/total number of cultures.
Posttransplant BM cells were obtained by flushing both femurs and tibias with RPMI 1640 medium (GIBCO) and were cultured in methylcellulose. For the first animal, late appearing (day 21) colonies (mixed, myeloid, and erythroid) were individually picked as previously described.15 Ten colonies were obtained and DNA was separately extracted from each. PCR amplification was performed as described above. The PCR products (60 μL) were subjected to electrophoresis in 1.8% agarose gel with ethidium bromide (0.5 ng/mL) staining and positive bands were noted. For the second animal, large numbers of mixed colonies were present in methylcellulose cultures, therefore, colonies from each mL of culture were pooled, resulting in 10 samples, each containing 3 to 7 colonies. PCR testing was performed in the same way. BM-derived, pooled colonies from a FVB normal mouse (not carrying the transgene) were used as negative control.
RESULTS
Hematopoietic Progenitor Cell Assays of FNPB Cells
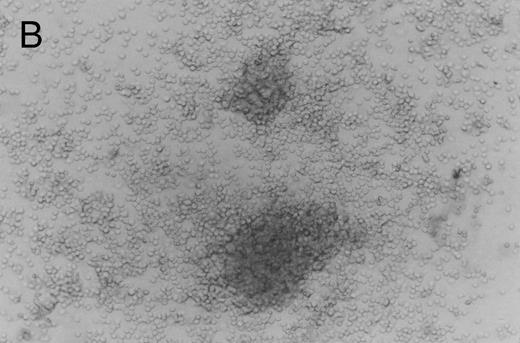
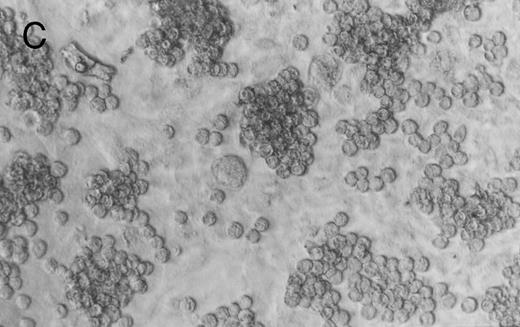
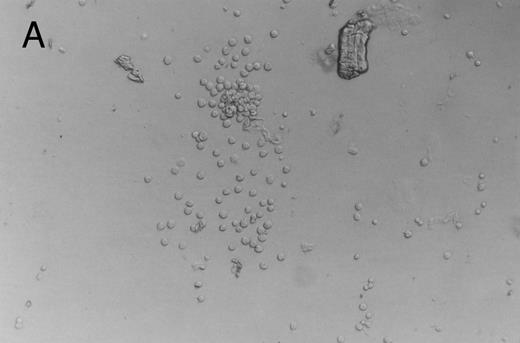
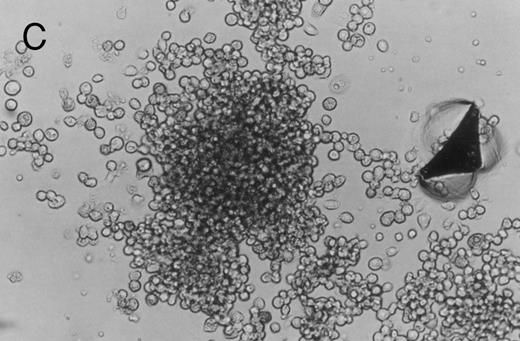
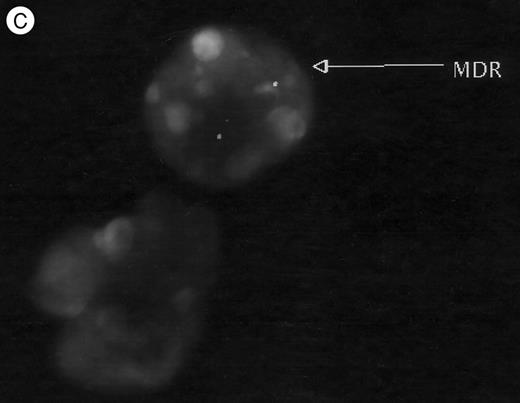
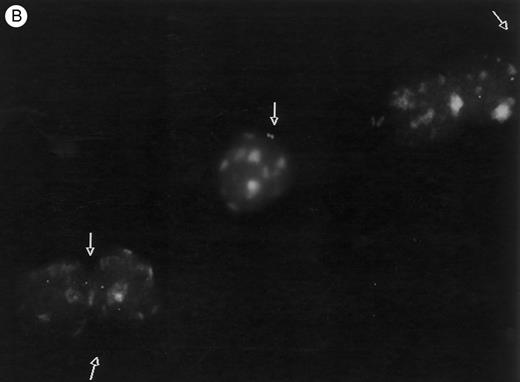
Methylcellulose cultures.Progenitor cell-derived colonies were present in methylcellulose cultures of FNPB mononuclear cells. The days of peak colony growth and the numbers of colonies (mean ± standard deviation [SD], range) are summarized in Table 1. Representative burst-forming unit-erythroid (BFU-E) and colony-forming unit–granulocyte-macrophage (CFU-GM) colonies are shown in Fig 1A and B. It is of particular interest that between days 19 and 45, a small number (1 to 3/105 cells plated) of large colonies with poorly differentiated cells were observed, as seen in Fig 1C. In two experiments, these large colonies were removed from the methylcellulose cultures, the cells were dispersed and replated in fresh methylcellulose cultures. Secondary colonies developed (data not shown). Therefore, these colonies appear to be primitive multipotential progenitor cells, perhaps analogous to the high proliferative potential colony forming cells (HPP-CFC).
Murine FNPB-derived colonies in methylcellulose cultures. (A) BFU-E colonies (day 8), (B) CFU-GM colony (day 8), (C) Late-developing colonies: very large colonies with poorly differentiated cells (day 28).
Murine FNPB-derived colonies in methylcellulose cultures. (A) BFU-E colonies (day 8), (B) CFU-GM colony (day 8), (C) Late-developing colonies: very large colonies with poorly differentiated cells (day 28).
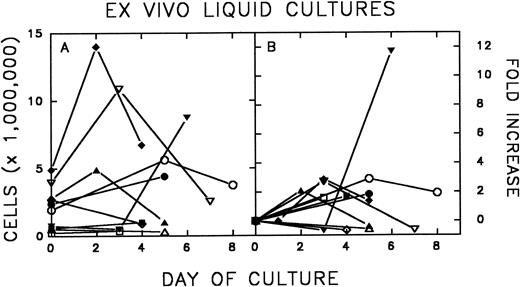
Ex vivo expansion of FNPB mononuclear cells.Figure 2 shows the expansion of FNPB mononuclear cells in liquid cultures. In eight of 10 experiments, there was an increase in the number of cells between days 2 and 6 of culture. Cell counts increased from 1.37- to 11.7-fold (average, 3.06, median, 2.0). Subsequently, the number of cells declined in four of eight cultures.
Murine FNPB cells were placed in liquid culture with hematopoietic growth factors: rMu SCF, rHu IL-3, and rHu IL-6 and were incubated for up to 8 days. Expansion, as measured by cell counts (A) and fold increase (B) after liquid culture, is shown.
Murine FNPB cells were placed in liquid culture with hematopoietic growth factors: rMu SCF, rHu IL-3, and rHu IL-6 and were incubated for up to 8 days. Expansion, as measured by cell counts (A) and fold increase (B) after liquid culture, is shown.
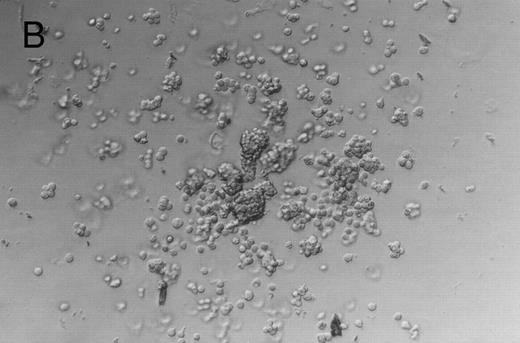
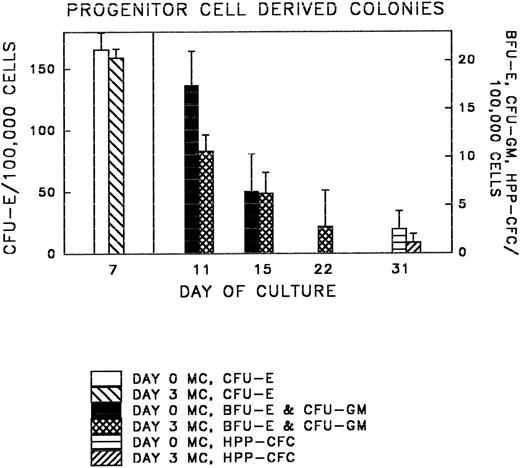
Following liquid culture, all cells were transferred to methylcellulose and the derived colonies were counted. Representative colonies are shown in Fig 3A through C; colony data are summarized in Table 1 and Fig 4. The number of colonies (CFU-E, BFU-E, and CFU-GM, and HPP-CFC) observed in methyl-cellulose cultures per 105 ex vivo cultured cells was similar to those produced by fresh FNPB mononuclear cells. In addition, the times at which different progenitor cell-derived colonies were seen (counted from the first day cells were in culture medium) was the same in both sets of cultures. These experiments demonstrate that FNPB mononuclear cells can be stimulated to undergo limited ex vivo expansion in medium containing stem cell factor (SCF ), IL-3, and IL-6 without changing their overall progenitor cell characteristics.
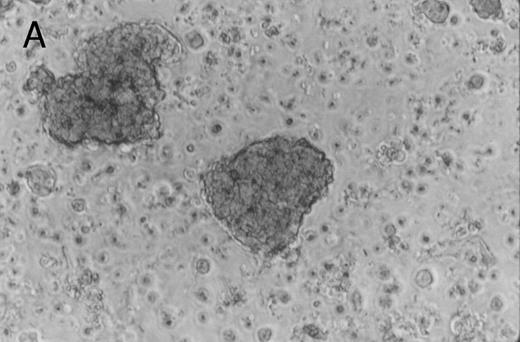
Murine FNPB-derived colonies in methylcellulose cultures after ex vivo culture with hematopoietic growth factors (rMu SCF, rHu IL-3, rHu IL-6) in liquid culture media. (A) CFU-GM (day 3), (B) BFU-E (day 3), (C) Large colonies containing large blast-like cells (day 23).
Murine FNPB-derived colonies in methylcellulose cultures after ex vivo culture with hematopoietic growth factors (rMu SCF, rHu IL-3, rHu IL-6) in liquid culture media. (A) CFU-GM (day 3), (B) BFU-E (day 3), (C) Large colonies containing large blast-like cells (day 23).
Colony (CFU-E, BFU-E, CFU-GM, and HPP-CFC) growth was compared in methylcellulose cultures of fresh, untreated FNPB cells (day 0 MC) and FNPB cells following 3-day ex vivo cultures (day 3 MC).
Colony (CFU-E, BFU-E, CFU-GM, and HPP-CFC) growth was compared in methylcellulose cultures of fresh, untreated FNPB cells (day 0 MC) and FNPB cells following 3-day ex vivo cultures (day 3 MC).
Transplantation Studies
Survival of transplant recipients.Data from the transplant experiments are shown in Table 2. All recipient mice were sublethally (750 cGy) irradiated. Five of seven mice in group 1, transplanted with fresh-untreated FNPB cells survived more than 30 days; two died at day 15 posttransplant. Three of eight mice in group 2, transplanted with donor cells that had been cultured ex vivo with hematopoietic growth factors, survived more than 30 days. The other mice died at days 8 (2 mice), 17 (1 mouse), and 18 (2 mice) after transplant. Controls were irradiated, but not transplanted. Three of five controls survived more than 30 days, the other two died on days 12 and 17 postirradiation. The transplant survivors appeared normal and healthy over a prolonged period of observation (170 to 250 days); one control died on day 80.
Donor-Type Engraftment of Murine Near-Term FNPB Cells in Sublethally Irradiated Adult Recipient Animals
| Time After Transplant . | Animal . | Peripheral Blood . | BM Colonies PCR . | |
|---|---|---|---|---|
| . | . | PCR/Hybridization (2 to 4 mo) . | FISH . | (>6 mo) . |
| . | . | . | (4 to 6 mo) . | . |
| Group 1 | 1 | pos | 3.7% | |
| Donor cells: fresh | 2 | pos | 2.0% | |
| Survivors: 5 of 7 | 3 | pos | 11.0% | |
| 4 | pos | 26.0% | ||
| 5 | pos | 6.3% | pos | |
| Group 2 | 1 | pos | 2.4% | |
| Donor cells: ex vivo culture | 2 | pos | 2.4% | |
| Survivors: 3 of 8* | 3 | pos | ND | pos |
| Time After Transplant . | Animal . | Peripheral Blood . | BM Colonies PCR . | |
|---|---|---|---|---|
| . | . | PCR/Hybridization (2 to 4 mo) . | FISH . | (>6 mo) . |
| . | . | . | (4 to 6 mo) . | . |
| Group 1 | 1 | pos | 3.7% | |
| Donor cells: fresh | 2 | pos | 2.0% | |
| Survivors: 5 of 7 | 3 | pos | 11.0% | |
| 4 | pos | 26.0% | ||
| 5 | pos | 6.3% | pos | |
| Group 2 | 1 | pos | 2.4% | |
| Donor cells: ex vivo culture | 2 | pos | 2.4% | |
| Survivors: 3 of 8* | 3 | pos | ND | pos |
Sublethally irradiated adult mice were transplanted with murine near-term FNPB cells, either fresh-untreated or after ex vivo culture with hematopoietic growth factors. Donor cells were genetically marked with the human MDR1 gene. Engraftment was tested by PCR amplification of the gene-marker in the peripheral blood of the survivors and by FISH analysis of their peripheral leukocytes. BM-derived colonies from two recipients were evaluated by PCR for the presence of the transgene.
Abbreviations: ND, not determined; pos, positive.
Spleen and bone marrow from 2 additional mice that died on day 8 posttransplant were positive for the MDR1 transgene by PCR analysis.
Engraftment of donor cells.To test for engraftment of donor cells, peripheral blood was collected from the survivors 2 to 4 months after transplantation, and the presence of donor cells was tested by PCR amplification of the MDR1 transgene and Southern blotting (Table 2). The results are shown in Fig 5. The five animals in group 1, after fresh FNPB transplants, are shown in lanes E, F, H, I, and K. The three survivors in group 2, which received ex vivo cultured cells, are shown in lanes G, L, and M.
Peripheral blood analysis of the eight transplant survivors. PCR amplification with MDR1 specific primers and Southern blot hybridization with a 32P-labeled human MDR1 cDNA probe were performed 2 to 4 months after transplantation. Lanes A and B represent negative controls: normal mouse spleen DNA. Lanes C and D represent positive controls: placental DNA MDR1 positive at 1:1,000 and 1:100 dilutions, respectively. Lanes E, F, H, I, and K represent the animals that received fresh-untreated donor cells. Lanes G, L, and M represent the animals that received ex vivo–cultured cells.
Peripheral blood analysis of the eight transplant survivors. PCR amplification with MDR1 specific primers and Southern blot hybridization with a 32P-labeled human MDR1 cDNA probe were performed 2 to 4 months after transplantation. Lanes A and B represent negative controls: normal mouse spleen DNA. Lanes C and D represent positive controls: placental DNA MDR1 positive at 1:1,000 and 1:100 dilutions, respectively. Lanes E, F, H, I, and K represent the animals that received fresh-untreated donor cells. Lanes G, L, and M represent the animals that received ex vivo–cultured cells.
The MDR1 sequence was detected in the leukocytes of all surviving recipients (N = 8) of FNPB transplants up to 130 days posttransplantation. In addition, necropsies were performed in two mice grafted with cultured cells that died on day 8 after transplant. PCR analysis performed on DNA extracted from spleen and BM cells also showed the presence of the transgene (data not shown).
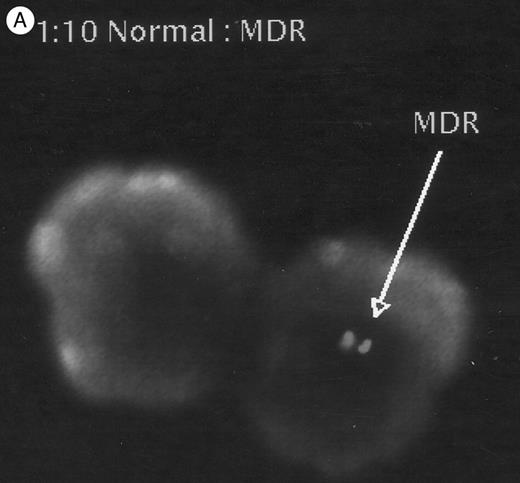
Chimerism of transplant recipients.Peripheral blood chimerism was evaluated by FISH in the recipients 4 to 6 months (129 to 196 days) after transplantation. Donor cells ranged from 2% to 26% of white cells in the animals that received fresh cells and there were 2.4% donor cells in the two animals grafted with ex vivo cultured FNPB cells (Table 2). Figure 6 shows cells positive for the MDR transgene from two animals (no. 4 and 5, respectively).
FISH analysis of peripheral blood. (A) MDR positive control (1:10 dilution), (B) and (C) two animals tested 4 to 6 months after transplantation: cells displaying positive signals (arrows), as well as negative cells are shown.
FISH analysis of peripheral blood. (A) MDR positive control (1:10 dilution), (B) and (C) two animals tested 4 to 6 months after transplantation: cells displaying positive signals (arrows), as well as negative cells are shown.
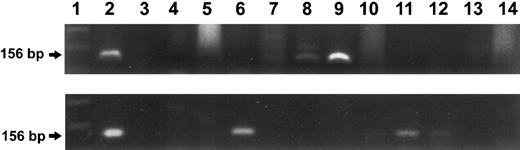
Long-term BM engraftment of donor cells.BM-derived colonies from two transplant recipients were analyzed for the presence of the MDR1 transgene 8 months (donor cells: fresh) and 14 months (donor cells: after ex vivo culture) after grafting. In both animals, the donor gene-marker was detected in the BM. In the first case, of the 10 individual colonies tested by PCR, three were found to carry the MDR1 gene. For the second animal, 10 samples of pooled colonies were tested and three were found positive for the transgene (Fig 7).
Analysis of bone marrow-derived colonies from two recipient animals. PCR amplification of the transgene using MDR1 specific primers and agarose gel electrophoresis are shown. Upper panel: recipient of fresh-untreated cells, tested 8 months after transplantation. Lane 1 represents the molecular size marker. Lane 2 represents the positive control (156-bp fragment). Lane 3 shows the negative control. Lanes 4 to 14 represent the BM-derived colonies. Three positive colonies were detected, as seen in lanes 7, 8, and 9. Lower panel: Recipient of ex vivo cultured cells, tested 14 months after transplant. Lane 1 represents the molecular size marker. Lane 2 represents the positive control. Lanes 3 to 13 represent the BM-derived colonies (pooled). Three positive samples were detected, as seen in Lanes 6, 11, and 12. Lane 14 shows the negative control (pooled colonies from normal, MDR-negative mouse).
Analysis of bone marrow-derived colonies from two recipient animals. PCR amplification of the transgene using MDR1 specific primers and agarose gel electrophoresis are shown. Upper panel: recipient of fresh-untreated cells, tested 8 months after transplantation. Lane 1 represents the molecular size marker. Lane 2 represents the positive control (156-bp fragment). Lane 3 shows the negative control. Lanes 4 to 14 represent the BM-derived colonies. Three positive colonies were detected, as seen in lanes 7, 8, and 9. Lower panel: Recipient of ex vivo cultured cells, tested 14 months after transplant. Lane 1 represents the molecular size marker. Lane 2 represents the positive control. Lanes 3 to 13 represent the BM-derived colonies (pooled). Three positive samples were detected, as seen in Lanes 6, 11, and 12. Lane 14 shows the negative control (pooled colonies from normal, MDR-negative mouse).
DISCUSSION
The work presented here demonstrates that syngeneic FNPB cells can engraft in sublethally irradiated adult recipients and that chimerism persists for several months after transplantation. Therefore, long-term survival of the graft can be achieved without complete myeloablation.
Our transplantation experiments were performed using donor cell doses of 0.5 to 1 × 106 nucleated cells/recipient, similar to those required for reconstitution after BMT (106 BM cells/recipient). Although smaller volumes of blood (10 to 20 μL of near-term fetal or newborn blood) were used in a previous report of murine newborn blood transplantation,21 when we grafted mice with such small volumes of FNPB, there were infections frequently with high mortality (data not shown).
Our transplantation experiments were designed to test the engraftment potential of FNPB cells. As such, the cell doses were not optimized to improve survival of the sublethally irradiated animals. It was recently shown in lethally irradiated recipients that increasing the dose of donor cells, by pooling blood from three newborn mice, can improve the survival rate.22
Marking of the donor cells with the MDR1 transgene allowed us to evaluate and follow donor-type engraftment. PCR amplification of DNA from peripheral blood leukocytes showed that all survivors carried the transgene. The assay used was qualitative, it only evaluated the presence of the donor gene-marker. Subsequently, the percentage of donor cells in the peripheral blood of the recipients was determined by FISH analysis, which indicated persistent chimerism at varying levels. Several experimental variables might influence chimerism, such as the time of collection of donor cells (late-fetal or early neonatal period), the exposure of cells to ex vivo culture systems, and the interval after transplantation.22 Nonetheless, all recipients tested demonstrated to be chimeras in their peripheral blood 4 to 6 months after transplant. Finally, BM engraftment was formally proven by detecting the transgene in BM-derived hematopoietic colonies from two recipients, one from fresh and one from previously cultured cells. Therefore, sustained, partial engraftment of the FNPB cells after sublethal irradiation of the recipients was shown.
Early in vivo studies demonstrated colony-forming units-spleen (CFU-S) in FNPB.23 In contrast to the extensive work published regarding the in vitro evaluation of murine BM-derived hematopoietic progenitors, no information is available on FNPB-derived progenitor cells or their capacity to engraft in syngeneic recipients. Furthermore, no data are available with respect to transplantation of cultured hematopoietic cells from FNPB. In our culture system, a remarkable “immaturity” of the FNPB-derived progenitor cells was evidenced by the late development of colonies in the methylcellulose assays and the blast-like morphology of the cells. It is possible that these “immature” progenitor cells take a longer time to engraft and this phenomenon accounts for the significant morbidity in the transplanted animals. Similarly, human PCB cultures contain a higher proportion of immature, late developing, multipotential colony-forming cells (CFU-GEMM and HPP-CFC) than adult BM cultures.24 25
In our ex vivo studies, we cultured FNPB mononuclear cells in liquid cultures under conditions similar to those used for expansion of murine BM cells10,11 and found that these cells responded to ex vivo stimulation with SCF, IL-3, and IL-6. Expansions observed in FNPB cultures were modest compared with those previously reported for adult mouse BM cultures26 and human cord blood cultures.15 27-30 Perhaps, the combination of hematopoietic growth factors employed in the current study was not optimal for expansion of FNPB cells. Future studies to investigate other combinations of cytokines are warranted.
The transplantation experiments were designed to test the long-term engraftment capacity of FNPB progenitor cells after ex vivo expansion. The liquid cultures were established with an equal amount of FNPB, as the volume used directly for transplantation (fresh) to each recipient. The results clearly demonstrate that ex vivo cultured FNPB progenitor cells are capable of sustained engraftment. Meaningful comparisons regarding cell doses, time of recovery of peripheral counts,10,11 and the long-term repopulating ability of hematopoietic cells obtained after ex vivo culture,31 as compared with fresh-untreated cells will, of course, require a larger series of transplants. The fact that murine fetal and neonatal hematopoieic cells do maintain their engraftment potential after ex vivo expansion suggests that similar results may also be possible for human PCB cells. The ability to numerically increase the progenitor cells contained in human umbilical cord blood, if so needed, may facilitate the use of these grafts for adult recipients.
As is the case with allogeneic BMT, the main indications of PCB transplantation are hematologic malignancies and genetic diseases. Approximately one third of all PCB transplants have been performed for inherited conditions. Traditionally, these patients have been receiving myeloablative preparative regimens, similar to the ones used for cancer patients. In the absence of a malignancy, the aim of the pretransplant conditioning is to achieve immunosuppression. A nonablative cytoreduction regimen might be sufficient in patients with genetic diseases, especially in conditions where the normal cells in the graft have a selective growth advantage.32 The data presented here indicate that FNPB-derived cells engraft after sublethal radiation and achieve clinically significant levels of chimerism in syngeneic settings. The work is being extended to semiallogeneic donor/recipient pairs.
Besides transplantation from a healthy donor, other aspects of the use of PCB in genetic therapy are under intensive laboratory investigation. For example, initial experiments have shown higher gene transfer rates in cord blood cells than in hematopoietic cells derived from adult bone marrow or adult peripheral blood.33 In addition, stable expression of the transduced genes in purified cord progenitor cells has been reported after prolonged in vitro cultures,7 making cord blood-derived stem cells an especially favorable target for gene transduction. With the rapid advance of this methodology, PCB may soon provide therapeutically useful autologous grafts for the correction of inherited diseases (immunodeficiencies, hemoglobinopathies, enzyme deficiencies, lysosomal storage disorders, etc). In this setting, it would be best to avoid complete myeloablation. The necessity for myeloablative radiation to “create marrow space” for the donor cells to engraft has been challenged recently34 and successful long-term BM engraftment has been reported in noncytoablated normal mice.35 However, these experiments required transplantation of very high donor cell doses (40 × 106 donor BM cells daily for 5 days), which may not be clinically practical. Just as in our experiments, recent work36 has shown that, thalassemic mice receiving (normal) congenic bone marrow transplants in conventional doses (2 × 106 BM cells/animal) after sublethal radiation experienced long-term engraftment and amelioration of the anemia. Clearly, correction of most relevant genetic diseases (eg, SCID, thalassemia) does not require complete marrow replacement,37 and the sublethal conditioning makes this approach a much safer alternative.
In our experiments, donor cells were infused either fresh, untreated, or after ex vivo expansion and both cell types engrafted successfully. The latter is important for gene transfer experiments because it has been shown that prestimulation of the cells with cytokines and cell cycling increase the efficiency of gene transduction.38
Our data demonstrate that partial BM reconstitution can be expected after transplantation of FNPB and that functional long-term marrow repopulating cells from FNPB can be sustained in appropriate ex vivo culture systems. Thus, murine FNPB, as an in vivo model for human PCB transplantation, can make an important contribution, similar to the one that the murine and canine models have made to human BMT.
ACKNOWLEDGMENT
We thank Dr Julie Asch, Dr Lisa Mueller, Michelle Geller, and Lily Kiang for their superb technical assistance. We thank Dr Richard Rosenfield for stimulating discussions and advice.
Supported in part by grants from the National Institutes of Health (HL 28381), Component Project, Columbia University Comprehensive Sickle Cell Center (to R.S.W.).
Address reprint requests to Rona S. Weinberg, PhD, Mount Sinai School of Medicine, Division of Hematology, Box 1079, 1 Gustave L. Levy Place, New York, NY 10029.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal