Abstract
OKT3 monoclonal antibody (MoAb) therapy is well established in the prevention and therapy of acute rejection in transplant patients. Unfortunately, this therapy is associated with several short-term (cytokine release syndrome) and long-term (infections, EBV-related lymphoma) side effects. Recently, we were able to demonstrate an association between the TNFα release following the first OKT3 MoAb infusions and the appearance of human cytomegalovirus (HCMV) reactivation several days later. In order to prevent this TNFα associated HCMV reactivation patients were additionally treated with pentoxifylline (PTX), a methylxanthine derivative that has been shown to suppress TNFα induction. Although the TNFα peak plasma level following OKT3 MoAb treatment was markedly reduced, the incidence of HCMV reactivation and HCMV disease was not influenced. In transient transfection experiments using HCMV immediate early enhancer/promoter CAT reporter gene constructs PTX enhanced the promoter activity independently from TNFα in premonocytic cells. Furthermore, PTX acted synergistically with TNFα. In virus-infected human embryonal lung fibroblasts HCMV replication was triggered in the presence of both PTX and TNFα, while either substance alone had only marginal effects. The stimulatory effect of PTX on the immediate early (IE) enhancer/promoter was mediated via CREB/ATF, a eukaryotic transcription factor that binds to the 19 bp sequence motif in the enhancer region, while TNFα stimulation was mediated by activation of the transcription factor NF-kB and its binding to the 18 bp sequence motif in the enhancer. These data suggest a potential side effect of cAMP-elevating drugs such as PTX.
REACTIVATION OF LATENT human cytomegalovirus (HCMV) is thought to be the main cause of severe active HCMV disease in immunosuppressed patients, eg, solid organ and bone marrow transplant recipients and AIDS patients.1 Monocytic cells or rather their progenitors in the bone marrow have been identified as a major site of HCMV latency.2,3 Reactivation of latent virus depends on the differentiation of progenitor cells into mature monocytes and macrophages, which are a known site of virus replication.4-8 Recently we suggested an association between elevated TNFα plasma levels and an increased incidence of HCMV antigenemia in transplant recipients9 and patients with septic disease.10 In vitro transient transfection experiments identified TNFα as a powerful cell specific stimulator of the major immediate early (IE) enhancer/promoter of HCMV in the undifferentiated granulocyte/monocyte progenitor cell line HL-60, which served as a model of latently infected cells in the bone marrow.11 TNFα-dependent stimulation of the promoter was mediated by activation of the eukaryotic transcription factor NF-kB and its binding to the 18-bp repetitive sequence motif in the enhancer region.12 In more differentiated premonocytic cells the positive TNFα effect was abrogated.11
The use of antilymphocyte antibody preparations has ultimately improved renal allograft survival but also caused an increase in opportunistic infections, particularly with HCMV9 and posttransplant lymphoproliferative disorders. Apart from these undesirable effects, a so-called “first dose reaction” is observed including symptoms such as fever, chills, malaise, myalgias, arthralgias, and headache. The pathogenesis of this syndrome has been attributed to an increased synthesis of different cytokines such as TNFα, IL-2, IL-6, or interferon gamma. Elevated TNFα secretion may explain why the incidence of active HCMV infections following treatment with OKT3 monoclonal antibody (MoAb) or antithymocyte globulin is higher than after steroid bolus or anti-CD4 MoAb.9
Recently it has been shown that pentoxifylline (PTX) reduces OKT3 MoAb-induced production of TNFα both in vitro and in vivo.13-15 PTX is a methylxanthine derivative reported to inhibit intracellular cyclic nucleotide phosphodiesterase; this results in an increased intracellular concentration of cyclic adenosine monophosphate (cAMP).16,17 Consequently, PTX significantly reduces TNFα protein synthesis and TNFα-mRNA transcription in murine macrophages and human monocytes through the cAMP pathway.18-20 As a result of this, it seemed quite possible that the administration of PTX would reduce the incidence of OKT3 MoAb-induced HCMV reactivation in renal transplant recipients by blocking TNFα secretion. In a clinical study, renal allograft recipients with late acute rejection were treated with OKT3 MoAb alone or in combination with PTX and monitored for active HCMV infection. Surprisingly, the incidence of patients developing HCMV antigenemia and disease was not significantly influenced by PTX compared with the control group, although PTX significantly reduced TNFα release in vivo. Because of this, we tested the effect of PTX, alone or in combination with TNFα, on the activity of the HCMV IE enhancer/promoter in premonocytic cells and on virus replication in permissive human embryonic lung fibroblasts.
PATIENTS AND METHODS
Patients.Seven long-term renal transplant patients (3 to 7 years posttransplantation) suffering from histology-proven steroid-resistant late acute rejection21 were included in this study. The pretreatment serum creatinine levels ranged between 245 and 550 μmol/mL. All patients received 5 mg/d OKT3 MoAb bolus injection for 7 consecutive days because they had not responded to the initial steroid-bolus therapy (5 mg/kg/d for 5 days) administered 2 weeks or more before the MoAb therapy. To reduce OKT3 MoAb-induced cytokine release all patients received a prophylactic dose of 1 mg/kg of methylprednisolone on the first day. In addition, four of seven patients received a 2-hour PTX infusion (Trental; Albert Roussel, Wiesbaden, Germany; 4 mg/kg/d intravenously [IV] starting 30 minutes before OKT3 MoAb application) on the first 2 days. There was no significant difference between the two groups regarding age, time after transplantation, pretreatment serum creatinine level, and HCMV serology (all were IgG-positive). The base-line immunosuppression consisting of cyclosporine A (blood levels between 120 and 150 ng/mL), azathioprine (1 mg/kg/d), and methylprednisolone (4-8 mg/d) was continued during OKT 3 MoAb therapy.
Cytokine Plasma Levels, HCMV Antigenemia, and HCMV Disease in Renal Allograft Recipients Treated With OKT3 MoAb and PTX in Combination or OKT3 MoAb Alone
| Patient . | TNFα Plasma Peak Level (pg/mL) . | IL-6 Plasma Peak Level (pg/mL) . | HCMV Antigenemia . | HCMV Disease . | Fever (d 1-3) . |
|---|---|---|---|---|---|
| OKT3 MoAB/PTX | |||||
| No 1 | 225 | 12 | + | + | + |
| No 2 | 1,382 | 125 | + | + | + |
| No 3 | 226 | 23 | + | + | + |
| No 4 | 270 | 25 | − | − | + |
| (median) | (median) | ||||
| Total | 248 | 24 | 3/4 | 3/4 | 4/4 |
| OKT3 MoAb | |||||
| No 5 | 854 | 225 | + | + | + |
| No 6 | 1,029 | 245 | + | − | + |
| No 7 | 1,211 | 491 | − | − | + |
| (median) | (median) | ||||
| Total | 1,029 | 245 | 2/3 | 1/3 | 3/3 |
| Patient . | TNFα Plasma Peak Level (pg/mL) . | IL-6 Plasma Peak Level (pg/mL) . | HCMV Antigenemia . | HCMV Disease . | Fever (d 1-3) . |
|---|---|---|---|---|---|
| OKT3 MoAB/PTX | |||||
| No 1 | 225 | 12 | + | + | + |
| No 2 | 1,382 | 125 | + | + | + |
| No 3 | 226 | 23 | + | + | + |
| No 4 | 270 | 25 | − | − | + |
| (median) | (median) | ||||
| Total | 248 | 24 | 3/4 | 3/4 | 4/4 |
| OKT3 MoAb | |||||
| No 5 | 854 | 225 | + | + | + |
| No 6 | 1,029 | 245 | + | − | + |
| No 7 | 1,211 | 491 | − | − | + |
| (median) | (median) | ||||
| Total | 1,029 | 245 | 2/3 | 1/3 | 3/3 |
Cytokine determination.For measuring cytokine plasma levels, EDTA blood samples were collected before PTX infusion, before the initial OKT3 MoAb bolus, as well as 1, 2, 4, 8, and 24 hours after the first and the second OKT3 MoAb administration. Blood samples were centrifuged immediately and the obtained plasma samples snap-frozen at −70°C until ELISA testing. IL-6 and TNFα plasma levels were determined by commercially available ELISA kits (Quantikine; DPC, Bad Neuheim, Germany) with a sensitivity of 3 pg/mL and 0.5 pg/mL, respectively.
HCMV antigen test.HCMV antigenemia was tested by immunocytology using MoAb recognizing IE-1 (B500) and pp65 antigen (both from Biotest Therapy, Dreieich, Germany) as described elsewhere.9 Briefly, mononuclear cells (PBMC) were prepared from citrated blood by standard density gradient centrifugation and washed in phosphate buffered saline (PBS). Cytospin preparations were obtained using a cytocentrifuge (Shandon, Heidelberg, Germany) and incubated with the appropriate MoAbs followed by the APAAP-staining technique (DAKO, Hamburg, Germany).
Cell lines and viruses.For transfection experiments we used the granulocyte-monocyte progenitor cell line HL-60 (ATCC CCL 240) and the premonocytic cell line THP-1 (ATCC TIB 202). Cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS, certified endotoxin-free; Biochrome, Berlin, Germany) at 37°C in a 5% humidified atmosphere. In some additional experiments primary human umbilical vein endothelial cells (HUVEC) were isolated and propagated according to the standard procedure.
HCMV strains were propagated in human lung embryonic fibroblasts (Fi 301) maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 7.5% FCS. Cells were tested for mycoplasma infection by the Mycoplasma Detection Kit (Boehringer Mannheim, Mannheim, Germany). Infection experiments were performed either with the reference strain, AD169, or low-passaged clinical isolates, C563 and C19, derived from a patient with confirmed HCMV encephalitis and from a patient with HCMV pneumonitis, respectively.
Plasmids, transfection, and CAT assay.The CAT reporter construct pRR55 contains the natural HCMV IE enhancer/promoter of strain AD169 between positions −671 and +52 relative to the IE-1/2 transcription start site.22 The plasmids p4-18PCAT, p4-19PCAT, p4-17PCAT, and p4-21PCAT carry an insertion of four copies of a synthetic oligonucleotide representing the 18-, 19-, 17-, and 21-bp enhancer motif, respectively. These insertions are located upstream of the minimal HCMV IE promoter of plasmid puPCAT. The construction of the plasmids was described elsewhere.23 All plasmids were kindly provided by T. Stamminger (Erlangen, Germany). Plasmid transfections of HL-60 and THP-1 cells by the dextran method as well as CAT assays were performed as described in detail elsewhere.11
Electrophoretic mobility shift assays (EMSA).Preparation of nuclear extracts from HL-60 or THP-1 cells was carried out as described in detail.12 For nuclear extract preparation cells were grown overnight in the presence or absence of 300 μg/mL PTX. TNFα (5 ng/mL = 100 U/mL) was added 3 hours before preparation of nuclear extracts. As target DNA for EMSA we used oligonucleotides representing either the 19- or the 18-bp sequence motif of the enhancer. Oligonucleotides were labeled by filling in recessed ends with Klenow enzyme (Boehringer Mannheim) and (α−32P)-dCTP (ICN Biochemicals, Meckenheim, Germany ). For electrophoretic mobility shift assays (EMSA) 9 μg of nuclear protein extract were incubated with 0.5 to 1.0 ng radiolabeled target DNA in 20 μL containing 10 mmol/L HEPES (pH 7.9), 50 mmol/L KCl, 5 mmol/L MgCl2 , 1 mmol/L DTT, 1 mmol/L spermidine, 1.2 μg poly(dI-dC)poly(dI-dC), and 10% glycerine for 20 minutes at room temperature before separation on 5% nondenatured polyacrylamide gel with 0.5 × TBE running buffer. For competition experiments nuclear extracts were preincubated for 5 minutes with the appropriate oligonucleotides before addition of radiolabeled target DNA. Rabbit polyclonal antisera specific to CREB-1, ATF-1, c-jun, and CREM-1 (TransCruz Gel Supershift reagents) were obtained from IC Chemikalien GmbH (Germany).
Plaque assay.Confluent monolayers of Fi301 cells were infected with HCMV at a multiplicity of infection of approximately 0.0002 viruses/cell. After adsorption for 1 hour at 37°C the monolayer was overlayed with medium containing 0.5% methocel (Fluka, Neu-Ulm, Germany) as well as increasing amounts of PTX and/or 100 U/mL TNFα. Medium was changed after 3 days and supplemented with the appropriate amount of PTX but not of TNFα. After 10 days the number of plaques were determined.
RESULTS
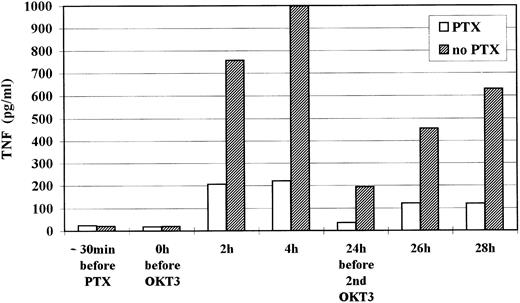
Pentoxifylline inhibits OKT3 antibody induced TNFα release but not OKT3-associated HCMV reactivation.Seven long-term renal allograft recipients with histology proven late acute rejection were treated with OKT3 MoAb (5 mg/d) for 7 days. Four of the seven patients were supplemented with PTX (4 mg/kg/d) between −30 minutes to +1.5 hours after OKT3 MoAb infusion at days 1 and 2. TNFα and IL-6 plasma levels were repeatedly determined at day 1 and day 2 post OKT3 MoAb treatment. Mononuclear blood cells were tested for HCMV antigens by APAAP immunocytology9 every 3 days. In the PTX group TNFα and IL-6 median peak plasma levels were drastically reduced when compared with the control group (248 pg/mL v 1,029 pg/mL and 23.6 pg/mL v 245 pg/mL, respectively) (Table 1). This effect was not due to a different kinetic of cytokine release as shown by serial measurements during the first 2 days (TNFα data are shown in Fig 1).
Kinetic of TNFα plasma level during the first 2 days of OKT3 MoAb treatment. Median levels of all 7 patients are shown.
Kinetic of TNFα plasma level during the first 2 days of OKT3 MoAb treatment. Median levels of all 7 patients are shown.
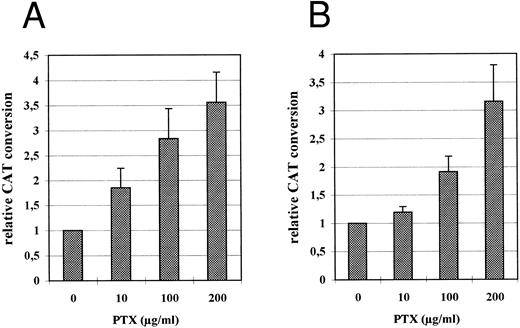
Relative CAT expression in HL-60 (A) and THP-1 (B) cells grown in the absence or in the presence of PTX. PTX was added to the culture medium immediately after transfection. Cell extracts for CAT assay were prepared 48 hours following transfection as described. The diagram represents CAT conversion rates obtained in four independent experiments (mean average ± SD).
Relative CAT expression in HL-60 (A) and THP-1 (B) cells grown in the absence or in the presence of PTX. PTX was added to the culture medium immediately after transfection. Cell extracts for CAT assay were prepared 48 hours following transfection as described. The diagram represents CAT conversion rates obtained in four independent experiments (mean average ± SD).
However, PTX had no effect on the incidence of HCMV antigenemia and HCMV disease. Before OKT 3 MoAb therapy, HCMV antigenemia was not detectable in any patient. In three of four patients in the PTX group and two of three patients in the control group, HCMV antigenemia was detected within 2 weeks after OKT3 MoAb therapy (days 15 to 21). There were no quantitative differences in the proportion of infected cells between the PTX and the control group (8-35 and 7-29 HCMV-IE/pp65 positive cells per 1,000 PBMC, respectively). The three patients in the PTX group developed fever and leukopenia concomitantly with the appearance of HCMV antigenemia or a few days later. In addition, one patient showed signs of invasive HCMV disease (pneumonitis). In the control group one patient developed HCMV disease (fever, leukopenia). All patients responded to ganciclovir therapy. No relation was seen between the TNFα plasma level and the development of HCMV disease. Because of these discouraging results we decided to discontinue the PTX study in OKT3 MoAb-treated patients.
PTX stimulates the HCMV IE enhancer/promoter activity in transiently transfected premonocytic cells.In order to study the influence of PTX on the HCMV IE enhancer/promoter both HL-60 and THP-1 cells were transfected with the CAT reporter gene plasmid pRR55 containing the HCMV major IE enhancer/promoter.11 The cells were then divided and cultured for 48 hours in the presence of increasing concentrations of PTX (added to the medium immediately after transfection) and without PTX (control). CAT expression directed by the HCMV IE enhancer/promoter was stimulated by PTX independently from the cell type and in a dose-dependent manner (Fig 2, A and B). In the presence of 200 μg/mL of PTX the activity of the HCMV IE promoter was stimulated by a factor between 3.5 and 5.5 in HL-60 cells (Fig 2A) and by a factor between 2.5 to 4 in THP-1 cells (Fig 2B).
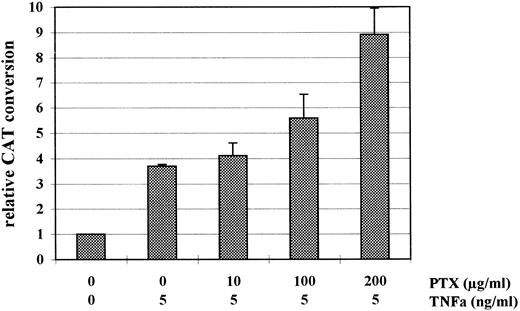
In HL-60 cells PTX and TNFα regulate IE enhancer/promoter activity independently from each other.In previous studies we could demonstrate that the cytokine TNFα is a powerful stimulator of the HCMV IE enhancer/promoter activity in transiently transfected HL-60 cells.11 Here we investigated the effect of PTX on the TNFα-mediated stimulation of this promoter. As shown in Fig 3 the positive effect of TNFα on IE enhancer/promoter-controlled CAT expression in HL-60 cells was increased by PTX in a dose-dependent manner. In the presence of 100 U/mL TNFα and 200 μg/mL PTX CAT expression was twice as high as in cells treated with 100 U/mL of TNFα alone.
Relative CAT expression in HL-60 cells incubated with TNFα and PTX in combination, with TNFα alone, or in the absence of either. PTX was added to the culture medium immediately after transfection and 1 hour before TNFα. After 48 hours at 37°C cell extracts were prepared for CAT assay. The diagram shows mean value of relative CAT conversion rates (±SD) measured in four independent experiments.
Relative CAT expression in HL-60 cells incubated with TNFα and PTX in combination, with TNFα alone, or in the absence of either. PTX was added to the culture medium immediately after transfection and 1 hour before TNFα. After 48 hours at 37°C cell extracts were prepared for CAT assay. The diagram shows mean value of relative CAT conversion rates (±SD) measured in four independent experiments.
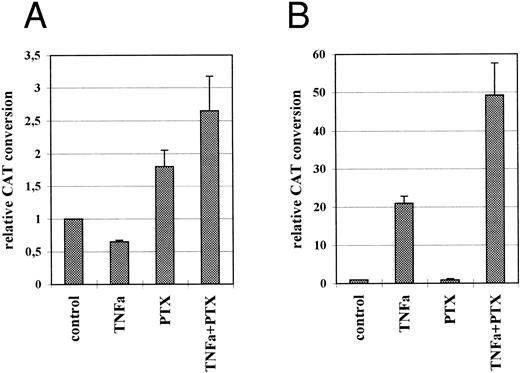
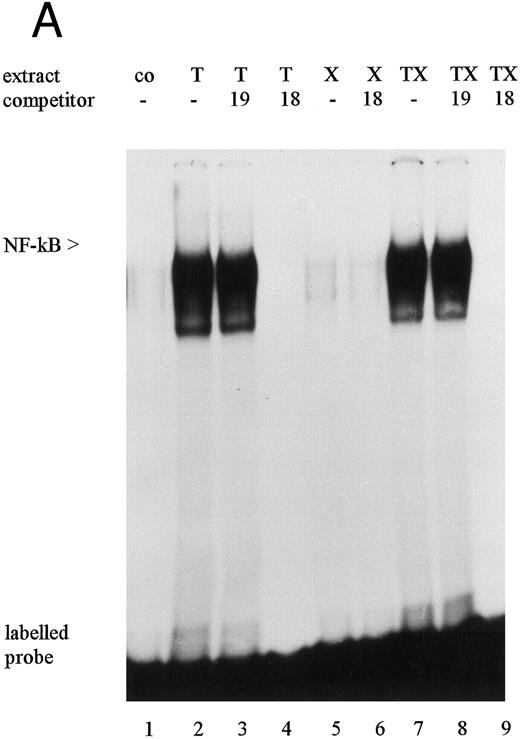
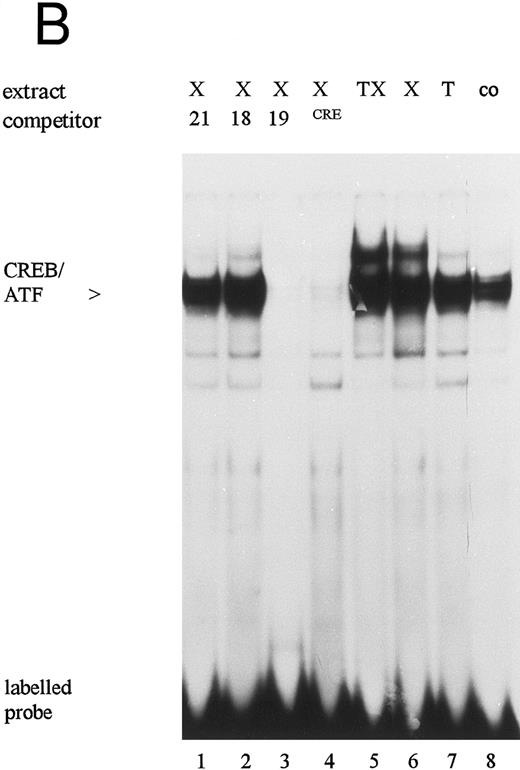
Stimulatory effect of PTX on the IE enhancer/promoter is mediated by the 19-bp sequence motif of the enhancer and phosphorylation of the CREB transcription factor.To identify the target sequence for the stimulatory effect of PTX, HL-60 cells were transiently transfected with the plasmid constructs p4-19PCAT, p4-18PCAT, p4-17PCAT, p4-21PCAT, or puPCAT containing the minimal IE promoter and four copies of defined repetitive sequence motifs of the enhancer.12,24 The transfected cells were incubated either in the presence or absence of PTX. For reference purposes, transfected cells were incubated with TNFα alone or in combination with PTX. As described earlier, base-line expression of CAT was only found in those cells transfected with the plasmids p4-18PCAT and p4-19PCAT.12 An increase of CAT expression due to PTX was only found in cells that had been transfected with plasmid p4-19PCAT, indicating that the stimulatory effect of PTX was mediated via the 19-bp sequence motif of the IE enhancer (Fig 4A). In the presence of 200 μg/mL PTX a two-fold increase of CAT expression was observed. In p4-19PCAT transfected THP-1 cells PTX induced stimulation by factors 3 to 4 (not shown). In contrast to this, the stimulatory effect of TNFα was mediated exclusively via the 18-bp sequence motif containing an NF-kB binding site (Fig 4B).12 In p4-19PCAT transfected HL-60 and THP-1 (not shown) cells TNFα suppressed base-line CAT expression by approximately 40% and 70%, respectively. Surprisingly, when p4-18PCAT transfected cells were treated with both TNFα and PTX, stimulation of CAT expression was much higher than in cells treated with TNFα alone. In p4-19PCAT transfected HL-60 cells grown in the presence of PTX and TNFα, stimulation of CAT expression was also slightly increased compared with cells incubated with PTX alone (1.8- vs 2.6-fold expression). In the electrophoretic mobility shift assay (EMSA) we were able to show that in TNFα- and TNFα/PTX-treated (Fig 5A, lane 2 and 7) HL-60 cells a protein is activated that specifically binds to the 18-bp sequence motif as shown by competition experiments using oligonucleotides containing either the 18-bp or the 19-bp sequence motif (Fig 5A, lanes 3, 4, 8, and 9). Recently, this protein has been identified as the eukaryotic transcription factor NF-kB.12 According to the above results PTX failed to induce NF-kB (Fig 5A, lane 5).
Relative CAT expression in untreated, TNFα-, PTX-, or TNFα/PTX-treated HL-60 cells transfected with the plasmids p4-19PCAT (A) or p4-18PCAT (B). Following transfection the cells were incubated for 48 hours in the absence or presence of TNFα (5 ng/mL) and/or PTX (200 μg/mL). PTX was added immediately after transfection and 1 hour before TNFα. The graph represents CAT conversion rates obtained in four independent experiments (mean average ± SD).
Relative CAT expression in untreated, TNFα-, PTX-, or TNFα/PTX-treated HL-60 cells transfected with the plasmids p4-19PCAT (A) or p4-18PCAT (B). Following transfection the cells were incubated for 48 hours in the absence or presence of TNFα (5 ng/mL) and/or PTX (200 μg/mL). PTX was added immediately after transfection and 1 hour before TNFα. The graph represents CAT conversion rates obtained in four independent experiments (mean average ± SD).
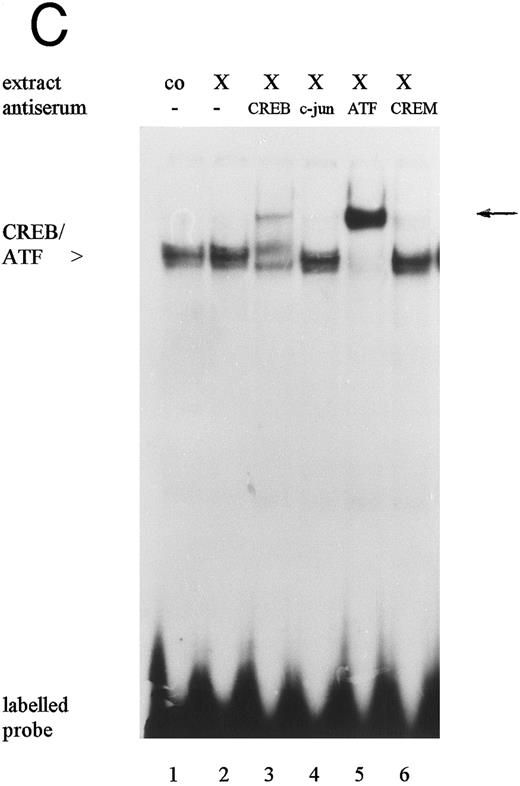
Electrophoretic mobility shift assays: Protein binding activity in nuclear extracts from untreated (co), TNFα- (T), PTX- (X), and TNFα/PTX (TX)-treated HL-60 cells to the 18-bp (A) or the 19-bp (B and C) sequence motif. Specificity of the DNA-protein interaction was determined by competition experiments (A and B) using unlabeled oligonucleotides containing the 18-bp (18), the 19-bp (19), or the 21-bp (21) sequence motifs of the IE enhancer or an oligonucleotide containing the CRE consensus sequence (CRE). For competition nuclear extracts were preincubated for 5 minutes with a 30 to 50 molar excess of unlabeled oligonucleotides before addition of the radiolabeled probe. The protein complex bound to the 19-bp sequence motif was characterized by antisera reactive to CREB-1, ATF-1, c-jun, or CREM-1 (C). The reaction mixture was supplemented with 1.5 μL antisera. Arrows on the right point to a new, highly retarded protein-DNA-antibody complex. The autoradiograph shows one representative experiment that was repeated at least three times.
Electrophoretic mobility shift assays: Protein binding activity in nuclear extracts from untreated (co), TNFα- (T), PTX- (X), and TNFα/PTX (TX)-treated HL-60 cells to the 18-bp (A) or the 19-bp (B and C) sequence motif. Specificity of the DNA-protein interaction was determined by competition experiments (A and B) using unlabeled oligonucleotides containing the 18-bp (18), the 19-bp (19), or the 21-bp (21) sequence motifs of the IE enhancer or an oligonucleotide containing the CRE consensus sequence (CRE). For competition nuclear extracts were preincubated for 5 minutes with a 30 to 50 molar excess of unlabeled oligonucleotides before addition of the radiolabeled probe. The protein complex bound to the 19-bp sequence motif was characterized by antisera reactive to CREB-1, ATF-1, c-jun, or CREM-1 (C). The reaction mixture was supplemented with 1.5 μL antisera. Arrows on the right point to a new, highly retarded protein-DNA-antibody complex. The autoradiograph shows one representative experiment that was repeated at least three times.
The 19-bp sequence motif contains a binding site for transcription factors of the CREB/ATF family.23,25 These transcription factors play a key role for the base-line activity of the HCMV IE enhancer/promoter in HeLa cells.23 Our EMSA results also showed that there is high constitutive expression of protein-binding activity to the 19-bp sequence motif in untreated HL-60 cells (Fig 5B, lane 8). Moreover, the 19-bp binding activity in nuclear extracts from HL-60 cells treated with either PTX or TNFα/PTX was increased when compared with that observed in untreated control cells or cells grown in the presence of TNFα (Fig 5B, lanes 5-8). In competition experiments specific complex formation was abrogated in the presence of unlabeled 19-bp motif-contained oligonucleotide or an oligonucleotide containing the consensus CRE element (SantaCruz Inc, CA) (Fig 5B, lanes 3 and 4) but not in the presence of oligonucleotides representing the 21- or the 18-bp sequence motif of the enhancer (lanes 1 and 2). Furthermore, antisera against ATF-1 and CREB-1 but not c-jun or CREM-1 (SantaCruz Inc) supershifted the DNA-protein complex (Fig 5C, lanes 3-6), suggesting that the proteins bound to the 19-bp sequence motif belong in fact to the CREB-1/ATF-1 family.
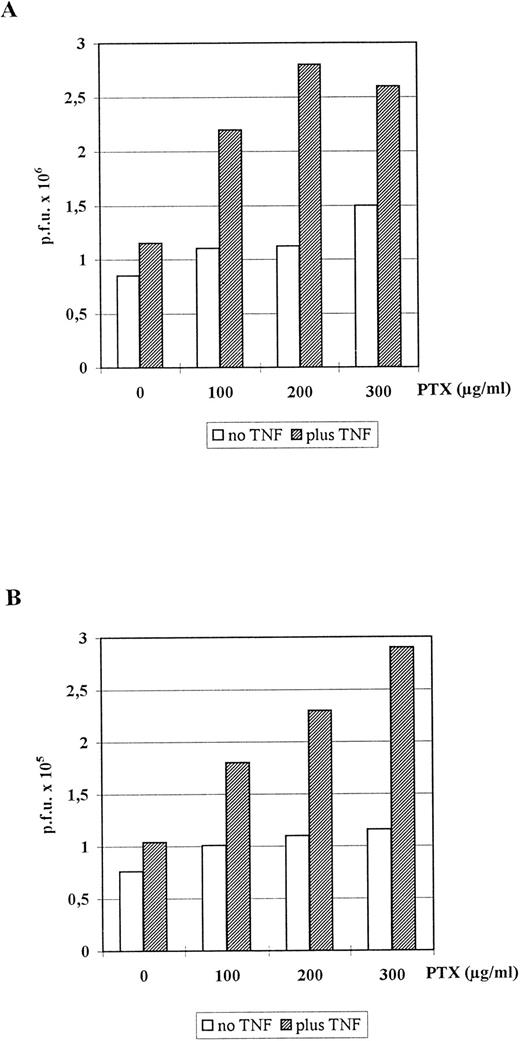
Synergistic stimulation of HCMV replication in human embryonal lung fibroblasts and HUVEC by PTX and TNFα.In order to study the influence of PTX and/or TNFα on HCMV replication, human embryonic lung fibroblasts (the most suitable cell for HCMV in vitro) were infected either with the reference strain AD169 (Fig 6A) or the low-passaged clinical isolate C563 (Fig 6B) and cultured in the presence of increasing amounts of PTX or without PTX and/or 100 U/mL TNFα. While either substance alone had only marginal stimulatory effects, virus replication was enhanced in the presence of both PTX and TNFα (Fig 6A and B). The stimulatory effect was dose-dependent with respect to PTX and independent from the virus strain used. Immunohistologic staining of the infected fibroblasts with the E13 MoAb specific for the HCMV IE 72-kD and 86-kD proteins indicated that antigen expression was significantly accelerated by both substances. On day 3 postinfection in PTX- and TNFα-treated cultures the numbers of IE-positive cells were approximately two to three times higher than in untreated control cultures. Cytopathic effects (CPE) were not detectable before day 6 in both groups. Then the ratio of the number of plaques in PTX + TNFα-treated/number of plaques in untreated control cultures remained stable at factor 2-3 (data for day 10 are shown in Fig 6). Moreover, plaques in control cultures were significantly smaller than in cultures treated with either PTX or TNFα or both substances (not shown).
Influence of TNFα and/or PTX on HCMV replication in human embryonal lung fibroblasts. Monolayers of Fi 301 cells were infected with either AD169 (A) or C563 (B). For plaque assay cells were incubated for 10 days at 37°C with one medium change at day 3. The graph represents data of one representative experiment that has been repeated four times.
Influence of TNFα and/or PTX on HCMV replication in human embryonal lung fibroblasts. Monolayers of Fi 301 cells were infected with either AD169 (A) or C563 (B). For plaque assay cells were incubated for 10 days at 37°C with one medium change at day 3. The graph represents data of one representative experiment that has been repeated four times.
Stimulation of HCMV antigen expression by TNFα and PTX was also observed in virus-infected HUVEC. HUVEC were infected with the clinical isolate C19. This low-passaged isolate is much more effective in infection of endothelial cells than the laboratory strain AD169 although virus spread and CPE could not be observed. Therefore, the number of infected and IE antigen expressing cells per well was counted after staining with the E13 MoAb (IE) or C10/C11 MoAb (pp65). Neither TNFα (99 positive cells/well) nor PTX (115/well) alone significantly increased the number of IE-positive cells compared with untreated controls (90/well). The combination of both substances, by contrast, elevated the number of infected cells by factor 1.5-2 (152/well). Results obtained after staining for pp65 matrix protein expression were quite similar (factor 1.6-1.9).
DISCUSSION
OKT3 MoAb therapy is well established in the prevention and therapy of acute rejection in transplant patients. Unfortunately, this therapy is associated with several short-term (cytokine release syndrome) and long-term (infections, EBV-related lymphoma) side effects.26-31 In a previous study we observed that all patients receiving OKT3 MoAb developed active HCMV infection 1 week or later following the first dose.9 Additional studies in vitro and in vivo suggested that TNFα has an important role in the reactivation of latent HCMV in granulocyte/monocyte progenitor cells resulting in active infection or even disease.9-12
In order to prevent this TNFα-associated HCMV reactivation in myeloid progenitor cells, we treated our patients in addition to OKT3 MoAb with PTX, a methylxanthine derivative that has been shown to suppress TNFα induction. Although the TNFα peak plasma level following OKT3 MoAb treatment was markedly reduced, the incidence of HCMV reactivation and HCMV disease was not influenced.
PTX has been shown to inhibit TNFα synthesis and secretion in macrophages, monocytes, and natural killer cells in vitro and in vivo.3,18-20,32,33 In clinical studies, PTX has been found to reduce the amount of TNFα released into the blood following OKT3 MoAb injection.14,15,32 34-36 In contrast to what we had expected, the incidence of active HCMV infection and disease in patients treated with PTX was not decreased compared with the control group. This could have been considered an ineffective blockade of TNFα release by PTX; however, the reduction of TNFα and (TNFα-induced) IL-6 plasma peak levels by factors 4 and 10, respectively, are in disagreement with this explanation.
Therefore, we wondered whether PTX has any direct effects on HCMV activity. In fact our data support a role of PTX in the process of HCMV antigen expression and replication. We were able to demonstrate that PTX, just like TNFα, is a powerful stimulator of the HCMV IE enhancer/promoter in transfected premonocytic cells in vitro. Unlike TNFα, which upregulates the IE enhancer/promoter only in the undifferentiated granulocyte/monocyte progenitor cell line HL-60 (serving as a model of latently infected cells in vivo), PTX enhances the promoter activity independently from the cell line and the differentiation stage of premonocytic cells. Moreover, in the presence of both substances the stimulatory effect on the IE enhancer/promoter-controlled CAT expression is additive. This observation suggests that both TNFα and PTX act on the HCMV IE enhancer/promoter via different and independent mechanisms. We were recently able to show that the stimulatory effect of TNFα in HL-60 cells is mediated via the activation and binding of the eukaryotic transcription factor NF-kB to the 18-bp repetitive sequence motif in the enhancer.12 The stimulatory effect of PTX, by contrast, was found to be independent from NF-kB. Transfection experiments using promoter constructs containing only the minimal IE promoter and single repetitive sequence motifs of the enhancer showed that the stimulatory effect of PTX is mediated by the 19-bp sequence motif in the enhancer containing a cAMP responsive element (CRE), which is able to bind members of the CREB/ATF transcription factor family.23,25 Using MoAbs recognizing individual members of this transcription factor family, binding of CREB-1 as well as ATF-1 to the 19-bp sequence motif was demonstrated in EMSA. The amounts of CREB-1/ATF-1 were shown to be increased in nuclear extracts from PTX or PTX/TNFα-treated cells. The transcriptional activity of CREB depends on the phosphorylation of CREB at serine 133,37 nevertheless, unphosphorylated CREB also binds DNA. We, therefore, propose that PTX stimulates the HCMV IE enhancer/promoter via the cAMP pathway leading to enhanced phosphorylation and activation of the transcription factors CREB-1/ATF-1. This hypothesis is supported by the finding that the PTX effect on the HCMV IE enhancer/promoter could be simulated by dibuturyl-cAMP, a cAMP-analogue (not shown). Nevertheless, when TNFα and PTX were used in combination, the stimulatory effects along both pathways were enhanced. This was shown by transfection experiments with constructs containing only the minimal promoter and either the 18-bp or the 19-bp sequence motifs. The mechanisms underlying this synergism, however, remain to be identified.
Because infection of HL-60 or THP-1 cells with HCMV is known to be very ineffective, the influence of PTX alone or in combination with TNFα on viral replication was tested in permissive embryonal lung fibroblast. It was shown that PTX and TNFα acted synergistically in stimulating HCMV replication in these cells while either substance alone had only marginal stimulatory effects. The effects of PTX and TNFα on virus replication were independent from the virus strain tested. Very similar results were obtained in HCMV-infected HUVEC. Immunocytologic staining of virus-infected HUVEC with the MoAbs E13 (reactive to HCMV IE1/2 proteins) or Clonab pp65 (reactive to the major matrix protein pp65) gave significantly higher numbers of antigen positive cells in cultures that had been incubated in the presence of both TNFα and PTX compared with cultures treated with either substance alone or untreated cells.
Presently, the high anti-inflammatory potential of cAMP-elevating drugs, particularly phosphodiesterase inhibitors like PTX, is being rediscovered. Just as for HCMV, TNFα is also a powerful stimulator of human immunodeficiency virus type 1 (HIV-1) replication and of HIV-LTR in immature monocytic cells.38,39 Stimulation of HIV-1 by TNFα is also mediated via activation of the transcription factor NF-kB and its binding to the viral LTR. Furthermore, synergistic stimulation of HIV by cAMP and TNFα from latently infected cells of monocyte-macrophage lineage has been described.40 The mechanism of this synergism was not brought to light because no cAMP-responsive element could be identified in the LTR of HIV-1. In vitro PTX has been shown to decrease HIV replication in PBMC and in cultured T cells as a consequence of TNFα suppression.41 Results from clinical trials testing PTX in the treatment of AIDS, however, are less clear.42-44 While PTX decreased TNFα mRNA levels in PBMC derived from AIDS patients, viral load and p24 expression were mostly unchanged.42
Other potential applications of PTX and related drugs (eg, other phosphodiesterase inhibitors) may include the treatment of acute (sepsis) and chronic (rheumatoid arthritis, chronic inflammatory bowel disease) inflammatory diseases. Clinical trials are currently carried out. Nevertheless, our data clearly point to a potentially harmful side effect of this group of drugs (phosphodiesterase inhibitors and other cAMP elevating drugs) — the reactivation of latent HCMV infection. This may be dangerous particularly in patients with a defective T-cell response. As a consequence, HCMV antigenemia should be carefully monitored in such patients.
ACKNOWLEDGMENT
We thank K. Muske and C. Liebenthal for excellent technical assistance and Dr F. Kern for helpful discussions during preparation of the manuscript.
Supported by a grant of Deutsche Forschungsgemeinschaft (DFG) Pr 337/1-2.
Address reprint requests to Hans-Dieter Volk, MD, Institute of Medical Immunology, Medical Faculty (Charité), Humboldt Universität zu Berlin, D-10089 Berlin, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal