Abstract
The existence of T cells capable of inhibiting in vitro hematopoiesis has been shown in aplastic anemia (AA), although whether such inhibition is mediated by a specific immune reaction involving an HLA allele remained unknown. We isolated a CD4+ Vβ21+ T-cell clone that was most dominant among Vβ21+ T cells in the bone marrow (BM) of an AA patient whose HLA-DRB1 alleles included 1501 and 0405. The T-cell clone named NT4.2 lysed an autologous Epstein-Barr virus-transformed lymphoblastoid cell line (LCL) and phytohemagglutinin-stimulated lymphocytes (PHA-blasts) as well as allogeneic LCLs sharing HLA-DRB1*0405. Cytotoxicity against LCL cells and PHA-blasts by NT4.2 was blocked by anti–HLA-DR monoclonal antibody (MoAb) or anti-CD3 MoAb. NT4.2 also lysed autologous BM mononuclear cells enriched with CD34+ cells that had been cultured for one week in the presence of colony-stimulating factors as well as allogeneic CD34+ cells of a normal individual carrying HLA-DRB1*0405, cultured in the same way. Moreover, NT4.2 strongly inhibited colony formation by hematopoietic progenitor cells derived from cultured CD34+ cells sharing HLA-DRB1*0405. These results indicate that the AA patient has T cells capable of killing hematopoietic cells in an HLA-DRB1*0405-restricted manner and that such cytotoxic T cells may contribute to the pathogenesis of AA.
TWENTY-FIVE YEARS AGO an immune mechanism was implicated in the development of aplastic anemia (AA) by Mathe et al.1 Since then, clinical evidence supporting immune mechanism(s) has accumulated.2 Recent reports of markedly high response rates to combined immunosuppressive therapy in patients with AA suggest that bone marrow (BM) failure in the majority of AA patients may be immune mediated.3,4 Various findings have been reported to account for immune-mediated suppression of hematopoiesis in AA in vitro. These include inhibition of hematopoietic progenitor cell growth by patient lymphocytes,5,6 overproduction of myelosuppressive cytokines by patient lymphocytes,7-9 and an increased proportion of activated suppressor T cells.10-12 In addition, correlation of some laboratory findings with the response to immunosuppressive therapy in patients with AA has indicated a pathogenic role for myelosuppressive T cells in vitro.13,14 However, these may be secondary phenomena of immune reactions irrelevant in the pathogenesis of AA.15 The target of these T cells remains entirely unknown, and it is unclear what induces such autoimmunity in AA patients.
Establishing T-cell lines or clones from an involved organ and studying their specificity is a common way to identify an unknown target antigen in organ-specific autoimmune diseases and neoplasms.16,17 Some patients with immune-mediated AA may have T cells capable of recognizing hematopoietic progenitor cells and inhibiting their growth in the BM.18 19 If these T cells were isolated and stably maintained in vitro, they could facilitate identification of target antigens mediating BM failure. To test these possibilities, we established T-cell clones from the BM of an untransfused AA patient whose hematopoietic function was dependent on administration of cyclosporine. One CD4+ clone lysed autologous hematopoietic cells, including cultured lymphocytes and BM mononuclear cells (BMMC) enriched for CD34+ cells, probably via recognition of a physiological peptide presented by HLA-DRB1*0405. The T-cell clone also inhibited progenitor cell growth derived from cultured CD34+ cells in an HLA-DRB1*0405–restricted manner.
MATERIALS AND METHODS
Isolation of BM T cells and CD34+ cells from a patient with immune-mediated AA.After informed consent was obtained, BM was aspirated from a 66-year-old untransfused man with AA after he relapsed with pancytopenia following a dose reduction of cyclosporine. BM mononuclear cells (BMMC) were prepared using density-gradient centrifugation. T cells were isolated from BMMC using rosette formation with sheep red blood cells. The CD4/CD8 ratio of BM T cells was 0.74. Non-T cells were incubated for 60 minutes in plastic flasks coated with monoclonal antibodies (MoAbs) against CD34 (Applied Immune Science, Santa Clara, CA), and CD34+ cells were obtained according to the manufacturer's instructions. This CD34+ cell fraction was 77% CD34+ and 93% HLA-DR positive. Although the cell fraction comprised a considerable number of CD34− cells, the cells in this fraction are termed CD34+ cells. BMMCs were again obtained from the patient when BM was aspirated for karyotype analysis after pancytopenia improved after dose escalation of cyclosporine. CD34+ cells were isolated in the same way and cryopreserved after aliquoting. This study was approved by the institutional human research committee.
Growth of BM T cells.BM T cells were suspended in RPMI 1640 supplemented with 2 mmol/L L-glutamine, minimal essential amino acids, sodium pyruvate, and kanamycin (all from GIBCO, Grand Island, NY; complete medium) plus 10% pooled AB serum. A total of 2 × 105 cells were cultured in round-bottom plastic tubes (Falcon, Oxnard, CA) with 105 irradiated (45 Gy) autologous CD34+ cells. After 7 days, recombinant interleukin-2 (IL-2; 100 IU/mL; Shionogi, Osaka, Japan) and the same number of irradiated CD34+ cells that had been cryopreserved were added to the culture and further incubated for 7 days. After culture, the number of viable T cells was determined using trypan-blue dye exclusion. Viable T cells were diluted with complete medium supplemented with 10% pooled AB serum, 1 μg/mL phytohemagglutinin (PHA; Wellcome, Dartford, UK), and 45 Gy-irradiated allogeneic peripheral blood mononuclear cells (PBMC; 5 × 105 mL). Twenty microliters of the cell suspension was plated in microtiter wells of Terasaki plates (Nunc, Roskilde, Denmark) and cultured in 5% CO2 . Ten days later, growing cultures were transferred to flat-bottom plates and further cultured. Isolated T-cell clones were maintained by regular supplementation with complete medium containing IL-2 and occasional restimulation with 45-Gy irradiated allogeneic PBMC and PHA.
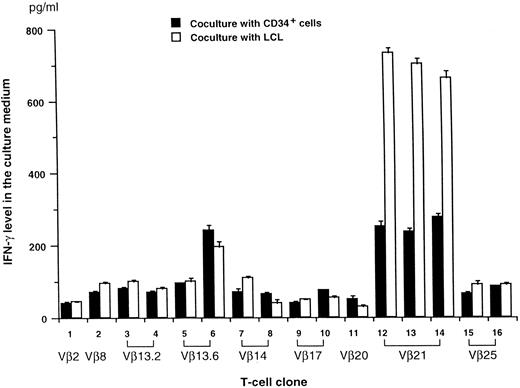
IFN-γ production by T-cell clones after coculture with auto CD34+ cells or EBV-transformed B-cell line (LCL) cells. Each T-cell clone (5 × 104 cells) was cultured with auto CD34+ cells or LCL cells (2 × 104 cells) for 20 hours in RPMI 1640 medium supplemented with 10% human AB serum and 100 IU/mL of IL2. IFN-γ concentrations in the culture supernatant (mean ± SE) were determined from duplicate cultures.
IFN-γ production by T-cell clones after coculture with auto CD34+ cells or EBV-transformed B-cell line (LCL) cells. Each T-cell clone (5 × 104 cells) was cultured with auto CD34+ cells or LCL cells (2 × 104 cells) for 20 hours in RPMI 1640 medium supplemented with 10% human AB serum and 100 IU/mL of IL2. IFN-γ concentrations in the culture supernatant (mean ± SE) were determined from duplicate cultures.
Establishment of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs).PBMC from patients with AA and normal individuals were depleted of T cells using the rosette formation method; 2 to 3 × 106 non-T cells were incubated in 10% fetal calf serum (FCS; GIBCO)-RPMI 1640 containing 10% culture medium of an EBV-producing cell line, B95-8, at 37°C for 2 hours. The EBV-infected cells were cultured for 3 weeks until transformed LCL cells grew. LCL cells were maintained by changing the medium every 4 to 5 days.
Typing for HLA-DRB1.High molecular weight DNA was extracted from leukocytes of patients or normal volunteers using a standard method. All patients gave informed consent for drawing of blood. The second exon of the DRB1 gene was amplified using the polymerase chain reaction (PCR) with specific primers, and each allele was typed using the restriction fragment length polymorphism method as described previously.20
Preparation of PHA-stimulated lymphocytes.PBMC from the patient were suspended in RPMI 1640 supplemented with 10% FCS and PHA (1 μg/mL) at a concentration of 2 × 105/mL. After 7 days, cultured lymphocytes (PHA-blasts) were washed twice with RPMI 1640 and used as target cells for a 51Cr-release assay.
Measurement of interferon-γ (IFN-γ) production by T-cell clones.Each T-cell clone (5 × 104 cells) was cultured with autologous CD34+ cells or LCL cells (2 × 104 cells) for 20 hours in complete medium supplemented with IL-2 (100 IU/mL) and 10% pooled AB serum. IFN-γ concentrations in the culture supernatant were determined from duplicate cultures using an enzyme-linked immunosorbent assay (Endogen, Cambridge, MA). The IFN-γ concentration in the culture medium of cloned T cells alone was less than 50 pg/mL.
3H-thymidine incorporation assay.A total of 5 × 104 T cells were cultured in 96-well U-bottomed plates (Nunc) with the same number of 45 Gy-irradiated autologous LCL cells. After 3 days of incubation, 1 μCi of 3H-thymidine (6.7 Ci/mmol; Dupont NEN Products, Boston, MA) was added to each well. Cultured cells were harvested after 6 hours, and 3H-thymidine incorporation was measured. The stimulation index of each T-cell clone against LCL cells was calculated from triplicate cultures by dividing incorporation against LCL cells by incorporation against autologous T cells.
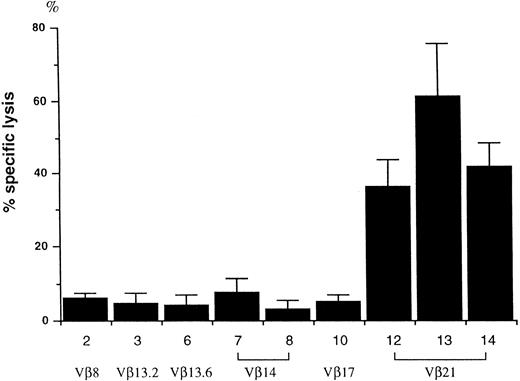
Cytotoxicity of T-cell clones against autologous LCL cells. A total of 5 × 104 cloned T cells were cultured with 5 × 10351Cr-labeled auto LCL cells for 4 hours, and 51Cr release was measured using a γ-counter. The percentage of specific lysis (mean ± SE) was determined from two separate experiments. T-cell clone numbers correspond to those in Fig 1.
Cytotoxicity of T-cell clones against autologous LCL cells. A total of 5 × 104 cloned T cells were cultured with 5 × 10351Cr-labeled auto LCL cells for 4 hours, and 51Cr release was measured using a γ-counter. The percentage of specific lysis (mean ± SE) was determined from two separate experiments. T-cell clone numbers correspond to those in Fig 1.
51Cr release assay.LCL cells, PHA-blasts, or CD34+ cells were incubated with 100 μCi of 51Cr at 37°C for 1 hour after washing with phosphate-buffered saline (PBS). Labeled cells were washed three times with PBS and suspended in complete medium supplemented with IL-2 (100 IU/mL) and 10% pooled AB serum. Cells (5 × 103 to 104) were incubated with various numbers of cloned T cells for 4 hours. 51Cr release into the medium was measured using a γ-counter. The percentage of specific lysis (mean ± standard error [SE]) obtained in the 51Cr release assay was determined from triplicate cultures as follows: 100 × (Experimental Release cpm − Spontaneous Release cpm)/(Maximum Release cpm − Spontaneous Release cpm).
Blocking of cytotoxicity by MoAbs.Mouse MoAbs were used as ascites or after being purified from ascites by protein A column (Pharmacia, Uppsala, Sweden) to block cytotoxicity by T cells against hematopoietic cells. Diluted 1/100 ascites or purified MoAbs (10 μg/mL) were added to target cells in 96-well plates and incubated for 30 minutes at 37°C before effector cells were added. MoAbs were HU-4 (anti-DR), HU-30 (anti-DR2), HU-11 (anti-DO; kindly provided by Dr Akemi Wakisaka, Hokkaido University, Japan), W6/32 (anti-HLA class I; American Type Culture Collection, Rockville, MD), B7-21 (anti-DP), and OKT3 (anti-CD3; Becton Dickinson, Mountain View, CA).
Determination of T-cell receptor Vβ usage by a T-cell clone.Cloned T cells were incubated with fluorescent MoAbs against 17 different variable regions of the T-cell receptor (TCR) β chain (Vβ; Vβ5.1, Vβ5.2, Vβ5.3, Vβ6.7, Vβ8, and Vβ12 from T Cell Sciences Inc, [Cambridge, MA]; Vβ3, Vβ6.1, Vβ9, Vβ11, Vβ13.1, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ20, Vβ21.3, and Vβ22, from Immunotech [Marseille, France]) and analyzed using a fluorescence-activated cell sorter scan (FACScan). For T-cell clones that did not react with any of these MoAbs, the Vβ family of each T-cell clone was determined using reverse dot blot hybridization as described elsewhere.21 Briefly, RNA was extracted from each clone, and double-strand cDNA was synthesized using a cDNA synthesis kit (BRL, Bethesda, MD) and a specific primer adapter. After adapter ligation followed by restriction enzyme digestion, cDNA was amplified using a Cβ primer and a primer adapter with PCR. Amplified cDNA products labeled with biotinylated deoxynucleotides were hybridized with 39 different oligonucleotides specific to each Vβ that had been enzymatically tailed at the 3′ terminus and attached to nylon membranes. The hybridization was visualized with a streptavidin-alkaline phosphatase conjugate (BRL) and a color development (NBT-BCIP; Promega, Madison, WI).
Single-strand conformation polymorphism analysis of amplified products derived from TCRVβ21 cDNA.Total RNA was extracted from BMMC of the patient, BMMC of six normal volunteers, including two with HLA-DRB1*0405 and two with HLA-DRB1*1501, and the three Vβ21+ T-cell clones. cDNA was synthesized from RNA using M-MLV reverse transcriptase (BRL) and oligo dT as a primer. TCRVβ21 cDNA was amplified with the PCR using a primer specific to Vβ21 (5′ GAGTGTGGCTTTTTGGTGCAA 3′) and a Cβ primer (5′ TCTACCCCAGGCCTCGGCGCTGACGAT 3′).22 The amplified products were heat denatured in a formamide solution containing 80% formamide, 20 mmol/L EDTA, and 0.1% bromphenol blue and electrophoresed on an 8% polyacrylamide gel.23 DNA was visualized using a silver stain kit (Daiichi Kagaku, Tokyo, Japan).
Suspension culture of CD34+cells.CD34+ cells (105) isolated from the patient and normal individuals were suspended in 1 mL of Iscove's modified Dulbecco's Medium (IMDM; GIBCO) supplemented with recombinant IL-3 (2 ng/mL), IL-6 (50 ng/mL), stem cell factor (10 ng/mL), granulocyte colony-stimulating factor (G-CSF, 100 ng/mL) (all were gifts from Kirin Brewery, Tokyo, Japan), and 10% FCS, and cultured in a 15-mL plastic tube (Falcon). After 7 days, cultured cells were harvested and used as target cells for the 51Cr release assay and hematopoietic progenitor cell assay.
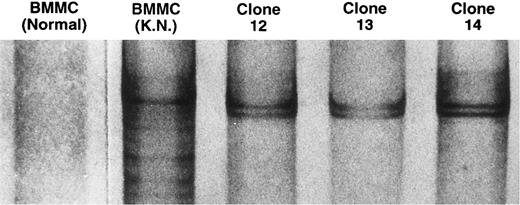
Single-strand conformation polymorphism analysis of TCR Vβ21 cDNA amplified with the PCR. The cDNA from BMMC of a normal individual, BMMC of the patient, and the three Vβ21+ T-cell clones were amplified with the PCR, using a primer specific to Vβ21 and a Cβ primer. The amplified products were heat-denatured in a formamide solution and electrophoresed on an 8% polyacrylamide gel. The DNA was visualized by silver staining.
Single-strand conformation polymorphism analysis of TCR Vβ21 cDNA amplified with the PCR. The cDNA from BMMC of a normal individual, BMMC of the patient, and the three Vβ21+ T-cell clones were amplified with the PCR, using a primer specific to Vβ21 and a Cβ primer. The amplified products were heat-denatured in a formamide solution and electrophoresed on an 8% polyacrylamide gel. The DNA was visualized by silver staining.
Hematopoietic progenitor cell assay.A total of 3 × 103 freshly isolated or cultured CD34+ cells, suspended in 0.3 mL of IMDM supplemented with 10% FCS were mixed with methylcellulose medium supplemented with various colony-stimulating factors (CSFs; StemCell Technologies, Vancouver, British Columbia, Canada). One milliliter of the cell mixture was plated on a 35-mm culture dish. In some experiments, 3 × 103 CD34+ cells were suspended in 0.3 mL of 10% FCS-IMDM with 3 × 104 cloned T cells from the patient and incubated at 37°C in a 15-mL plastic tube (Falcon). After 4 hours, the cell suspension was added with 2.7 mL of the methylcellulose medium, and, after shaking the tube vigorously, 1 mL of the cell mixture was plated on a 35-mm culture dish. After 14 days, colony-forming unit-granulocyte macrophage (CFU-GM)-derived colonies and burst-forming unit-erythroid (BFU-E)–derived colonies were counted under an inverted microscope.
RESULTS
TCRVβ of T-cell clones and response to autologous hematopoietic cells.A total of 16 CD4+ T-cell clones were established from BM T cells and were stably maintained for greater than 1 year. All clones were tested and found to be Mycoplasma-free. Clones included three Vβ21+, two Vβ14+, two Vβ17+, two Vβ13.2+, two Vβ13.6+, two Vβ25+, one Vβ20+, one Vβ2+, and one Vβ8+ (Fig 1). When these clones were tested against autologous CD34+ cells, only three Vβ21+ clones and one Vβ13.6+ clone produced more than 200 pg/mL of IFN-γ in culture medium after coculture with autologous CD34+ cells (Fig 1). We reasoned that, if these CD4+ T cells recognized a self-peptide presented by HLA class II antigen on CD34+ cells, they might respond to other autologous hematopoietic cells expressing HLA class II antigens. In agreement with this hypothesis, these four T-cell clones produced more IFN-γ when cocultured with LCL cells (Fig 1). Because the T-cell clones appeared to show similar responses against autologous LCL and CD34+ cells, LCL cells were used as targets in the functional analysis of the T-cell clones.
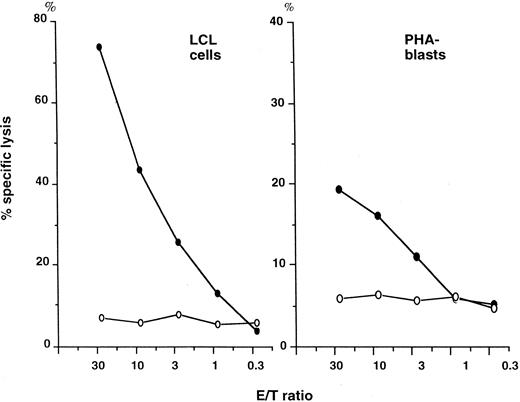
Cytotoxicity of T-cell clones against auto LCL cells and PHA-stimulated peripheral blood mononuclear cells (PHA-blasts). Auto LCL cells and peripheral blood mononuclear cells that were cultured with 1% PHA in 10% FCS-RPMI 1640 medium for 7 days (PHA-blasts) were incubated with differing numbers of cloned T cells in a 4-hour cytotoxicity assay. (•) NT4.2 (clone 12); (○) NT27 (clone 10).
Cytotoxicity of T-cell clones against auto LCL cells and PHA-stimulated peripheral blood mononuclear cells (PHA-blasts). Auto LCL cells and peripheral blood mononuclear cells that were cultured with 1% PHA in 10% FCS-RPMI 1640 medium for 7 days (PHA-blasts) were incubated with differing numbers of cloned T cells in a 4-hour cytotoxicity assay. (•) NT4.2 (clone 12); (○) NT27 (clone 10).
Cytotoxicity of T-cell clones against autologous LCL cells.When the T-cell clones were tested for their proliferative response to autologous LCL cells using 3H-thymidine incorporation, none of the clones exhibited an outstanding response; the stimulation index was comparable among all 16 T-cell clones, ranging from 2.5 to 3.3 (data not shown). However, cytotoxicity against LCL cells varied with each clone (Fig 2). Among the 9 T-cell clones examined, only the 3 Vβ21+ clones killed LCL cells with more than 35% specific lysis at an effector/target ratio of 10.
Single-strand conformation polymorphism analysis of TCRVβ21 cDNA amplified with PCR.The amplified products from the three Vβ21+ T-cell clones showed the same mobility shift, suggesting that these clones were of a common origin (Fig 3). This clone was named NT4.2, which was CD3+ CD4+ CD8− CD56+. The amplified products derived from BMMC cDNA from six normal individuals failed to exhibit any bands, indicating an absence of clonally expanded T cells among Vβ21+ T cells. In contrast, the amplified products derived from the BM of our patient exhibited several distinct bands, indicating an oligoclonal proliferation of Vβ21 T cells in the BM. Moreover, the mobility shift of the most distinct band was the same as that of NT4.2, indicating dominant proliferation of NT4.2 in vivo. When the patient's BM was examined after pancytopenia improved after dose escalation of cyclosporine, no distinct band was detectable in Vβ21 cDNA amplified with PCR (data not shown).
Cytotoxicity of T-cell clones against autologous LCL cells and PHA-stimulated PBMC (PHA-blasts).When autologous LCL cells were incubated with differing numbers of cloned T cells in a 4-hour cytotoxicity assay, NT4.2 cells lysed these cells in a dose-dependent fashion. Cytotoxicity reached 74% at an effector/target ratio of 30, whereas the percentage-specific lysis of LCL cells by clone 10 (NT27) cells was only 7% at this ratio (Fig 4). NT4.2 cells failed to lyse freshly isolated autologous PBMC and K562 cells (data not shown) but did lyse autologous PHA-blasts in a dose-dependent fashion. These findings suggested that killing of autologous LCL cells by NT4.2 cells was not mediated by EBV-related antigens.
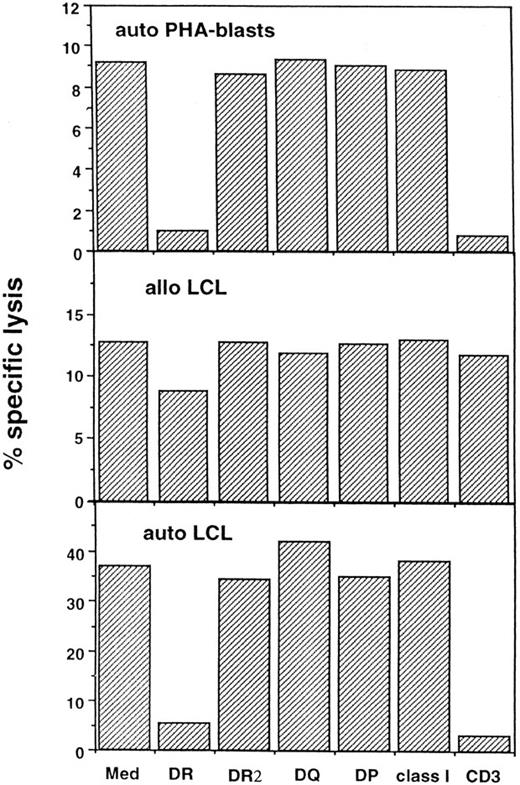
Blocking of cytotoxicity mediated by NT4.2 cells against autologous (auto) and allogeneic (allo) lymphocytes.Cytotoxicity mediated by NT4.2 cells against the auto LCL cells and PHA-blasts was blocked to a similar degree by the addition of either anti–HLA-DR MoAb or anti-CD3 MoAb (Fig 5). Cytotoxicity was not affected by the addition of either anti–HLA-DR2 MoAb, anti–HLA-DQ MoAb, anti–HLA-DP MoAb, or anti-HLA class I MoAb. NT4.2 cells also lysed one of four allo LCLs that did not share an HLA-DRB1 allele with the patient. Because cytotoxicity against this LCL (DRB1*0901, 0901) was not blocked by anti-HLA MoAb or OKT3, this would appear to be a nonspecific event, irrelevant to the interaction between HLA and the TCR.
Blocking of cytotoxicity mediated by NT4.2 cells against auto LCL cells, allo LCL cells, and auto PHA-blasts. A total of 5 × 104 NT4.2 cells were incubated with 5 × 10351Cr-labeled target cells in medium (Med) alone or with addition of the indicated MoAbs: anti–HLA-DR, DQ, DP, anti-HLA class I, and anti-CD3, ascites 1/100; anti-HLA DR2, purified MoAb 10 mg/mL. The mean percentage of specific lysis was determined from triplicate cultures after 4 hours of incubation.
Blocking of cytotoxicity mediated by NT4.2 cells against auto LCL cells, allo LCL cells, and auto PHA-blasts. A total of 5 × 104 NT4.2 cells were incubated with 5 × 10351Cr-labeled target cells in medium (Med) alone or with addition of the indicated MoAbs: anti–HLA-DR, DQ, DP, anti-HLA class I, and anti-CD3, ascites 1/100; anti-HLA DR2, purified MoAb 10 mg/mL. The mean percentage of specific lysis was determined from triplicate cultures after 4 hours of incubation.
Cytotoxicity of NT4.2 cells against allo LCLs that share HLA-DRB1*1501 or DRB1*0405.NT4.2 cells failed to lyse allo LCLs that shared DRB1*1501 with our patient, except for those whose remaining DRB1 allele was DRB1*0405 or DRB1*0410, which differs from DRB1*0405 at only two residues on the β chain (Fig 6). By contrast, the CD4+ clone killed allo LCLs homologous with DRB1*0405 from normal individuals. These findings suggested that the target antigen of NT4.2 cells was presented by DR4 and that it is not unique to this patient but is also shared by normal individuals carrying HLA-DRB1*0405.
Cytotoxicity of NT4.2 cells against allo LCLs that share HLA-DRB1*1501 or HLA-DRBI*0405. A total of 5 × 104 NT4.2 cells were incubated with 5 × 10351Cr-labeled auto LCL cells or allo LCL cells from aplastic anemia patients and normal individuals that share HLA-DRB1*1501 or HLA-DRB1*0405, and 51Cr release was determined. Data are expressed as a mean of triplicate cultures.
Cytotoxicity of NT4.2 cells against allo LCLs that share HLA-DRB1*1501 or HLA-DRBI*0405. A total of 5 × 104 NT4.2 cells were incubated with 5 × 10351Cr-labeled auto LCL cells or allo LCL cells from aplastic anemia patients and normal individuals that share HLA-DRB1*1501 or HLA-DRB1*0405, and 51Cr release was determined. Data are expressed as a mean of triplicate cultures.
Cytotoxicity of NT4.2 cells against CD34+ cells.NT4.2 cells did not show cytotoxicity against freshly isolated CD34+ cells despite the fact that most of them expressed HLA-DR (Table 1A). Because auto PBMC needed to be cultured to be susceptible to killing by NT4.2, 105 CD34+ cells were cultured in the medium containing various CSFs for 7 days and then tested for cytotoxicity by NT4.2. Seven days later, 0.8 to 2.2 × 105 cells were recovered. The T-cell clone lysed cultured CD34+ cells by up to 15% (Table 1A). Another T-cell clone, NT27, did not lyse CD34+ cells. The proportion of CD34+ cells in the cultured cell fraction could not be determined because of the limited number of cells. When cultured allo CD34+ cells from three normal individuals were used as target cells, NT4.2 showed greater than 10% cytotoxicity only against CD34+ cells from the normal individual (A) carrying HLA-DRB1*0405 (Table 1B). The proportion of CD34+ cells in the cultured cell fraction from individual A in two experiments was 46.3% and 46.5%.
Cytotoxicity of T-Cell Clones Against CD34+ Cells
| Target . | Effector . | |
|---|---|---|
| . | NT4.2 . | NT27 . |
| A | ||
| Freshly obtained autologous CD34+ cells | 2.4 | 2.1 |
| Cultured autologous CD34+ cells | 14.8 | 3.2 |
| B | ||
| Cultured allogeneic CD34+ cells from patient A | 10.2 | NT |
| Cultured allogeneic CD34+ cells from patient B | 2.8 | NT |
| Cultured allogeneic CD34+ cells from patient C | 3.1 | NT |
| Target . | Effector . | |
|---|---|---|
| . | NT4.2 . | NT27 . |
| A | ||
| Freshly obtained autologous CD34+ cells | 2.4 | 2.1 |
| Cultured autologous CD34+ cells | 14.8 | 3.2 |
| B | ||
| Cultured allogeneic CD34+ cells from patient A | 10.2 | NT |
| Cultured allogeneic CD34+ cells from patient B | 2.8 | NT |
| Cultured allogeneic CD34+ cells from patient C | 3.1 | NT |
Specific lysis of CD34+ cells from the patient and normal individuals (patient A, HLA-DRB1*0405, 0901; patient B, HLA-DRB1*0901, 0803; patient C, HLA-DRB1*0803, 1302) by cloned T cells was measured using a 51Cr release assay in triplicate at effector/target ratio 10:1. Data are expressed as mean percentage specific lysis.
Abbreviation: NT, not tested.
Effect of T-cell clones on colony formation by auto CD34+ cells.To examine the effects of cloned T cells on hematopoietic progenitor cell growth, cultured or uncultured auto CD34+ cells were preincubated with cloned T cells and then cultured in methylcellulose medium supplemented with CSFs. Although the addition of NT4.2 cells slightly reduced the number of CFU-GM colonies derived from freshly isolated CD34+ cells, the degree of reduction was marginal (Table 2). By contrast, the addition of NT4.2 cells to the cultured CD34+ cells markedly reduced the number of colonies derived from both BFU-E and CFU-GM. The number of colonies was partially recovered by the addition of anti–HLA-DR MoAb into the culture with NT4.2 cells. When the 4-hour incubation of CD34+ cells with NT4.2 cells in the suspension culture before adding methylcellulose medium was omitted, the decrease in the number of colonies was not seen.
Effect of T-Cell Clones on Autologous Hematopoietic Progenitor Cell Growth
| Cells Plated for Progenitor Cell Assay . | T-Cell Clone Added . | Preincubation . | CFU-GM . | BFU-E . |
|---|---|---|---|---|
| Experiment 1 | ||||
| Freshly obtained CD34+ cells | — | − | 232 ± 17 | 192 ± 12 |
| NT4.2 | − | 194 ± 25 | 152 ± 16 | |
| NT4.2 | + | 201 ± 12 | 144 ± 16 | |
| NT27 | + | 194 ± 19 | 166 ± 11 | |
| Cultured CD34+ cells | — | − | 98 ± 13 | 7 ± 2 |
| NT4.2 | − | 74 ± 6 | 6 ± 2 | |
| NT4.2 | + | 2 ± 1 | 1 ± 1 | |
| NT27 | + | 78 ± 4 | 8 ± 2 | |
| Experiment 2 | ||||
| Cryopreserved-thawed CD34+ cells* | — | − | 92 ± 6 | 40 ± 3 |
| NT4.2 | − | 80 ± 5 | 34 ± 3 | |
| NT4.2 | + | 81 ± 5 | 31 ± 5 | |
| Cultured CD34+ cells | — | − | 78 ± 6 | 19 ± 3 |
| NT4.2 | − | 70 ± 2 | 15 ± 1 | |
| NT4.2 | + | 3 ± 1 | 0 | |
| NT4.2 + DR MoAb† | + | 40 ± 7 | 6 ± 1 |
| Cells Plated for Progenitor Cell Assay . | T-Cell Clone Added . | Preincubation . | CFU-GM . | BFU-E . |
|---|---|---|---|---|
| Experiment 1 | ||||
| Freshly obtained CD34+ cells | — | − | 232 ± 17 | 192 ± 12 |
| NT4.2 | − | 194 ± 25 | 152 ± 16 | |
| NT4.2 | + | 201 ± 12 | 144 ± 16 | |
| NT27 | + | 194 ± 19 | 166 ± 11 | |
| Cultured CD34+ cells | — | − | 98 ± 13 | 7 ± 2 |
| NT4.2 | − | 74 ± 6 | 6 ± 2 | |
| NT4.2 | + | 2 ± 1 | 1 ± 1 | |
| NT27 | + | 78 ± 4 | 8 ± 2 | |
| Experiment 2 | ||||
| Cryopreserved-thawed CD34+ cells* | — | − | 92 ± 6 | 40 ± 3 |
| NT4.2 | − | 80 ± 5 | 34 ± 3 | |
| NT4.2 | + | 81 ± 5 | 31 ± 5 | |
| Cultured CD34+ cells | — | − | 78 ± 6 | 19 ± 3 |
| NT4.2 | − | 70 ± 2 | 15 ± 1 | |
| NT4.2 | + | 3 ± 1 | 0 | |
| NT4.2 + DR MoAb† | + | 40 ± 7 | 6 ± 1 |
A total of 3 × 103 freshly isolated CD34+ cells or CD34+ cells cultured in the presence of colony-stimulating factors were incubated in medium alone or with 3 × 104 cloned T cells for 4 hours (preincubation +) and then mixed with methylcellulose medium supplemented with growth factors. Some CD34+ cells were mixed with cloned T cells just before addition of the methylcellulose medium (preincubation −). Data are expressed as mean ± standard error of duplicate cultures.
An aliquot of autologous CD34+ cells that had been cryopreserved was used in Experiment 2.
Anti–HLA-DR MoAb, ascites 1/100, was added to the culture of CD34+ cells with NT4.2.
Effect of T-cell clones on colony formation by allo cultured CD34+ cells.When allo CD34+ cells from a normal individual carrying HLA-DRB1*0405 (A) were tested for susceptibility to suppression by NT4.2, colony formation was suppressed by more than 70% (Table 3). This suppression was not observed when the 4-hour preincubation was omitted. Suppression of colony formation by CD34+ cells from normal individuals (B and C) not carrying HLA-DRB1*0405 was less than 25%.
Effect of T-Cell Clones on Colony Formation by Allogeneic CD34+ Cells
| Donor of CD34+ Cells . | Preincubation . | . | CFU-GM . | BFU-E . |
|---|---|---|---|---|
| A | Control | 168 ± 12 | 79 ± 6 | |
| − | 142 ± 9 | 69 ± 4 | ||
| + | 40 ± 5 | 9 ± 2 | ||
| B | Control | 106 ± 7 | 73 ± 5 | |
| − | 99 ± 6 | 70 ± 6 | ||
| + | 80 ± 5 | 66 ± 4 | ||
| C | Control | 184 ± 12 | 91 ± 7 | |
| − | 168 ± 10 | 88 ± 3 | ||
| + | 160 ± 7 | 78 ± 5 |
| Donor of CD34+ Cells . | Preincubation . | . | CFU-GM . | BFU-E . |
|---|---|---|---|---|
| A | Control | 168 ± 12 | 79 ± 6 | |
| − | 142 ± 9 | 69 ± 4 | ||
| + | 40 ± 5 | 9 ± 2 | ||
| B | Control | 106 ± 7 | 73 ± 5 | |
| − | 99 ± 6 | 70 ± 6 | ||
| + | 80 ± 5 | 66 ± 4 | ||
| C | Control | 184 ± 12 | 91 ± 7 | |
| − | 168 ± 10 | 88 ± 3 | ||
| + | 160 ± 7 | 78 ± 5 |
A total of 3 × 103 allogeneic CD34+ cells cultured in the presence of colony-stimulating factors were incubated in medium with (preincubation +) or without (control) 3 × 104 NT4.2 cells for 4 hours and then mixed with methylcellulose medium for colony assay. In some experiments, CD34+ cells were mixed with NT4.2 cells just before addition to methylcellulose medium (preincubation −). Data are expressed as mean ± standard error of duplicate cultures. Patient A, HLA-DRB1*0405, 0901; patient B, HLA-DRB1*0901, 0803; patient C, HLA-DRB1*0803, 1302.
DISCUSSION
Several T-cell clones capable of inhibiting in vitro hematopoiesis have been isolated from patients with AA.18,19 Moebius et al18 found that a CD4+CD8+ T-cell clone from peripheral blood of a patient with AA lysed auto and allo LCLs in an HLA-DP–restricted fashion and inhibited the growth of hematopoietic progenitor cells. In this study, cytotoxicity of the CD4+CD8+ T-cell clone was not restricted by a certain HLA-DP allele and inhibition of hematopoietic progenitor cells was nonspecific. A CD4+Vβ17+ T-cell clone that we isolated from a transfused AA patient in a previous study secreted IFN-γ in response to auto CD34+ cells and inhibited the growth of hematopoietic progenitor cells from CD34+ cells.19 However, because only a limited number of auto CD34+ cells were used as target cells, restriction of the inhibition by an HLA allele could not be examined. In the present study, we identified a CD4+Vβ21+ T-cell clone (NT4.2) in the BM of a patient with cyclosporine-dependent AA that is capable of killing cultured lymphocytes and BMMC enriched for CD34+ cells in an HLA-DRB1*0405-restricted manner. Thus, NT4.2 is the first T-cell clone isolated from an AA patient that requires HLA-DRB1 identity with the hematopoietic cells to exert cytotoxicity for them.
NT4.2 showed moderate cytotoxicity against CD34+ cells cultured in the presence of CSFs for 1 week. The T-cell clone markedly inhibited growth of hematopoietic progenitor cells derived from the cultured CD34+ cells by direct cell-cell contact. Inhibition against hematopoietic progenitor cells was partially abrogated by addition of MoAb against HLA-DR. Thus, it is possible that NT4.2 can lyse cultured CD34+ progenitor cells by a mechanism similar to that responsible for lysis of LCLs and PHA-blasts.24 However, this T-cell clone failed to kill a freshly obtained CD34+ cell population that was 77% pure. It is known that some antigen-presenting cells, such as dendritic cells, can be generated from CD34+ cells during the culture.25 These antigen-presenting cells may stimulate NT4.2 during the preincubation time before methylcellulose culture to produce IFN-γ that inhibits colony formation. Thus, CD34+ cells may not be the true target of NT4.2, and inhibition of hematopoietic progenitor cells by NT4.2 may be indirect. Because it is virtually impossible to obtain a sufficiently high number of pure CD34+ cells to be used as target cells in the cytotoxicity assay by using cell sorting, a leukemic cell line with HLA-DRB1*0405 sharing the characteristic of hematopoietic progenitor cells seems to be required to determine whether NT4.2 can directly kill hematopoietic progenitor cells.
Little is known about target antigens of myelosuppressive T cells in AA because T-cell clones specifically recognizing hematopoietic cells via an HLA such as NT4.2 had not been available. Studying this T-cell clone could facilitate identification of such target antigens. Given its cytotoxicity toward autohematopoietic cells in the absence of exogenous antigens, NT4.2 is thought to recognize a ubiquitous self peptide bound to HLA-DR4 as a result of failure in maintaining tolerance. It is possible that cytotoxicity was directed not against an endogenous antigen but rather against an exogenous serum component, because all hematopoietic cells susceptible to killing by NT4.2 had been cultured in medium containing human serum or FCS in this study. One possible candidate is a stress protein. Yoshino et al26 have recently shown that tumor-infiltrating CD4+ T cells react to LCL cells expressing heat shock protein 70 in an HLA-DR restricted manner. In autoimmune diseases such as rheumatoid arthritis27 and Behcet's disease,28 self cells that express heat shock proteins have been shown to be recognized and injured by autoreactive T cells directed to the heat shock protein-derived epitope in the context of major histocompatibility antigens. It is possible that CD4+ T cells recognizing stressed hematopoietic cells through heat shock proteins exist in the BM of immune-mediated AA. Whether the killing of LCL cells by NT4.2 is mediated by such stress proteins is currently under investigation.
Because NT4.2 was isolated after stimulation of BM T cells with irradiated auto CD34+ cells, it is possible that proliferation of this T-cell clone would only be an in vitro “artifact” irrelevant to the pathogenesis of AA. Acquisition of cytotoxicities by CD4+ T-cell clones has been implicated in in vitro long-term cultures.29 Similar T-cell clones cytotoxic against auto hematopoietic cells may be generated from BM T cells from a normal individual because T cells reactive against auto hematopoietic cells have been detected in the circulation of normal patients.30,31 It is also possible that NT4.2 has been induced by the administration of CyA because the patient had been treated with CyA at the time of marrow aspiration.32 However, in the BM of normal individuals, there was no sign of clonal proliferation of Vβ21+ T cells as shown by single-strand conformation polymorphism (SSCP) analysis. NT4.2 was identical to the most dominant T-cell clone among the Vβ21+ T cells of this patient's BM. The distinct band in the SSCP gel detected in this patient does not represent a physiological skewing of TCR Vβ21 usage associated with the patient's HLA-DRB1* allele because Vβ21 cDNA from four unaffected individuals possessing DRB1*0405 or DRB1*1501 did not produce such distinct bands in the SSCP gel. Evidence for oligoclonal proliferation of Vβ21+ T cells in the BM was no longer detected when the patient's blood counts recovered after dose escalation of cyclosporine. Moreover, cloning efficiency of this T-cell clone was as high as 3 of 16, indicating preferential expansion of the clone in response to stimulation with auto CD34+ cells in vitro. In addition, NT4.2 was a T-cell clone with a CD4+CD56+ phenotype, which could hardly be established from normal individuals.33 These findings suggest that NT4.2 is not a T cell provoked to proliferate primarily in vitro, but a T-cell clone that had been activated by certain antigens to proliferate in the BM of immune-mediated AA.
The patient with AA in the present study carried an HLA-class II haplotype (DRB1*1501-DQA1*0102-DQB1*0602), which is associated with susceptibility to cyclosporine-dependent AA.34 Because killing of the auto LCL by NT4.2 depended on HLA-DR, we suspected that, of his DRB1 alleles, DRB1*1501 would be responsible for the cytotoxicity. However, the failure of HLA-DR2 MoAb to block cytotoxicity and the selective killing of allo LCLs with DRB1*0405 clearly suggested that a target antigen may be presented by HLA-DR4. DRB1*0405 is the most common DR4-associated subtype among Japanese, and is strongly associated with rheumatoid arthritis in Japanese.35,36 This DRB1 allele and DRB1*0410, which differs from DRB1*0405 at only two residues on the β chain, was found in 5 of 13 patients with cyclosporine-dependent AA.34 Thus, it is possible that DRB1*0405 may also be associated with immune mechanisms causing AA.
CD4+ cells with cytotoxic activity have been shown in certain disease states, such as autoimmune diseases,16 viral infections,37 and malignancies.38 CD4+ cytotoxic lymphocytes (CTL) are also known to kill auto antigen-presenting cells in the presence of exogenous antigen.24 This cytotoxicity has implicated CD4+ CTL as modulators of the immune response. However, recent studies using murine and human systems have documented the important role of CD4+ CTL in defense against foreign pathogens and transplanted allografts.37,39 NT4.2 did not require the presence of exogenous antigens for killing of auto LCL cells. To our knowledge, this is the first CD4+ CTL against auto antigen-presenting cells that was not pulsed with exogenous antigens. Moebius et al previously established a CD4+CD8+ T-cell clone with autocytotoxic activity similar to NT4.2 from a patient with AA.18 Although its phenotype differs from NT4.2, it is intriguing that CD4+ CTL with similar activities against auto LCL cells could be established in patients with AA.
Previous studies on T lymphocytes of AA patients have emphasized pathogenic roles of CD8+ T cells rather than CD4+ T cells.9-12 Peripheral blood T cells capable of suppressing in vitro growth of hematopoietic progenitor cells were mainly CD8+ T cells.40,41 In the BM of AA patients, the number of activated CD8+ T cells has been shown to be relatively high.12 In the present study, T-cell clones isolated from the patient's BM were all CD4+ despite the decreased CD4/CD8 ratio (0.74) in the BM. The absence of a CD8+ T-cell clone may be caused by culturing of BM T cells with irradiated auto CD34+ cells before T-cell cloning, because culturing of T cells with auto hematopoietic cells with HLA-DR favors proliferation of CD4+ T cells.42 However, recent studies on BM biopsy specimens from AA patients using immunohistochemical staining showed that the CD4/CD8 ratio was not necessarily decreased, even when the ratio in aspirated BM was decreased.43 In a rodent model, a small number of allo CD4+ cytotoxic T cells was shown to produce profound atrophy of BM by cell-cell contact.39 In addition, NT4.2 had a surface phenotype of CD4+CD56+ that has been associated with higher cytotoxicity and more abundant IFNγ secretion than CD4+CD56− T cells.33 Thus, CD4+ T cells like NT4.2 may have an important role in the pathogenesis of immune-mediated AA. Further study of many untransfused patients with cyclosporine-dependent AA will help clarify the role of CD4+ T cells capable of killing auto hematopoietic cells in an HLA-DR–restricted manner.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture (06671080), Japan; by a Grant-in-Aid for Intractable Haemopoietic Disease Research from the Ministry of Health and Welfare, Japan; and by the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Address reprint requests to Shinji Nakao, MD, Third Department of Medicine, Kanazawa University School of Medicine, 13-1 Takaramachi, Kanazawa, Ishikawa 920, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal