Abstract
Hydroxyurea (HU) can increase fetal hemoglobin (HbF) in sickle cell anemia (HbSS). To identify determinants of the HbF response, we studied 150 HU-treated patients grouped by quartiles of change in HbF from baseline to 2 years. Half of the HU-assigned patients had long-term increments in HbF. In the top two quartiles, HbF increased to 18.1% and 8.8%. These patients had the highest baseline neutrophil and reticulocyte counts, and largest treatment-associated decrements in these counts. In the lower two quartiles, 2-year HbF levels (4.2% and 3.9%) and blood counts changed little from baseline. In the highest HbF response quartile, myelosuppression developed in less than 6 months, compliance was best, and final doses of HU were 15 to 22.5 mg/kg. All four quartiles had substantial increases of F cells in the first year. This was maintained for 2 years only in the top three quartiles. Leukocyte and reticulocyte counts decreased initially in all quartiles, but drifted back toward baseline levels in the lowest HbF response quartile. Initial HbF level and phenotype of the F-cell production (FCP) locus were not associated with HbF response, but absence of a Central African Republic (CAR) haplotype was. Bone marrow ability to withstand HU treatment may be important for sustained HbF increases during HU treatment of HbSS.
BOTH CLINICAL and laboratory features of sickle cell anemia (HbSS) are influenced by fetal hemoglobin (HbF) concentration.1-3 Patients with higher HbF levels have fewer pain episodes and longer survival. Hydroxyurea (HU) therapy can increase HbF levels in HbSS.4,5 These observations suggested that this drug might temper the clinical course of this disease, and recently, HU was shown to reduce the number of painful episodes, hospitalizations, acute chest events, and amount of blood transfused in adults with HbSS.6
HbF levels vary greatly among patients with HbSS. HbF production can be influenced by determinants linked, or in cis, to the β-globin gene cluster and by elements in trans to these genes. Among cis-acting determinants, β-globin gene haplotype — defined by the nonrandom association of restriction endonuclease sites amid the β-globin gene cluster — serves as a marker for the genetic background inherited with the sickle hemoglobin gene.3,7,8 Each haplotype is associated with other polymorphic variations in the β-globin gene cluster. These are concentrated in evolutionarily conserved regions rich in erythroid-specific and ubiquitous transcription factor binding sites and are likely to denote the cis-acting elements that regulate gene expression.9 Among potential trans-acting elements,10-13 an X-linked “F-cell” production (FCP) locus has been studied most intensively. In one study, this locus accounted for approximately 40% of HbF variability in HbSS, while the β-globin gene haplotype explained less than 10%.14
In HbSS patients treated with HU, the amplitude and rapidity of the HbF response among patients differed — some had drug-induced HbF levels near 40%, while others had little or only minor changes in HbF concentrations.4,5,15 16 To attempt to identify factors that influence the HbF response, to HU we examined the following: (1) genetic elements — haplotype of the β-globin gene cluster and α-globin genes, and phenotype of the FCP locus; (2) patient characteristics at baseline — age, gender, and blood cell counts; and (3) follow-up characteristics — adherence, toxicity, and blood cell counts.
MATERIALS AND METHODS
Study plan.The Multicenter Study of Hydroxyurea (MSH) was a double-blinded, placebo-controlled study of the efficacy of HU therapy in HbSS that used the intention-to-treat principle. The primary analysis tested the effect of HU on the annual frequency of acute vasoocclusive (painful) crisis. Entry criteria, detection of painful episodes, and data analysis have been described previously.6,17 Moderate to severe disease — a criterion for patient entry into this trial — was defined as at least three painful episodes in the year preceding enrollment. The titration of doses of HU was described previously.6 17 HU was given daily, in a single dose starting at 15 mg/kg, and increased by 5 mg/kg every 12 weeks up to a maximum-tolerated dose of 35 mg/kg, unless toxicity developed. Toxicity was defined as a neutrophil count less than 2,000/μL, reticulocyte count less than 80,000/μL, platelet count less than 80,000/μL, or hemoglobin concentration less than 4.5 g/dL. When toxicity occurred, treatment was stopped until blood counts recovered. HU was then resumed at a dose 2.5 mg/kg lower than the toxic dose.
MSH began in January 1992 with 299 patients enrolled — 152 randomly assigned to HU and 147 to placebo. The study ended 4 months ahead of schedule in January 1995 because of evidence of efficacy of HU compared with placebo in the reduction of the frequency of vasoocclusive crises.6 The minimum length of follow-up evaluation for patients with HbF measured at the end of the study was 21 months (maximum, 38 months; mean, 28). A maximum-tolerated dose was not determined in 70 (43%) patients.
Patients.Two hundred ninety-five of 299 patients enrolled in MSH were homozygous for the βS mutation. By DNA analysis, three patients had sickle hemoglobin (HbS)-βo thalassemia and one had HbS-β+ thalassemia (this subject had a very low percentage of HbA that was not seen on the initial hemoglobin electrophoresis). Two subjects with βo thalassemia had the IVS-1 position 2, T→C mutation, and one had an IVS-1, G→A mutation. The patient with β+ thalassemia had an IVS-1, position 5, G→C mutation.18 Eight individuals died and eight did not return for final HbF studies. The present analyses are based on data from 279 βS homozygotes (143 assigned to HU).
Cell counts.Blood cell counts were performed by standard methods at a central laboratory. Leukocyte counts were corrected for the presence of nucleated red blood cells. Reticulocytes were counted by incubating blood with Auramine O (Sigma, St Louis, MO), followed by flow cytometry.19 Counts were performed at least three times pretreatment, every 2 weeks during follow-up dose titration, and every 4 weeks after the maximum-tolerated dose of HU was reached.
DNA studies.Blood samples were obtained from all 299 participants before treatment initiation and DNA was prepared from their leukocytes.20 β-Globin gene haplotype — assigned after examining seven restriction endonuclease sites within the β-globin gene cluster (XmnI 5′ to Gγ, HindIII within Gγ and Aγ, HincII within and 3′ to ψβ, Hinf1 5′ to β, HpaI 3′ to β) and α-globin genotype — ascertained by Southern blot analysis — were determined as previously described.3,21 Homozygosity for the HbS mutation was established as described22 by polymerase chain reaction (PCR) amplification of a β-globin gene fragment encompassing codon β6, digestion of this fragment with Dde I (in place of OxaNI), and detection of the digestion products on ethidium bromide–stained agarose gels.
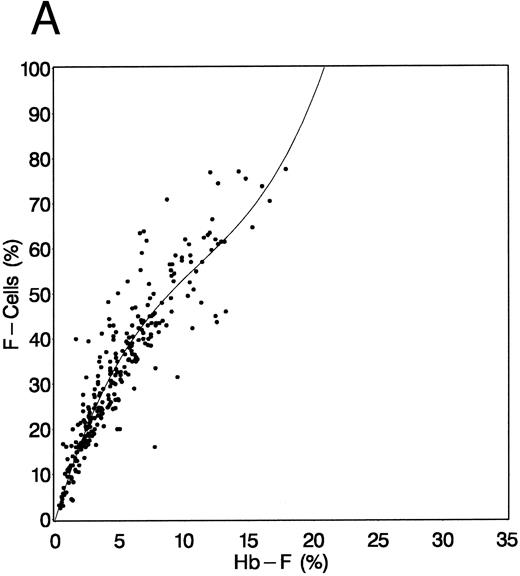
Fetal hemoglobin studies.HbF was measured by alkali denaturation at least twice pretreatment and twice at 2 years of treatment.23 Samples were collected during clinic visits for serial measurements of HbF and F cells (erythrocytes containing HbF), but for technical reasons, serial HbF measurements could not be completed. F cells were measured by flow cytometry every 4 weeks for the first half of the study and every 8 weeks after that.24,25 Because serial HbF measurements were not available during follow-up evaluation, F cells were used as a surrogate measure. The relationship observed between F cells and HbF (measured by alkali denaturation) at baseline and at 24 months is shown in Fig 1. This relationship was best described by a cubic equation with no intercept (ie, the regression line was forced through the origin). Although regression coefficient estimates of the relationship at baseline (Fig 1A, R2 = .967, root mean square error [RMSE] = 6.71) were slightly different from that at 2 years (Fig 1B, R2 = .979, RMSE = 6.76), a common regression was estimated as follows: F cells = 9.4372(HbF) − 0.4445(HbF)2 + 0.0082(HbF)3. Similarly, HbF can be estimated from F cells as follows: HbF = 0.1516(F cells) - 0.0017(F cells)2 + 0.00003(F cells)3; R2 = .956. The regression of F cells on HbF was not different between HU-assigned patients and placebo-assigned patients at either baseline or 2 years (data not shown). HbF was not estimated from F cells for the results that follow; the F-cell measurements were used directly. HbF-containing reticulocytes (F reticulocytes) were counted at least twice before treatment and twice after 2 years of treatment.24-26 FCP locus phenotype was assigned as previously described.13 This locus has two major alleles — high (H) and low (L). Females were classified as having the H/H phenotype if they had ≥ 24% F reticulocytes, the H/L phenotype if F reticulocytes were 12% to less than 24%, and the L/L phenotype if F reticulocytes were less than 12%. In HbSS males, the L phenotype had less than 12%, and the H phenotype ≥ 12% F reticulocytes.
Relationship between baseline (A) and 2-year (B) HbF levels and F-cell counts. HbF was measured by alkali denaturation and F cells by flow cytometry on samples obtained at baseline and at the conclusion of 2 years of HU therapy. (A) Relationship at baseline and (B) at 2 years of follow-up. In (B), data were pooled from patients assigned to HU and those assigned to placebo. See text for explanation.
Relationship between baseline (A) and 2-year (B) HbF levels and F-cell counts. HbF was measured by alkali denaturation and F cells by flow cytometry on samples obtained at baseline and at the conclusion of 2 years of HU therapy. (A) Relationship at baseline and (B) at 2 years of follow-up. In (B), data were pooled from patients assigned to HU and those assigned to placebo. See text for explanation.
Adherence.Adherence to therapy was assessed by four measures: (1) capsule counts of the returned bottles were used to compute the percentage of capsules taken; (2) the percentage of all scheduled 2-week follow-up clinic visits completed was calculated; (3) qualitative assays for HU in the serum were scheduled once every 8 weeks and the percent positive assays was computed27; and (4) the number of times a patient experienced toxic myelosuppression (see earlier) during follow-up evaluation was counted. Because of the rapid clearance of HU from the serum,4 a negative assay was not specific for nonadherence. As about one third of placebo-assigned patients — for unexplained reasons, perhaps the natural cyclic variation in leukocyte counts — experienced counts considered toxic once during follow-up evaluation, and that few of these patients had two instances of toxic blood counts, the occurrence of at least two episodes of toxic myelosuppression during the follow-up period was deemed specific for a HU-induced effect.
Statistical methods.Categorical variables, including dichotomous ones, are presented as percentage distributions and were compared using the chi-square test of homogeneity of distributions.28 Continuous variables are presented as means ± SD and were compared by analysis of variance and linear regression,29 or are presented graphically as medians and interquartile ranges. The relationships of laboratory results to age, gender, β-globin gene haplotype, α-globin genotype, and the FCP locus were analyzed using stepwise multiple regression. To test whether the difference in HbF change between HU and placebo was similar in different groups of patients (eg, men and women), multiple regression was performed with HbF as the outcome and independent terms that included treatment assignment, baseline and patient characteristics (main terms), and terms for interaction between treatment and main terms. To adjust for multiple tests of data, two-sided tests with P values between .01 and .001 were considered to provide some evidence, and tests with P values less than .001 were considered to provide strong evidence of differences.
A single pretreatment value for each laboratory determination was computed by averaging all pretreatment values of that variable for each patient. A single 2-year value was computed by averaging available data collected after 21 months of treatment; no patient had blood collected for testing after 38 months from the start of treatment. For values between baseline and 2 years, all available values of each determination were averaged within 8-week periods.
Patients assigned to HU who had a 2-year HbF value were grouped according to change in HbF from baseline to 2 years, corresponding roughly to quartiles of the distribution: quartile 1, losses of greater than 0.3% HbF (n = 34); quartile 2, losses of 0.3% to gains of less than 1.7% (n = 38); quartile 3, gains of 1.7% to less than 6.5% (n = 36); and quartile 4, gains of 6.5% or greater (n = 35). Percentage distributions or means (±SD) of patient characteristics (gender, age, baseline laboratory tests, etc), follow-up characteristics (adherence to protocol and HU dose), and co-outcomes (change in other laboratory values) are presented for the four quartiles.
To examine the effect of HU over time on HbF, F cells, mean corpuscular volume (MCV), reticulocytes, and neutrophils, the generalized estimating equations method was used for comparisons between HU and placebo and among quartiles of the HbF response.30 These models test whether repeated follow-up values are different between HU and placebo groups or among the four quartiles, and at what time points there are differences.
Analyses were performed using SAS software (SAS, Cary, NC) on an MSH data file updated in July 1995.
RESULTS
Baseline values.Table 1 shows baseline blood counts and HbF measurements according to treatment assignment, gender, age, β-globin gene haplotype, and FCP phenotype. HU- and placebo-assigned patients had similar values at baseline. In regression models, the H-FCP phenotype was associated with higher HbF (adjusted P < .001, β = 3.7) and a higher hemoglobin concentration (adjusted P < .001, β = 0.7) and MCV (adjusted P < .001, β = 3.8). Adjusted for FCP phenotype and other variables, female gender was associated with a higher HbF (adjusted P = .01, β = 0.8), lower hemoglobin concentration (adjusted P < .001, β = 0.9), and higher MCV (adjusted P = .003, β = 2.7), but not F reticulocytes or F cells. Older age was associated with lower F cells (adjusted P = .01, β = 0.2), lower hemoglobin levels (adjusted P = .002, β = 0.2) and higher MCV (P = .002, β = 0.2). Neutrophil counts were similar in all groups.
Baseline Values of Hematologic and HbF Measurements
| Variable . | n . | Hb (g/dL) . | MCV (FI) . | HbF (%) . | F Reticulocytes (%) . | F Cells (%) . | Reticulocytes (×103/μL) . | WBC (×109/L) . |
|---|---|---|---|---|---|---|---|---|
| HU | 150 | 8.4 (1.4) | 93.8 (9.1) | 5.0 (3.5) | 14.6 (8.0) | 32.7 (16.6) | 328 (97) | 12.5 (3.4) |
| Placebo | 145 | 8.5 (1.2) | 93.3 (8.5) | 5.2 (3.6) | 14.5 (7.6) | 32.4 (16.8) | 323 (87) | 12.3 (3.2) |
| Male | 143 | 8.9 (1.4) | 91.5 (8.4) | 4.1 (2.9) | 13.2 (7.9) | 28.1 (15.7) | 332 (83) | 12.3 (3.4) |
| Female | 152 | 8.1 (1.1) | 95.5 (8.7) | 6.0 (3.8) | 15.8 (7.5) | 36.7 (16.6) | 319 (100) | 12.6 (3.2) |
| <30 yr | 149 | 8.6 (1.3) | 91.9 (8.6) | 5.0 (3.5) | 14.2 (7.5) | 33.4 (17.0) | 336 (87) | 12.8 (3.3) |
| ≥30 yr | 146 | 8.3 (1.3) | 95.2 (8.7) | 5.1 (3.5) | 14.8 (8.1) | 31.7 (16.4) | 315 (96) | 12.0 (3.3) |
| SEN | ||||||||
| 0* | 244 | 8.4 (1.3) | 93.4 (8.5) | 4.9 (3.5) | 14.0 (7.9) | 31.7 (17.0) | 325 (94) | 12.5 (3.3) |
| 1, 2 | 51 | 8.7 (1.2) | 94.1 (10.2) | 6.1 (3.6) | 16.8 (7.0) | 36.7 (14.4) | 329 (83) | 12.1 (3.3) |
| CAR | ||||||||
| 0* | 203 | 8.6 (1.3) | 93.9 (8.7) | 5.3 (3.6) | 15.1 (7.9) | 33.7 (16.2) | 326 (95) | 12.5 (3.3) |
| 1, 2 | 92 | 8.3 (1.3) | 92.8 (9.0) | 4.6 (3.4) | 13.3 (7.6) | 30.1 (17.5) | 322 (87) | 12.2 (3.2) |
| FCP | ||||||||
| 0† | 131 | 8.2 (1.3) | 90.4 (8.2) | 2.8 (2.0) | 7.8 (2.9) | 20.5 (10.9) | 326 (92) | 12.5 (3.2) |
| 1 | 141 | 8.7 (1.3) | 95.9 (8.1) | 6.3 (2.9) | 18.3 (5.0) | 39.4 (12.5) | 330 (88) | 12.3 (3.3) |
| 2 | 23 | 9.0 (1.0) | 97.5 (10.2) | 11.0 (3.1) | 29.2 (3.5) | 59.1 (11.3) | 293 (112) | 12.4 (3.8) |
| Variable . | n . | Hb (g/dL) . | MCV (FI) . | HbF (%) . | F Reticulocytes (%) . | F Cells (%) . | Reticulocytes (×103/μL) . | WBC (×109/L) . |
|---|---|---|---|---|---|---|---|---|
| HU | 150 | 8.4 (1.4) | 93.8 (9.1) | 5.0 (3.5) | 14.6 (8.0) | 32.7 (16.6) | 328 (97) | 12.5 (3.4) |
| Placebo | 145 | 8.5 (1.2) | 93.3 (8.5) | 5.2 (3.6) | 14.5 (7.6) | 32.4 (16.8) | 323 (87) | 12.3 (3.2) |
| Male | 143 | 8.9 (1.4) | 91.5 (8.4) | 4.1 (2.9) | 13.2 (7.9) | 28.1 (15.7) | 332 (83) | 12.3 (3.4) |
| Female | 152 | 8.1 (1.1) | 95.5 (8.7) | 6.0 (3.8) | 15.8 (7.5) | 36.7 (16.6) | 319 (100) | 12.6 (3.2) |
| <30 yr | 149 | 8.6 (1.3) | 91.9 (8.6) | 5.0 (3.5) | 14.2 (7.5) | 33.4 (17.0) | 336 (87) | 12.8 (3.3) |
| ≥30 yr | 146 | 8.3 (1.3) | 95.2 (8.7) | 5.1 (3.5) | 14.8 (8.1) | 31.7 (16.4) | 315 (96) | 12.0 (3.3) |
| SEN | ||||||||
| 0* | 244 | 8.4 (1.3) | 93.4 (8.5) | 4.9 (3.5) | 14.0 (7.9) | 31.7 (17.0) | 325 (94) | 12.5 (3.3) |
| 1, 2 | 51 | 8.7 (1.2) | 94.1 (10.2) | 6.1 (3.6) | 16.8 (7.0) | 36.7 (14.4) | 329 (83) | 12.1 (3.3) |
| CAR | ||||||||
| 0* | 203 | 8.6 (1.3) | 93.9 (8.7) | 5.3 (3.6) | 15.1 (7.9) | 33.7 (16.2) | 326 (95) | 12.5 (3.3) |
| 1, 2 | 92 | 8.3 (1.3) | 92.8 (9.0) | 4.6 (3.4) | 13.3 (7.6) | 30.1 (17.5) | 322 (87) | 12.2 (3.2) |
| FCP | ||||||||
| 0† | 131 | 8.2 (1.3) | 90.4 (8.2) | 2.8 (2.0) | 7.8 (2.9) | 20.5 (10.9) | 326 (92) | 12.5 (3.2) |
| 1 | 141 | 8.7 (1.3) | 95.9 (8.1) | 6.3 (2.9) | 18.3 (5.0) | 39.4 (12.5) | 330 (88) | 12.3 (3.3) |
| 2 | 23 | 9.0 (1.0) | 97.5 (10.2) | 11.0 (3.1) | 29.2 (3.5) | 59.1 (11.3) | 293 (112) | 12.4 (3.8) |
Values are mean (±1 SD) of all MSH patients at baseline.
Abbreviations: SEN, Senegal haplotype; CAR, Central African Republic haplotype; FCP, F-cell production locus phenotype.
0, Absence of a haplotype; 1 or 2, heterozygosity or homozygosity for a haplotype.
0, Male hemizygotes and female homozygotes for the “L” FCP locus phenotype; 1, individuals hemizygous for the H phenotype or combined heterozygotes for the H/L phenotype; 2, indicates homozygosity for the H phenotype.
The prevalence of α thalassemia in the MSH (data not shown) was comparable to other reports of patients with HbSS.3 31-33 Patients with α thalassemia had a lower MCV (adjusted P < .001), lower reticulocyte counts (adjusted P < .001), and higher hemoglobin levels (adjusted P = .014) than individuals with a normal α-globin genotype (data not shown). The presence of α thalassemia did not affect HbF level or F cells.
β-Globin gene haplotypes.Table 2 shows the β-globin gene haplotypes in all MSH patients with HbSS and within each treatment group. Included for the purpose of comparison is the haplotype distribution in the largest group of African-American HbSS patients reported to date.3 Haplotype distributions in the HU and placebo arms of MSH were similar.6 Haplotype distributions in MSH patients and the comparison population of HbSS patients were also alike. However, for each of the three most common haplotypes — where sufficient numbers of patients allowed analysis — the percent HbF in each haplotype of the comparison group was higher than the baseline HbF in the MSH patients (Table 3).
Haplotype Distribution in All Patients and by Treatment Group
| Variable . | Total MSH Group . | Comparison Group* . | HU MSH Group . | Placebo MSH Group . |
|---|---|---|---|---|
| . | (n = 295) . | (n = 468) . | (n = 143) . | (n = 135) . |
| Mean age (yr) | 30.5 | 31 | 31 | 30 |
| BEN/BEN | 40 | 33 | 37 | 44 |
| BEN/CAR | 21 | 22 | 21 | 21 |
| BEN/SEN | 12 | 13 | 13 | 12 |
| SEN/SEN | <1 | 4 | — | — |
| SEN/CAR | 3 | 6 | 3 | 3 |
| CAR/CAR | 3 | 4 | 3 | 3 |
| Other† | 20 | 18 | 23 | 17 |
| Variable . | Total MSH Group . | Comparison Group* . | HU MSH Group . | Placebo MSH Group . |
|---|---|---|---|---|
| . | (n = 295) . | (n = 468) . | (n = 143) . | (n = 135) . |
| Mean age (yr) | 30.5 | 31 | 31 | 30 |
| BEN/BEN | 40 | 33 | 37 | 44 |
| BEN/CAR | 21 | 22 | 21 | 21 |
| BEN/SEN | 12 | 13 | 13 | 12 |
| SEN/SEN | <1 | 4 | — | — |
| SEN/CAR | 3 | 6 | 3 | 3 |
| CAR/CAR | 3 | 4 | 3 | 3 |
| Other† | 20 | 18 | 23 | 17 |
The percentage of patients with a given haplotype is shown.
The comparison group is a study of 468 patients with HbSS who were regular attenders of their respective clinics in the urban northeast and semirural south.3
Other includes patients heterozygous or homozygous for atypical and Cameroon haplotypes.
HbF at Baseline in MSH Patients and Comparison Group
| Haplotype . | HbF (Comparison) . | HbF (MSH) . | P . |
|---|---|---|---|
| BEN/BEN | 7.1 ± 4.5 | 4.9 ± 3.2 | <.001 |
| BEN/CAR | 6.9 ± 5.0 | 4.7 ± 3.6 | <.001 |
| SEN/BEN | 9.6 ± 5.5 | 6.1 ± 3.8 | <.001 |
| Haplotype . | HbF (Comparison) . | HbF (MSH) . | P . |
|---|---|---|---|
| BEN/BEN | 7.1 ± 4.5 | 4.9 ± 3.2 | <.001 |
| BEN/CAR | 6.9 ± 5.0 | 4.7 ± 3.6 | <.001 |
| SEN/BEN | 9.6 ± 5.5 | 6.1 ± 3.8 | <.001 |
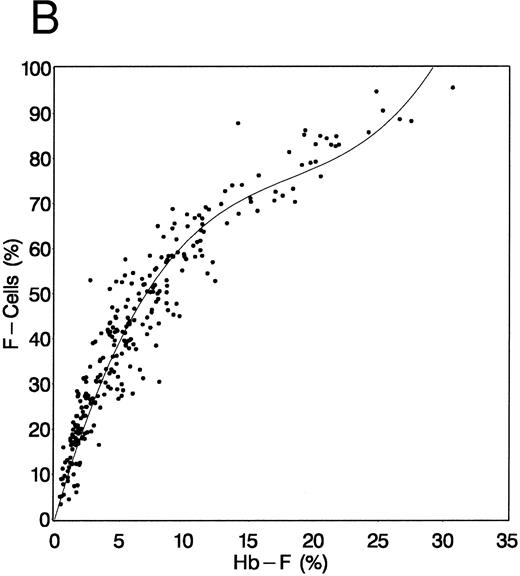
HbF and F-cell responses at 24 months.Comparisons of baseline and 2-year levels of HbF (A, placebo group; B, HU group) and F cells (C, placebo group; D, HU group) are shown in Fig 2. In HU-treated — but not in placebo-assigned patients — HbF and F-cell levels after treatment are increased from baseline (2-year mean change in HbF, 3.6% ± 5.4% v −0.4% ± 2.0% in placebo; P < .001). Some patients assigned to HU had similar HbF and percent F cells before and after treatment, although many of these individuals had increased F cells early during their follow-up evaluation; others had a sustained increase in these measurements.
Baseline and 2-year HbF (A and B) and F-cell (C and D) values in placebo-assigned and HU-treated patients. Baseline values (X-axis) and 2-year values (Y-axis) along with their regression lines are shown for the HU-assigned (B and D) and placebo-assigned (A and C) groups. Only in the HU-assigned patients is there an increase in HbF (B) and F cells (D).
Baseline and 2-year HbF (A and B) and F-cell (C and D) values in placebo-assigned and HU-treated patients. Baseline values (X-axis) and 2-year values (Y-axis) along with their regression lines are shown for the HU-assigned (B and D) and placebo-assigned (A and C) groups. Only in the HU-assigned patients is there an increase in HbF (B) and F cells (D).
To determine if there were baseline characteristics that were predictive of the HbF and F-cell response at the conclusion of treatment, we examined age, gender, and baseline laboratory values according to quartiles of HbF change at 2 years of observation. These results are shown in Table 4. At baseline, all quartiles had similar HbF levels. Adding the 2-year change to the baseline level, HbF increased to a mean of 18.1% (11.7% increase) in quartile 4 (n = 35) and to 8.8% (3.7% increase) in quartile 3 (n = 36), but changed little in quartiles 1 and 2 (n = 34 and 38). At 2 years, HbF levels were unchanged in patients assigned to placebo (Fig 2A). HbF change was not associated with age, gender, or the FCP locus phenotype. Quartiles 3 and 4 tended to have shorter time to myelosuppression (P < .001), higher percent positive HU serum assay (P = .011) and capsule count adherence (P = .015), more patients on a 15- to 22.5-mg/kg dose of HU (P < .001), higher baseline neutrophil (P = .017) and reticulocyte counts (P < .001), and fewer patients with one or two Central African Republic (CAR) haplotype chromosomes (P = .014) (Tables 4 and 5). Individuals with the largest increments in HbF had the greatest increases in total hemoglobin and MCV and greatest decreases in neutrophils and reticulocytes.
Baseline and Two-Year Change in Laboratory Determinations by Two-Year HbF Quartile
| Variable . | HU Total . | HU Quartile 1 (n = 34) . | HU Quartile 2 (n = 38) . | HU Quartile 3 (n = 36) . | HU Quartile 4 (n = 35) . | Placebo . |
|---|---|---|---|---|---|---|
| . | (n = 143) . | . | . | . | . | (n = 135) . |
| CAR haplotype (%) | 41 | 45 | 28 | 20 | ||
| HbF (%) | 5.1 (3.5) | 5.4 (3.2) | 3.6 (2.4) | 5.1 (4.0) | 6.4 (3.8) | 5.2 (3.6) |
| Δ2-yr | 3.6 (5.4) | −1.5 (1.1) | 0.6 (0.6) | 3.7 (1.4) | 11.7 (3.8) | −0.4 (2.0) |
| F cells (%) | 33.0 (16.7) | 33.9 (15.4) | 25.9 (13.2) | 33.5 (19.9) | 39.6 (15.6) | 32.8 (16.9) |
| Δ2-yr | 15.2 (17.3) | −1.1 (9.3) | 8.1 (9.2) | 17.7 (11.7) | 36.2 (12.0) | 2.3 (7.1) |
| F reticulocytes | 14.7 (8.0) | 15.6 (9.1) | 13.1 (7.0) | 13.1 (7.4) | 17.2 (7.9) | 14.7 (7.6) |
| Δ2-yr | 2.7 (5.7) | −0.6 (2.5) | 0.4 (2.6) | 3.0 (3.9) | 8.2 (7.8) | −0.1 (2.4) |
| MCV (fl) | 93.6 (9.3) | 94.0 (8.7) | 92.8 (11.2) | 92.3 (9.7) | 95.3 (7.1) | 93.3 (8.6) |
| Δ2-yr | 9.7 (11.2) | −1.4 (5.2) | 4.0 (7.1) | 13.3 (6.7) | 22.8 (7.0) | −0.4 (4.8) |
| Neutrophils4-150 | 6.8 (2.3) | 5.9 (1.8) | 6.9 (2.4) | 6.7 (1.8) | 7.7 (2.6) | 6.7 (2.3) |
| Δ2-yr | −1.9 (2.4) | −0.3 (1.7) | −1.0 (2.0) | −2.2 (1.5) | −4.1 (2.3) | −0.4 (2.2) |
| Reticulocytes4-151 | 327 (100) | 316 (120) | 322 (96) | 306 (104) | 367 (64) | 324 (94) |
| Δ2-yr | −97 (107) | −37 (59) | −37 (85) | −95 (81) | −223 (73) | −21 (72) |
| Variable . | HU Total . | HU Quartile 1 (n = 34) . | HU Quartile 2 (n = 38) . | HU Quartile 3 (n = 36) . | HU Quartile 4 (n = 35) . | Placebo . |
|---|---|---|---|---|---|---|
| . | (n = 143) . | . | . | . | . | (n = 135) . |
| CAR haplotype (%) | 41 | 45 | 28 | 20 | ||
| HbF (%) | 5.1 (3.5) | 5.4 (3.2) | 3.6 (2.4) | 5.1 (4.0) | 6.4 (3.8) | 5.2 (3.6) |
| Δ2-yr | 3.6 (5.4) | −1.5 (1.1) | 0.6 (0.6) | 3.7 (1.4) | 11.7 (3.8) | −0.4 (2.0) |
| F cells (%) | 33.0 (16.7) | 33.9 (15.4) | 25.9 (13.2) | 33.5 (19.9) | 39.6 (15.6) | 32.8 (16.9) |
| Δ2-yr | 15.2 (17.3) | −1.1 (9.3) | 8.1 (9.2) | 17.7 (11.7) | 36.2 (12.0) | 2.3 (7.1) |
| F reticulocytes | 14.7 (8.0) | 15.6 (9.1) | 13.1 (7.0) | 13.1 (7.4) | 17.2 (7.9) | 14.7 (7.6) |
| Δ2-yr | 2.7 (5.7) | −0.6 (2.5) | 0.4 (2.6) | 3.0 (3.9) | 8.2 (7.8) | −0.1 (2.4) |
| MCV (fl) | 93.6 (9.3) | 94.0 (8.7) | 92.8 (11.2) | 92.3 (9.7) | 95.3 (7.1) | 93.3 (8.6) |
| Δ2-yr | 9.7 (11.2) | −1.4 (5.2) | 4.0 (7.1) | 13.3 (6.7) | 22.8 (7.0) | −0.4 (4.8) |
| Neutrophils4-150 | 6.8 (2.3) | 5.9 (1.8) | 6.9 (2.4) | 6.7 (1.8) | 7.7 (2.6) | 6.7 (2.3) |
| Δ2-yr | −1.9 (2.4) | −0.3 (1.7) | −1.0 (2.0) | −2.2 (1.5) | −4.1 (2.3) | −0.4 (2.2) |
| Reticulocytes4-151 | 327 (100) | 316 (120) | 322 (96) | 306 (104) | 367 (64) | 324 (94) |
| Δ2-yr | −97 (107) | −37 (59) | −37 (85) | −95 (81) | −223 (73) | −21 (72) |
Values are means (±1 SD) of the baseline value (top) and 2-year posttreatment change (Δ2-yr) (bottom) grouped according to the quartile of change of HbF at 2 years of treatment. There were no age differences among the 4 quartiles.
Neutrophil count × 103/μL.
Reticulocytes × 103/μL.
Toxicity, Dose of HU, Adherence and Two-Year Crisis Rate by HbF Quartile After Two Years of Treatment
| Variable . | Quartile 1 (n = 34) . | Quartile 2 (n = 38) . | Quartile 3 (n = 36) . | Quartile 4 (n = 35) . |
|---|---|---|---|---|
| Toxic > 2 | 47 | 58 | 86 | 94 |
| HU dose (mg/kg)5-150 | % | |||
| <15 | 50 | 34 | 50 | 12 |
| 15-22.5 | 6 | 13 | 22 | 66 |
| 25-32.5 | 9 | 16 | 20 | 20 |
| 35 | 35 | 37 | 8 | 3 |
| ≥80% Visits5-151 | 85 | 90 | 97 | 94 |
| ≥80% Caps5-152 | 56 | 71 | 81 | 83 |
| ≥50% HUρ | 14 | 24 | 28 | 46 |
| Crises/yr5-154 | 6.5 ± 5.9 | 7.2 ± 10.1 | 3.1 ± 5.2 | 2.3 ± 4.5 |
| Variable . | Quartile 1 (n = 34) . | Quartile 2 (n = 38) . | Quartile 3 (n = 36) . | Quartile 4 (n = 35) . |
|---|---|---|---|---|
| Toxic > 2 | 47 | 58 | 86 | 94 |
| HU dose (mg/kg)5-150 | % | |||
| <15 | 50 | 34 | 50 | 12 |
| 15-22.5 | 6 | 13 | 22 | 66 |
| 25-32.5 | 9 | 16 | 20 | 20 |
| 35 | 35 | 37 | 8 | 3 |
| ≥80% Visits5-151 | 85 | 90 | 97 | 94 |
| ≥80% Caps5-152 | 56 | 71 | 81 | 83 |
| ≥50% HUρ | 14 | 24 | 28 | 46 |
| Crises/yr5-154 | 6.5 ± 5.9 | 7.2 ± 10.1 | 3.1 ± 5.2 | 2.3 ± 4.5 |
Toxicity was defined as a neutrophil count < 2,000/μL, reticulocyte count < 80,000/μL, platelet count < 80,000/μL, or hemoglobin concentration < 4.5 g/dL.
Percent of patients on a given dose of HU.
≥80% Visits indicates that ≥80% of scheduled clinic visits were kept.
≥80% Caps indicates that ≥80% of the prescribed HU capsules were taken.
ρ Indicates that HU was present in the serum on ≥50% of HU serum assays.
Crises per year expressed as the 2-year crisis rate ±1 SD.
Patients in quartile 1 formed a heterogeneous group of individuals, some of whom were not maintained on treatment according to the protocol (Table 5). In some, treatment was stopped because of refusal to continue (3 patients), pregnancy (2), toxicity on three occasions at a HU dose of 2.5 mg/kg/d (3), chronic transfusion (2), and fulminant hepatitis (1). Twelve patients (35%) in quartile 1 were prescribed 35 mg/kg, the highest dose of HU allowed. Of these 12 patients, 11 were “toxic” twice or more and one was “toxic” once; 10 had positive HU serum assays only 10% to 55% of the time, and 11 still were not on a stable dose after 2 years. Fifty percent of patients in this quartile received less than 15 mg/kg. This quartile adhered least to the study protocol as suggested by the counts of capsules taken, numbers of positive serum HU assay results, and low frequency of toxic blood counts (Table 5). In this heterogeneous group of patients, one third were not prescribed continuing HU because of restrictions imposed by the study design. Some individuals in quartile 1 followed the treatment protocol, but became “toxic” on low doses of HU. Others were given increasing doses up to 35 mg/kg and experienced toxicity, but only a limited HbF increase. The unusual distribution of HU doses in quartiles 1 and 4 suggest that intermediate doses suffice for patients with the greatest HbF increases.
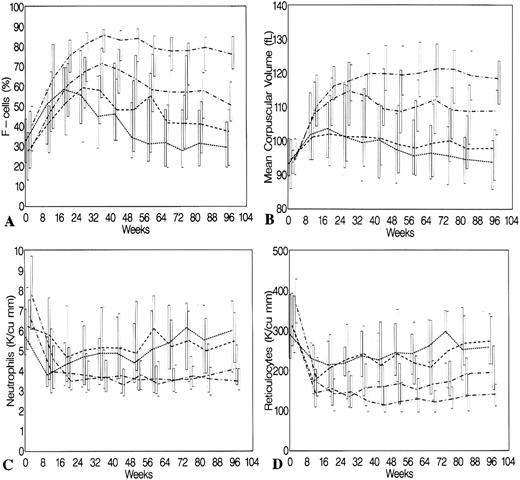
Of particular interest, but not a primary goal of this study, is the relationship between HbF or F-cell increments and painful episodes. The pain crisis rate in the four quartiles of HbF response are shown in Table 5. There is an inverse relationship between HbF response and the 2-year crisis rate (Spearman r = .416, P = .07). Response to HU as measured by HbF is highly related to response in MCV, neutrophils, and reticulocytes (Fig 3).
Changes in F cells (A), MCV (B), neutrophil count (C), and reticulocyte count (D) during 2 years of treatment with HU according to the quartile of percent change in HbF at 2 years. Boxes indicate the 75th and 25th percentiles, which are connected at the median values. (•• — • •) fourth quartile; ( — • — ) third quartile; (––––) second quartile ( — — — — ); first quartile (……..).
Changes in F cells (A), MCV (B), neutrophil count (C), and reticulocyte count (D) during 2 years of treatment with HU according to the quartile of percent change in HbF at 2 years. Boxes indicate the 75th and 25th percentiles, which are connected at the median values. (•• — • •) fourth quartile; ( — • — ) third quartile; (––––) second quartile ( — — — — ); first quartile (……..).
An analysis of change of HbF according to the baseline and follow-up characteristics is shown in Table 6. Patients who did not have a CAR haplotype had a mean change in HbF of 4.3% ± 5.6% compared with 2.3% ± 4.8% for patients with at least one CAR haplotype (P = .04; treatment × CAR interaction, P = .05); patients with reticulocyte counts greater than 300 × 103/μL had a mean change in HbF of 4.8% ± 6.0% versus 1.8% ± 3.8% for patients with lower reticulocyte counts (P < .001; treatment × reticulocyte interaction, P < .001); patients with at least 7,500 neutrophils/μL had a mean change in HbF of 5.3% ± 6.3% versus 2.7% ± 4.8% for patients with lower neutrophil counts (P = .006; treatment × neutrophil interaction, P = .014).
Two-Year HbF Change by Baseline and Follow-Up Characteristics
| . | Hydroxyurea . | Placebo . | Int P . | |||||
|---|---|---|---|---|---|---|---|---|
| . | n . | Mean . | SD . | P . | n . | Mean . | SD . | . |
| Overall | 143 | 3.6 | 5.4 | 135 | −0.4 | 2.0 | — | |
| Baseline | ||||||||
| Male | 74 | 3.0 | 4.8 | 71 | −0.4 | 2.5 | ||
| Female | 69 | 4.1 | 5.9 | .22 | 64 | −0.4 | 4.1 | .27 |
| 0 CAR6-150 | 95 | 4.3 | 5.6 | 96 | −0.4 | 1.8 | ||
| 1-2 CAR | 48 | 2.3 | 4.8 | .04 | 39 | −0.3 | 2.6 | .05 |
| L,L/H FCP6-150 | 130 | 3.4 | 5.5 | 127 | −0.3 | 2.1 | ||
| H/H FCP6-151 | 13 | 4.7 | 5.4 | .43 | 8 | −1.1 | 1.2 | .27 |
| Retic < 300K6-152 | 58 | 1.8 | 3.8 | 59 | 0.0 | 2.1 | ||
| Retic ≥ 300K | 85 | 4.8 | 6.0 | .001 | 77 | −0.7 | 2.0 | .0001 |
| Neuts < 7.5Kρ | 94 | 2.7 | 4.8 | 89 | −0.4 | 2.2 | ||
| Neuts ≥ 7.5K | 49 | 5.3 | 6.3 | .006 | 47 | −0.4 | 1.8 | .01 |
| HbF < 7.5% | 116 | 3.2 | 5.2 | 107 | −0.3 | 1.5 | ||
| HbF ≥ 7.5% | 27 | 5.3 | 6.2 | .07 | 29 | −0.9 | 3.4 | .03 |
| Follow-up | ||||||||
| <80% Adh6-154 | 39 | 2.0 | 5.0 | 42 | −0.3 | 2.0 | ||
| ≥80% Adh | 104 | 4.2 | 5.5 | .03 | 93 | −0.4 | 2.1 | .02 |
| 0-1 toxic6-155 | 41 | 0.6 | 3.8 | 111 | −0.4 | 2.2 | ||
| 2+ toxic | 112 | 4.8 | 5.5 | .0001 | 24 | −0.5 | 1.5 | .0002 |
| . | Hydroxyurea . | Placebo . | Int P . | |||||
|---|---|---|---|---|---|---|---|---|
| . | n . | Mean . | SD . | P . | n . | Mean . | SD . | . |
| Overall | 143 | 3.6 | 5.4 | 135 | −0.4 | 2.0 | — | |
| Baseline | ||||||||
| Male | 74 | 3.0 | 4.8 | 71 | −0.4 | 2.5 | ||
| Female | 69 | 4.1 | 5.9 | .22 | 64 | −0.4 | 4.1 | .27 |
| 0 CAR6-150 | 95 | 4.3 | 5.6 | 96 | −0.4 | 1.8 | ||
| 1-2 CAR | 48 | 2.3 | 4.8 | .04 | 39 | −0.3 | 2.6 | .05 |
| L,L/H FCP6-150 | 130 | 3.4 | 5.5 | 127 | −0.3 | 2.1 | ||
| H/H FCP6-151 | 13 | 4.7 | 5.4 | .43 | 8 | −1.1 | 1.2 | .27 |
| Retic < 300K6-152 | 58 | 1.8 | 3.8 | 59 | 0.0 | 2.1 | ||
| Retic ≥ 300K | 85 | 4.8 | 6.0 | .001 | 77 | −0.7 | 2.0 | .0001 |
| Neuts < 7.5Kρ | 94 | 2.7 | 4.8 | 89 | −0.4 | 2.2 | ||
| Neuts ≥ 7.5K | 49 | 5.3 | 6.3 | .006 | 47 | −0.4 | 1.8 | .01 |
| HbF < 7.5% | 116 | 3.2 | 5.2 | 107 | −0.3 | 1.5 | ||
| HbF ≥ 7.5% | 27 | 5.3 | 6.2 | .07 | 29 | −0.9 | 3.4 | .03 |
| Follow-up | ||||||||
| <80% Adh6-154 | 39 | 2.0 | 5.0 | 42 | −0.3 | 2.0 | ||
| ≥80% Adh | 104 | 4.2 | 5.5 | .03 | 93 | −0.4 | 2.1 | .02 |
| 0-1 toxic6-155 | 41 | 0.6 | 3.8 | 111 | −0.4 | 2.2 | ||
| 2+ toxic | 112 | 4.8 | 5.5 | .0001 | 24 | −0.5 | 1.5 | .0002 |
Mean and SD of the 2-year change in HbF are shown according to cross-classifications of treatment assignment group and the baseline and follow-up characteristics. The mean HbF is tested between the 2 levels of each baseline or follow-up characteristic for the HU and placebo group (P). The interaction (Int) P value tests whether the difference between HU and placebo is the same in both levels of the baseline or follow-up characteristic.
L,L/H, homozygotes, hemizygotes, and heterozygotes for the L or low phenotype.
H/H, homozygotes and hemizygotes for the H or high phenotype.
Reticulocyte count < or ≥300,000/μL.
ρ Neutrophil count < or ≥7,500/μL.
Less than or ≥80% capsule count adherence.
One or no episodes of toxicity or two or more toxic episodes.
Among follow-up characteristics, patients with ≥80% adherence had a 4.2% ± 5.5% mean increase in HbF versus 2.02% ± 5.0% in individuals with less than 80% adherence. Patients with toxic counts two or more times had changes in HbF of 4.62% ± 5.5% versus 0.62% ± 3.8% for patients with no more than one episode of toxicity (P < .001).
Longitudinal studies.The distributions of levels of F cells, MCV, neutrophil counts, and reticulocyte counts at 8-week intervals for the four quartiles of HbF response are shown in Fig 3. In the placebo group, the distributions at each time point for MCV and neutrophil count are similar to the distribution at baseline. However, there was an early and sustained increase in F cells from baseline in patients assigned to placebo, with a mean increase of 5% by 20 weeks, 8% by 36 weeks, and then a slight decline, although to levels above baseline (P = .001 at all time points through 2 years [global P < .001]). Also in the placebo group, reticulocytes decreased over 2 years by 5 × 103 to 20 × 103/μL (global P < .001). HU-treated patients showed substantial increases compared with placebo-assigned patients in F cells and MCV, and substantial decreases in reticulocyte count and neutrophil count over 2 years (all global tests; P < .001). HU-treated patients had an increase in F cells at 32 weeks of treatment (64.9 ± 20.5) compared with placebo patients (39.6 ± 20.8) (Fig 3). Using the conversion formula, this would equate to a HbF difference between these groups of 10.9% ± 2.7% versus 5.2% ± 2.7%.
Comparing quartiles 2, 3, and 4 of HbF change to quartile 1 as a reference group, quartile 2 F cells diverged from quartile 1 at 52 weeks and remained higher for the duration of observation (P = .0001). Patients in quartiles 2, 3, and 4 attained earlier, more pronounced, and more sustained increases in F cells compared with quartile 1.
Adherence.Individuals with the best HbF response were “toxic” more often and earlier than patients whose HbF level increased little (Table 5). Patients in quartile 1 completed fewer clinic visits than those in quartiles 2, 3, and 4. Eighty-one percent and 83% of patients in quartiles 3 and 4 were reported to be taking ≥80% of their prescribed HU capsules compared with 56% and 71% of individuals in quartiles 1 and 2. Serum HU assays were positive ≥50% of the time in 46% of quartile 4 patients, but in only 14% of individuals in quartile 1.
Sixty-six percent of patients in quartile 4 received 15 to 22.5 mg/kg of HU as their last dose of drug, compared with 6%, 13%, and 22% of quartiles 1, 2, and 3. Only 12% and 3% of patients in quartile 4 received the lowest and highest HU doses, compared with 50% and 35% of patients in quartile 1 (Table 5).
DISCUSSION
These studies were initiated to provide a detailed description of the variation of HbF level after treatment with HU, to examine how changes in HbF were modulated, and to find out which patients were likely to have sustained increments in HbF. We focused on (1) genetic elements that might regulate, or mark, regions that regulate γ-globin gene expression, such as the FCP locus and haplotypes of the β- and α-globin gene clusters; (2) patient characteristics such as age, gender, and baseline hematologic measurements; and (3) follow-up characteristics, including adherence to therapy, occurrence of toxicity, and the dose of HU. Patients enrolled in the MSH had to meet eligibility requirements that limited participants to adults with moderate to severe disease (three or more pain crises per year). Because of that selection and since the sample size was relatively small, suggestive trends observed between MSH laboratory measurements and possible predictors of HbF response in the present analysis are not necessarily the same as what might be found in the broader HbSS population. Because HU or placebo were randomly assigned, changes in laboratory measurements can be causally attributed to the action of HU, as can crisis rates. However, because of our experimental design, proving a direct association between laboratory changes — one outcome — and crisis rate — another outcome, is more problematical. An analysis of the observed relationship of crisis rate with laboratory changes and patient characteristics will be reported separately.34
Consonant with prior studies, we found that HU administration was associated with some increase in HbF levels in most patients with HbSS. Most patients had an initial substantial increase in F cells. At 32 weeks of observation, F cells were nearly 65% in HU patients, compared with 40% in controls (Fig 3). The increases in HbF level at 2 years were greatest in patients with baseline reticulocyte counts ≥ 300 × 103/μL, neutrophil counts ≥ 7,500/μL, and two or more episodes of study-defined myelotoxicity. Also, related to the HbF response was ≥ 80% adherence based on capsule counts and the absence of a CAR haplotype. However, after 2 years, half our patients had no increase, or only a trivial increment in HbF. This could be due to variation in genetic elements that influence HbF production, a bone marrow insufficient to weather the stress of HU as we used it, poor adherence to the treatment regimen, our method of dose titration, or differences in drug bioavailabilty and metabolism among our patients. Our data do not allow us to distinguish among these possibilities.
Genetic variability among patients with HbSS came into focus when it was possible to discriminate among different chromosomes containing the HbS mutation.7,8,35-37 In HbSS, three haplotypes — Benin, CAR, and Senegal — are predominant. Cameroon and atypical haplotypes are less frequent.3,7,37 Studies of the β-globin gene cluster haplotype in adult HbSS showed that haplotype interacted with gender in the modulation of HbF levels and magnitude of anemia.3 The moderate to severely affected patients who participated in the MSH had a distribution of haplotypes similar to that reported in another large North American study of HbSS (Table 2).3 They were not more likely to have a CAR haplotype — previously associated with severe disease complications38-41 — or less likely to have the Senegal haplotype that has been associated with a more benign course.7 Suggesting that cis-acting elements modulate, in part, the HbF response to HU, was the trend in CAR haplotype prevalence in the lower quartiles of HbF response (Table 4).
Baseline HbF levels were lower in MSH patients than in a previously described HbSS population (Table 3). However, as HbF was measured by different methods and in different laboratories for these two populations, this observation should be interpreted with caution. While the distribution of haplotypes in the MSH and in the previously described population — one selected for disease severity and the other likely to have a more representative disease spectrum — were similar, the differences in HbF levels suggest that some γ-globin gene regulation is not linked to the β-globin gene haplotype. FCP locus phenotype was a determinant of baseline HbF levels, but did not predict the HbF response to HU. In earlier studies of a small number of patients, it was suggested that individuals with low baseline HbF and F-reticulocyte levels were less likely to have an increase in HbF,16 an observation we could not confirm. While baseline HbF, F-cells, and F-reticulocyte counts were all slightly higher in the highest quartile of change in HbF, these differences were not significant (Table 4).
Myelosuppression — perhaps a prerequisite for HU to increase HbF — is determined by an interplay between the myelotoxic effects of the drug and the proliferative capacity of the bone marrow. In our analyses, patients with the highest baseline granulocyte and reticulocyte counts — who also had the largest decreases in these counts during treatment — had the greatest increases in HbF. Previously, the initial leukocyte count and change in leukocytes with treatment were also determinants of the final HbF level after treatment with HU.4
Due to eligibility requirements, our patients had high annual crisis rates. Crisis rate is inversely proportional to HbF level.1 The observed increases in HbF levels and decreases in reticulocyte counts in placebo-assigned patients may represent a regression to the mean. Other laboratory measures in the placebo group, including hemoglobin concentration and neutrophil counts, varied little across time.
Most patients responded initially to HU with increased numbers of F cells, but there was a divergence between patients who were long-term responders and those who did not sustain an F-cell response to treatment (Fig 3). Among patients with little change in final HbF level, neutrophil and reticulocyte counts returned toward baseline and percent F cells decreased. Patients in quartiles 1 and 2 had early changes in F cells. The changes in quartile 2 were different from placebo-assigned patients throughout the observation period, while in quartile 1, differences from the placebo group were only present for 1 year, suggesting that these early, and possibly important responses, could not be maintained. Blood cell counts decrease with aging in HbSS, an observation that may reflect cumulative vasoocclusive damage to the bone marrow.42 43 Perhaps, because of marrow scarring, some patients are unable to tolerate continual myelosuppressive doses of HU.
We propose that baseline HbF levels are influenced by the FCP locus, elements linked to the β-globin gene-cluster haplotype, age and gender, and other undefined factors. The ability to respond to HU is dependent on bone marrow “reserve,” defined operationally as the capacity of the marrow to withstand moderate doses of HU with acceptable myelotoxicity. Baseline reticulocyte and neutrophil counts may reflect marrow “reserve.” Sustained HbF increases during HU treatment can occur in individuals with bone marrow “reserve” sufficient to cope with the myelotoxicity of this agent.
Because crisis rate is inversely associated with F-cell response (Table 5) and F-cell response is associated with response in MCV, neutrophils, reticulocytes, and possibly other parameters, crisis rate is not necessarily attributable only to HbF changes. These data are discussed in detail in another report.34 It is possible that HU may have therapeutic effects in HbSS beyond its influence on HbF.44-47
As we used HU, early F-cell response occurred in nearly all patients, but substantial HbF increases were maintained in half of our patients. In these individuals, HbF responses to HU are likely to be clinically important (Table 5). In earlier studies, some patients did not respond to treatment or had a minor increase in HbF. However, the dosing regimens, final doses achieved, and length of treatment in these studies differed from ours.4,5 Other drug regimens — for example, pulse therapy,5 the addition of hematopoietic growth factors to HU,48 49 or beginning HU at younger ages when marrow damage is likely to be less severe — should be further investigated.
Waldenström's macroglobulinemia. Dense fibrillar and globular material (arrows) fills dilated cisternae of rough endoplasmic reticulum in a bone marrow plasma cell. By light microscopic immunoperoxidase, these inclusions contained IgM-κ, the same monoclonal Ig that was detected in the patient's serum. N, nucleus; M, mitochondria. (Original magnification × 12,500.) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
Waldenström's macroglobulinemia. Dense fibrillar and globular material (arrows) fills dilated cisternae of rough endoplasmic reticulum in a bone marrow plasma cell. By light microscopic immunoperoxidase, these inclusions contained IgM-κ, the same monoclonal Ig that was detected in the patient's serum. N, nucleus; M, mitochondria. (Original magnification × 12,500.) (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Hospital, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215.)
ACKNOWLEDGMENT
We thank the Technicon Division of Bayer Pharmaceuticals for the generous loan of an H*1 blood cell counter.
APPENDIX
Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia
1. Clinical Centers
University of North Carolina, Chapel Hill, NC: E. Orringer, S. Jones, D. Strayhorn (19). Duke University, Durham, NC: W. Rosse, G. Phillips (deceased), D. Peace, A. Johnson-Telfair (16). Medical College of Georgia, Augusta, GA: P. Milner, A. Kutlar, A. Tracy (15). Thomas Jefferson University, Philadelphia, PA: S.K. Ballas, G.E. Allen, J. Moshang, B. Scott (21 ). University of Mississippi, Jackson, MS: M. Steinberg, A. Anderson, V. Sabahi (19). University of Miami, Miami, FL: C. Pegelow, D. Temple, E. Case, R. Harrell, S. Childerie (12). San Francisco General Hospital, San Francisco, CA: S. Embury, B. Schmidt, D. Davies (6). University of Illinois, Chicago, IL: M. Koshy, N. Talischy-Zahed, L. Dorn, G. Pendarvis, M. McGee (57). Michael Reese Hospital, Chicago, IL: M. Telfer, A. Davis (11). Howard University, Washington, DC: O. Castro, H. Finke, E. Perlin, J. Siteman (20). University of Medicine and Dentistry of New Jersey, Newark, NJ: P. Gascon, P. di Paolo, S. Gargiulo (10). Emory University, Atlanta, GA: J. Eckman, J. Howard Bailey, A. Platt, L. Waller (14). St Luke's-Roosevelt Medical Center, New York, NY: G. Ramirez, V. Knors, S. Hernandez, E.M. Rodriguez, E. Wilkes (18). Children's Hospital of Oakland, Oakland, CA: E. Vichinsky, S. Claster, A. Earles, K. Kleman, K. McLaughlin (5). Medical College of Virginia, Richmond, VA: P. Swerdlow, W. Smith, B. Maddox, L. Usry, A. Brenner, K. Williams, R. O'Brien, K. Genther (19). Case-Western Reserve University, Cleveland, OH: S. Shurin, B. Berman, K. Chiarucci, L. Keverline (5). Hospital for Sick Children, Toronto, Ontario: N. Olivieri, D. Shaw, N. Lewis (6). Brigham and Women's Hospital, Boston, MA: K. Bridges, B. Tynan, C. Winograd (5). Interfaith Medical Center, Brooklyn, NY: R. Bellevue, H. Dosik, M. Sheikhai, P. Ryans, H. Souffrant (8). University of Alabama, Birmingham, AL: J. Prchal, J. Braddock, T. MCardle (8). University of Pittsburgh, Pittsburgh, PA: T. Carlos, A. Schmotzer, D. Gardner (5).
Numbers in parenthesis represent numbers of patients enrolled in the study. Dr Olivieri is a Career Scientist of the Ontario Ministry of Health.
2. Central Office Staff (Johns Hopkins University, Baltimore, MD)
Principal Investigator: Samuel Charache, MD; Deputy Principal Investigator: Richard Moore, MD; Coinvestigator: George Dover, MD; Director, Treatment Distribution Center: Richard Moore, MD; Director, Core Laboratory: George Dover, MD; Director, DNA Laboratory: Martin Steinberg, MD; Health Psychologist: Marilyn Bergner (deceased), Craig Ewart, PhD; Study Coordinator: Susan Eckert; Budget Analyst: Carol Lent, Joanne Ullrich; Assistant Coordinator: Laura Fishpaw, Gema Tirado; Systems Analyst: J. Ibson; Technical Staff: Timothy Moeller, Tina Nagel.
3. Data Coordinating Center (Maryland Medical Research Institute, Baltimore, MD)
Principal lnvestigator: Michael Terrin, MD; Deputy Director: Franca B. Barton MS (Hyg); Senior Biostatistician: Robert P. McMahon, PhD; Systems Analyst: Carol Handy; Coordinators: Dorothy Harris, Martha Canner, MS, Joyce Depkin, MA, Nancy Meinert, RD; Computing Staff: Margie Carroll, Rose Giro, Susan Karabelas, Cheryl Kelly.
4. Crisis Review Committee (*member since beginning of study)
Active Members: Meyer Heyman, MD (Chair), Peter Beilinson, MD, Malcolm Druskin, MD, Peter Ellis, MD, William A. Flood, MD, Sheldon Kravitz, MD, Sophie Lanzkron, MD, Victor Lorica, MD, Alison Moliterno, MD, Albert Nahum, MD, John A. Nesbitt III, MD, Lawrence Rosenthal, MD, PhD, William Sharfman, MD, Michael Streiff, MD, Matthew Wachsman, MD, PhD; Former Members: Paul Bray, MD, Chi Van Dang, MD, James Casella, MD, Maura McGuire, MD, Lisa Patrick, MD, Heinz Schaad, MD, Claudia Steiner, MD.
5. Data and Safety Monitoring Board
Cage Johnson, MD (Chair), Arthur Bank, MD, Gary Cutter, PhD, Edward Davis, PhD, Ola Huntley, EdD, Lawrence Lessin, MD, Orah Platt, MD, Marian Gray Secundy, ACSW, PhD.
6. Project Office (National Heart, Lung, and Blood Institute, Bethesda, MD)
Duane Bonds, MD (Project Officer), Clarice Reid, MD, Nancy Geller, PhD, Myron Waclawiw, PhD.
Supported by National Heart, Lung and Blood Institute Cooperative Agreements No. UO1-HL 45692 and UO1-HL 45696, National Institutes of Health GCRC Grants No. RR 00046, RR 00065, and RR 00083, Research Funds of the Department of Veterans Affairs, and a grant from Bristol Myers Squibb.
Presented in part at the annual meeting of the American Society of Hematology, Seattle, WA, December 2-5, 1995, and published in abstract form (Blood 86:418a, 1995).
Address reprint requests to Martin H. Steinberg, MD, Associate Chief of Staff for Research, VA Medical Center, 1500 E Woodrow Wilson Dr, Jackson, MS 39216.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal