Abstract
Interleukin-10 (IL-10) has been reported to be a negative cytokine for monocytes/macrophages. In the present study, we showed that IL-10 is rather a positive cytokine and augments the growth and differentiation of human monocytes stimulated with macrophage colony-stimulating factor (M-CSF ). Highly purified adherent human monocytes were cultured for 7 days with M-CSF in the presence or absence of IL-10. The number of recovered cells increased in the culture of monocytes with M-CSF + IL-10 compared to the culture with M-CSF alone. IL-10 alone was not enough to maintain the survival and differentiation of monocytes into macrophages. Morphological change cultured in M-CSF was also accelerated by addition of IL-10, and macrophages cultured in M-CSF + IL-10 were more elongated compared to macrophages cultured with M-CSF alone. Binding of 125I-M-CSF to monocytes incubated with M-CSF + IL-10 was about 1.7-fold higher than that to monocytes incubated with M-CSF alone. In accordance with the binding study, Northern blot analysis showed that the levels of the expression of c-fms, M-CSF receptor, mRNA in macrophages cultured in M-CSF + IL-10 were higher than that in macrophages cultured in M-CSF alone. Macrophages cultured in M-CSF + IL-10 expressed higher level of FcγRI, II, III, and showed augmented Fcγ receptor mediated phagocytosis. The former also produced higher level of H2O2 and O−2 , when stimulated with zymosan, and of IL-6 when stimulated with lipopolysaccharide compared to the latter. These results taken together suggest that IL-10 augments the growth and differentiation of human monocytes cultured in M-CSF.
INTERLEUKIN-10 (IL-10) is a cytokine produced by T cells, B cells, and monocytes/macrophages. IL-10 shows both positive and negative immunoregulatory functions; it inhibits the synthesis of cytokines such as interferon-γ, IL-1α in Th1 cells,1,2 downregulates constitutive and IFN-γ or IL-4–induced expressions of MHC class II antigen,3,4 and the production of H2O25 or NO−26 in mouse peritoneal macrophages. These results indicate that IL-10 is an inhibitory cytokine against many kinds of cells. However, IL-10 has also enhancing effects on various cells. IL-10 stimulates the growth of B cells7 and mast cells.8 Moreover, IL-10 induces Fcγ receptor I (FcγRI) expression which relates to the clearance of immune complexes and antibody-dependent cellular cytotoxicity (ADCC) activity of human monocytes, and stimulates ADCC.3
Macrophage colony-stimulating factor (M-CSF ) is a hematopoietic glycoprotein that stimulates the proliferation and differentiation of bone marrow progenitor cells into mature cells. M-CSF plays an important role in monocyte/macrophage homeostasis9-11 and promotes the production of other cytokines by monocyte/macrophages.12,13 These properties are indispensable to the antimicrobial defense of host animals against bacteria and fungi14 and inducing cytotoxicity of the host cells against certain tumor cell targets via ADCC.15 16
In this study, we determined the effects of IL-10, and M-CSF on the survival, growth and differentiation of human monocytes. We show that IL-10 alone, in contrast to M-CSF alone, does not stimulate the survival, growth, and differentiation into macrophages of human monocytes, but IL-10 can enhance those of monocytes stimulated with M-CSF. Furthermore, we show that macrophages generated from human monocytes by M-CSF plus IL-10 are superior in several functions such as reactive oxygen intermediate (ROI) or IL-6 production and FcγR-mediated phagocytosis than macrophages generated by M-CSF alone. IL-10 together with M-CSF increased the expression of M-CSF receptors. These results provide the evidence that IL-10 acts as an enhancing cytokine on monocytes survival, growth and differentiation by cooperating with M-CSF.
MATERIALS AND METHODS
Cytokines.Recombinant human M-CSF (M-CSF ) was purified as described.12 rhGM-CSF and rhIL-10 were obtained from Genzyme Corp (Cambridge, MA).
Preparation of human monocytes.Peripheral blood mononuclear cells were obtained from the venous blood drawn from normal healthy volunteers with their informed consent as described.17 Briefly, PBMC were isolated by centrifugation on a Ficoll-Metrizoate density gradient (Lymphoprep; Nycomed, Oslo, Norway) and suspended in RPMI 1640 medium (GIBCO, Grand Island, NY) containing 7.5% (heat-inactivated fetal calf serum [FCS], GIBCO), 100 μg/mL streptomycin and 100 U/mL penicillin. The FCS contained 0.003 ng of lipopolysaccharide per mL as assessed by the limulus amebocyte lysate test. PBMC were placed into monocyte-isolating plates (MSP-P; Japan Immunoreserch Laboratories Co, Ltd, Takasaki, Japan) and incubated for 2 hours at 37°C in a humidified 5% CO2 atmosphere. The resultant adherent cells were collected by vigorous pipetting. More than 96% of the cells were judged to be monocytes by morphology, CD14 positively (LeuM3; Becton Dickinson, San Jose, CA) and nonspecific esterase staining.
Adherent cell counting.The cetrimide-Coulter counter procedure described by Chen et al18 was used to count adherent macrophages. In brief, macrophage cultures were depleted of medium by aspiration, then resuspended in warm cetrimide solution for 3 minutes at 37°C to lyse adherent macrophages and liberate intact nuclei. The nuclei were then counted using a Coulter counter.
Assay for O−2 production. The chemiluminesense (CL) analyzer Biolumat (LB9505; Berthod, Germany) was used to measure CL activity generated by macrophages.19 Macrophages cultured with M-CSF and IL-10 or both for 7 days, were dislodged in the presence of 0.2% Lidocaine, washed, and suspended in Hanks' balanced salt solution with Lucigenin (final concentration, 1 mmol/L). Test vials containing 0.5 mL of sample were initially warmed in an incubator at 37°C for 10 minutes. Reactions were started by adding 1.0 mg/mL of zymosan. CL activities of the stimulated macrophages were measured continuously for 30 to 60 minutes, recorded on a TV monitor, and stored on a disk for analysis.
Assay for H2O2 production.The cellular release of H2O2 was detected by the semiautomated microassay reported by Harpe and Nathan.20 In brief, human monocytes (5 × 104 per well in 100 μL) were cultured in 96-well flat-bottom tissue culture plates in the presence of M-CSF plus IL-10 or M-CSF alone for 7 days. The culture medium was removed from the plates and the adherent cells were rinsed with phosphate-buffered saline (PBS). The assay mixture was prepared before use from stock solutions and consisted of 30 μmol/L scopoletin (Sigma, St Louis, MO), 1 mmol/L NaN3 , 1 purpurogallin U/mL horseradish peroxides (Sigma), in Krebs-Ringer phosphate buffer (145 mmol/L NaCl, 4.86 mmol/L KCl, 0.54 mmol/L CaCl2 , 1.22 mmol/L MgSO4 , 5.7 mmol/L sodium phosphate) containing 5.5 mmol/L glucose. Zymosan (1 mg/mL) was the stimulus. Immediately after adding zymosan, the plate was placed in a fluorometer and the fluorescence for each well was recorded (0 minutes). The plate was maintained at 37°C and after 60 minutes the fluorescence in each well was again measured. H2O2 release was calculated from the loss of fluorescence, using the formula:
where E0 is the initial fluorescence reading for the well; E60 is the fluorescence reading at 60 minutes; W is the fluorescence recorded in an empty well; C0 and C60 are the mean fluorescence readings in the cell-free control wells at 0 and 60 minutes, respectively; and S is the amount of scopletin (3 nmol) added to each well at the start of assay.
Phagocytosis of EA.Fc receptor (FcR)-mediated phagocytosis was examined by a modification of the described procedure.21 Briefly cells were washed once and incubated with 1 mL of 0.4% EA (sheep erythrocytes sensitized with IgG of rabbit anti-sheep erythrocytes antiserum) at 37°C. Following 20 minutes or 1 hour incubation, noninternalized EA were lyzed with ammonium chloride. The percentage of cells ingesting more than one EA was determined by counting at least 300 cells, and the results are expressed as the mean and the standard deviations of triplicate.
Northern blots.Total RNA, isolated using RNAzol-B (TEL-TEST, INC, Friendswood, TX), was fractionated on a 1% agarose formaldehyde gel, transferred to nylon membrane (Hybond-N; Amersham, Arlington Heights, IL), and immobilized by ultraviolet cross-linking.22 Membranes were prehybridized with hybridization buffer (50% deionized formamide, 0.72 mol/L NaCl, 50 mmol/L sodium phosphate, 5 mmol/L Na2-EDTA, 0.5% SDS, 5× denhalt, 40 μg/mL S.S.DNA, 10% dextran) for 2 hours. A c-fms DNA probe (Oncogene Science, Manhasset, NY) and a human GAPDH DNA probe were radioisotopically labeled using Multiprime DNA labeling system (Amersham) and [α-32P]-dCTP (Amersham). The membranes were hybridized overnight in hybridization buffer, washed twice for 10 minutes at 42°C with 2× SSPE (0.36 mol/L NaCl, 20 mmol/L sodium phosphate, 0.2 mmol/L Na2-EDTA, pH 7.7), for 30 minutes at 42°C in 0.1% sodium dodecyl sulfate (SDS)/1× SSPE, twice for 30 minutes at room temperature in 0.1% SDS/0.1 × SSPE, and then exposed to film (Eastman Kodak, Rochester, NY) for autoradiography at −80°C.
Assay for M-CSF–binding receptor.M-CSF was iodinated as described.23 In brief, M-CSF was radioiodinated with chloramine T at a specific activity of 3.0 × 108 cpm/μg. Macrophages (1 × 105) induced with M-CSF plus IL-10 or with M-CSF alone, were incubated in 0.1 mL of culture medium containing an appropriate amount of radioiodinated M-CSF at 4°C for 6 hours. The cells were washed twice with cold PBS and dissolved in 0.5N NaOH, then the radioactivity was measured in a liquid scintilation counter. To obtain the specific binding of M-CSF, a 100-fold excess of unlabeled M-CSF was added to the above reaction mixture and the radioactivity bound to the cells was subtracted from the value obtained in the absence of unlabeled M-CSF.
Assay for lymphokines.The production of IL-6 by monocytes was determined by means of IL-6–specific enzyme-linked immunosorbent assay (ELISA). IL-6–specific ELISA kit was obtained from Amersham International plc (Bucks, UK).
Analysis of cell surface markers.Expression of cell surface were analyzed by direct immunofluorescence. Monocytes were cultured with M-CSF in the presence or absence of IL-10 for 7 days and harvested by vigorous pipetting with Lidocaine. After washing with PBS, cells were incubated with normal Goat serum to block nonspecific binding at 4°C for 30 minutes, then were stained with fluorescein isothiocyanate (FITC)-conjugated CD14 (LeuM3; Becton Dickinson), HLA-DR (NU-la; Nitirei, Tokyo), CD16 (3G8; Immunotech, Marseille, France), CD32 (2003; Pharmingen, San Diego, CA), CD64 (10.1; Pharmingen, San Diego, CA) and CD11b (BEAR1; Immunotech), then analyzed by flow cytometry (EPICS). For all experiments, an FITC-conjugated irrelevant control monoclonal antibody (MoAb) of the same IgG isotype was included.
Statistical analysis.The significance of all assays was assessed by Student's t-test.
RESULTS
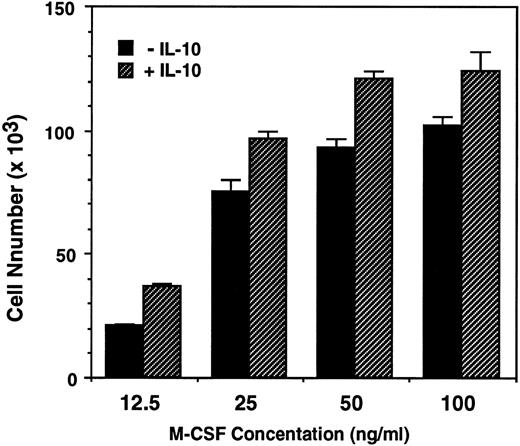
IL-10 enhance M-CSF–dependent monocytes survival, growth, and differentiation into macrophages.To determine the effects of IL-10 and M-CSF on the survival and growth of human monocytes, monocytes were incubated for 7 days with these cytokines, and the numbers of recovered cells was counted. M-CSF (50 ng/mL) alone stimulated the monocyte survival, and the number of recovered cells was almost the same as that of inoculated cells. In contrast to M-CSF, IL-10 (1 to 100 ng/mL), alone is ineffective and almost all of the monocytes died as well as in culture of monocytes incubated with medium alone. However, IL-10, enhanced the effect of M-CSF in a dose-dependent manner, and the number of recovered cells in monocytes cultured with IL-10 + M-CSF were significantly higher than that in monocytes cultured with M-CSF alone (1.3 times increase was observed at more than 10 ng/mL of IL-10, but 1 ng/mL of IL-10 did not show significant effect) (Table 1). When monocytes were incubated with graded dose of M-CSF (12.5 to 100 ng/mL), the number of recovered cells depended directly on the concentrations of M-CSF. At the dose of 12.5 or 25 ng/mL, the number of recovered cells was rather lower than that of inoculated cells, but at the dose of 100 ng/mL, the number was rather increased and is 1.2 times of the inoculated cells. These results suggest that M-CSF can stimulate not only the survival but also the growth of monocytes. In these cultures, IL-10 (10 ng/mL) enhanced the recovered cell number at any dose of M-CSF and the number of recovered cells in monocytes cultured with M-CSF (100 ng/mL) + IL-10 was about 1.5 times of the inoculated cells (Fig 1).
IL-10 Enhances the M-CSF–Dependent Growth of Human Monocytes
| IL-10 (ng/mL) . | Cell No. (×104) . | |
|---|---|---|
| . | − M-CSF . | + M-CSF . |
| 0 | 0.5 ± 0.2 | 9.3 ± 0.8 |
| 1 | 0.6 ± 0.3 | 10.8 ± 0.6 |
| 10 | 0.2 ± 0.2 | 12.1 ± 0.6* |
| 100 | 0.1 ± 0.2 | 12.4 ± 0.3* |
| IL-10 (ng/mL) . | Cell No. (×104) . | |
|---|---|---|
| . | − M-CSF . | + M-CSF . |
| 0 | 0.5 ± 0.2 | 9.3 ± 0.8 |
| 1 | 0.6 ± 0.3 | 10.8 ± 0.6 |
| 10 | 0.2 ± 0.2 | 12.1 ± 0.6* |
| 100 | 0.1 ± 0.2 | 12.4 ± 0.3* |
Monocytes (8.8 × 104) in 24-well tissue culture plates were incubated with IL-10 (1, 10, 100 ng/mL), M-CSF (50 ng/mL), M-CSF + IL-10 or medium alone for 7 days. The cells were counted using cetrimide as described in Materials and Methods. Data are expressed as the mean ± SD from three wells in triplicate experiments.
P < .05 versus IL-10, 0 ng/mL.
IL-10 enhances the M-CSF–dependent growth of human monocytes. Monocytes (8.8 × 104) in 24-well tissue culture plates were incubated with increasing concentration of M-CSF (12.5 to 100 ng/mL) in the presence or absence of IL-10 (10 ng/mL) for 7 days. The recovered cells were counted using cetrimide as described in Materials and Methods. Data are expressed as the mean of triplicated wells (+SD) from a representative experiments of three performed.
IL-10 enhances the M-CSF–dependent growth of human monocytes. Monocytes (8.8 × 104) in 24-well tissue culture plates were incubated with increasing concentration of M-CSF (12.5 to 100 ng/mL) in the presence or absence of IL-10 (10 ng/mL) for 7 days. The recovered cells were counted using cetrimide as described in Materials and Methods. Data are expressed as the mean of triplicated wells (+SD) from a representative experiments of three performed.
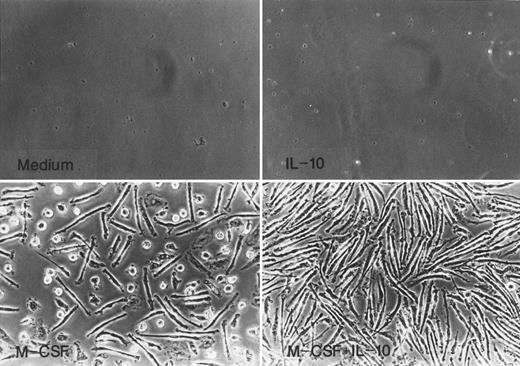
Monocytes cultured for 7 days with M-CSF or M-CSF + IL-10 underwent morphological change characteristic of monocytes to macrophage differentiation such as increase in size and adherence as shown in Fig 2. Most cells recovered from culture with M-CSF were rather elongated and showed spindle-like morphology, and the remaining cells were round. In contrast, almost all of the cells cultured in M-CSF + IL-10 showed elongated spindle-like morphology and no round cells exist.
Morphology of monocytes cultured in M-CSF or M-CSF + IL-10. Monocytes were incubated with rhIL-10 (25 ng/mL), rhM-CSF (50 ng/mL), M-CSF + IL-10, or medium alone for 7 days.
Morphology of monocytes cultured in M-CSF or M-CSF + IL-10. Monocytes were incubated with rhIL-10 (25 ng/mL), rhM-CSF (50 ng/mL), M-CSF + IL-10, or medium alone for 7 days.
These results taken together indicate that IL-10 is unable to stimulate the survival, growth and differentiation of monocytes, but IL-10 can cooperate with M-CSF and increased the survival, growth, and differentiation into macrophages of monocytes.
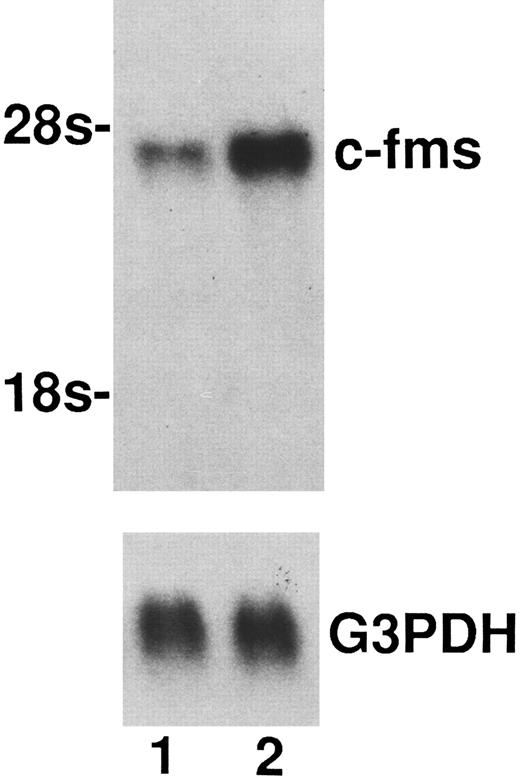
Upregulation of M-CSF receptor in macrophages generated from monocytes cultured with M-CSF + IL-10.As a reason for the enhancing effect of IL-10 on M-CSF cultured monocytes growth and differentiation into macrophage, it is possible to consider that IL-10 upregulate the c-fms, M-CSF receptor. To clarify this possibility, binding assay to M-CSF receptor using 125I-M-CSF and Northern blot analysis of c-fms mRNA expression in macrophages generated form monocytes cultured with M-CSF + IL-10 or M-CSF alone were performed. As shown in Table 2, the binding of 125I-M-CSF to the macrophages cultured in M-CSF + IL-10 was about 1.7-fold higher than that to the macrophages cultured in M-CSF alone. In accordance with these data, macrophages cultured in M-CSF + IL-10 expressed higher level of c-fms mRNA compared to macrophages cultured in M-CSF alone (Fig 3).
Increased M-CSF Receptor Levels on Macrophages Generated From Monocyte Cultured With M-CSF + IL-10
| . | 125I-M-CSF Specific Binding (CPM/105 cells) . | |
|---|---|---|
| . | M-CSF . | M-CSF + IL-10 . |
| Exp. 1 | 4,254 | 6,964 |
| Exp. 2 | 3,400 | 6,588 |
| Exp. 3 | 2,903 | 4,848 |
| . | 125I-M-CSF Specific Binding (CPM/105 cells) . | |
|---|---|---|
| . | M-CSF . | M-CSF + IL-10 . |
| Exp. 1 | 4,254 | 6,964 |
| Exp. 2 | 3,400 | 6,588 |
| Exp. 3 | 2,903 | 4,848 |
Monocytes were incubated with rhM-CSF (50 ng/mL) plus IL-10 (25 ng/mL) or M-CSF alone for 1 week, then 125I-M-CSF binding was assessed as described in Materials and Methods. Data are results from three independent experiments.
Enhancement of the expression of c-fms mRNA by IL-10. Total RNAs (15 μg) from macrophages generated from monocytes cultured for 7 days with M-CSF + IL-10 or M-CSF alone were loaded in each lane. Total RNAs was processed for Northern blotting as described in Materials and Methods. Lane 1, mRNA from macrophages cultured in M-CSF (50 ng/mL) alone. Lane 2, mRNA from macrophages cultured in M-CSF (50 ng/mL) + IL-10 (25 ng/mL). Arrow indicates rRNA size markers. Evenness of loading was assessed by hybridization with a GAPDH probe. One representative experiment of three is shown.
Enhancement of the expression of c-fms mRNA by IL-10. Total RNAs (15 μg) from macrophages generated from monocytes cultured for 7 days with M-CSF + IL-10 or M-CSF alone were loaded in each lane. Total RNAs was processed for Northern blotting as described in Materials and Methods. Lane 1, mRNA from macrophages cultured in M-CSF (50 ng/mL) alone. Lane 2, mRNA from macrophages cultured in M-CSF (50 ng/mL) + IL-10 (25 ng/mL). Arrow indicates rRNA size markers. Evenness of loading was assessed by hybridization with a GAPDH probe. One representative experiment of three is shown.
These results taken together suggest that at least one of the reasons of the cooperativity between IL-10 and M-CSF appears to arise through the upregulation of c-fms at both in mRNA and protein level by IL-10.
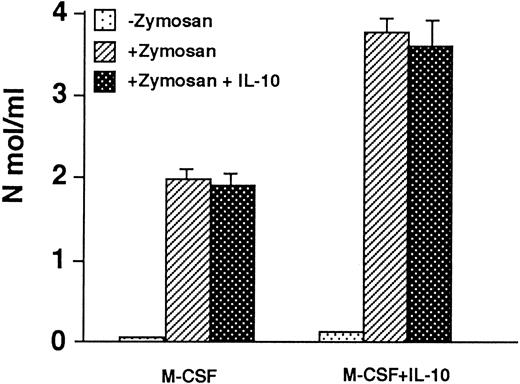
Macrophages cultured in M-CSF + IL-10 show enhanced H2O2 and O−2 production compared to macrophage cultured in M-CSF alone. It was reported that IL-10 inhibits O−2 or H2O2 production by monocyte/macrophage. Therefore, we examined the O−2 or H2O2 production by macrophages cultured in M-CSF + IL-10 or M-CSF alone. The macrophages were stimulated by zymosan in the presence or absence of IL-10. Without zymosan stimulation, both macrophages produced only a negligible amount of H2O2 . When both macrophages were stimulated with zymosan, they produced a significant amount of H2O2 . However, the level of zymosan stimulated H2O2 production by macrophages cultured in M-CSF + IL-10 was twofold higher than that by macrophages cultured in M-CSF alone (Fig 4). When both macrophages were stimulated with zymosan in the presence of IL-10, the production of H2O2 was not suppressed. Similar results were obtained for O−2 production (Table 3).
H2O2 production in macrophages generated from monocytes cultured with M-CSF + IL-10 or M-CSF alone. Macrophages (5 × 105) generated from monocytes cultured with M-CSF (50 ng/mL) or M-CSF + IL-10 (25 ng/mL) were washed with PBS, then stimulated by zymosan (1 mg/mL) in the presence or absence of IL-10. The cellular release of H2O2 was detected as described in Materials and Methods. Data are expressed as the mean of triplicate wells (+SD) from representative experiment of three performed.
H2O2 production in macrophages generated from monocytes cultured with M-CSF + IL-10 or M-CSF alone. Macrophages (5 × 105) generated from monocytes cultured with M-CSF (50 ng/mL) or M-CSF + IL-10 (25 ng/mL) were washed with PBS, then stimulated by zymosan (1 mg/mL) in the presence or absence of IL-10. The cellular release of H2O2 was detected as described in Materials and Methods. Data are expressed as the mean of triplicate wells (+SD) from representative experiment of three performed.
Zymosan Stimulated O2-Production in Monocytes-Derived Macrophage
| Mφ Recovered From Culture With . | IL-10 . | Peak Count (×106 CPM) . | ||
|---|---|---|---|---|
| . | . | Exp. 1 . | Exp. 2 . | Exp. 3 . |
| M-CSF | − | 16.4 | 15.1 | 12.0 |
| + | 15.4 | 15.5 | 12.2 | |
| M-CSF + IL-10 | − | 26.5 | 25.4 | 24.9 |
| + | 27.1 | 25.0 | 24.3 | |
| Mφ Recovered From Culture With . | IL-10 . | Peak Count (×106 CPM) . | ||
|---|---|---|---|---|
| . | . | Exp. 1 . | Exp. 2 . | Exp. 3 . |
| M-CSF | − | 16.4 | 15.1 | 12.0 |
| + | 15.4 | 15.5 | 12.2 | |
| M-CSF + IL-10 | − | 26.5 | 25.4 | 24.9 |
| + | 27.1 | 25.0 | 24.3 | |
Monocytes were incubated with M-CSF (50 ng/mL) + IL-10 (25 ng/mL) or M-CSF alone for 7 days. Recovered macrophages were washed, then the reaction was started by adding zymosan (1 mg/mL) in the presence or absence of IL-10. CL of these macrophages (5 × 105) was measured as described in Materials and Methods. Data are results from three independent experiments.
These results indicate that macrophages cultured in M-CSF + IL-10 possess an augmented ROI producing ability compared to macrophages cultured in M-CSF alone.
Enhancement of FcγR-mediated phagocytic activity in macrophages cultured in M-CSF + IL-10.To compare the phagocytic activity in macrophages cultured in M-CSF + IL-10 and macrophages cultured in M-CSF alone, both macrophage were incubated with EA for 20 minutes or 60 minutes, and the percentage of cells phagocytized EA was determined. One hundred percent of both macrophages phagocytized EA within 1 hour. However, the level of phagocytosis of EA at 20 minutes in macrophages cultured in M-CSF + IL-10 was significantly higher than that in macrophages cultured in M-CSF alone (Table 4). Addition of IL-10 during the assay did not change phagocytosis of these cells.
FcγR-Mediated Phagocytosis Activity of Macrophages Generated From Monocytes Cultured With M-CSF or M-CSF + IL-10
| Mφ Recovered From Culture With . | IL-10 . | Phagocytosis of EA (%) . | |
|---|---|---|---|
| . | . | 20 min . | 60 min . |
| M-CSF | − | 64.8 ± 6.8 | 100 |
| + | 65.1 ± 4.7 | 100 | |
| M-CSF + IL-10 | − | 82.8 ± 8.24-150 | 100 |
| + | 84.1 ± 4.94-150 | 100 | |
| Mφ Recovered From Culture With . | IL-10 . | Phagocytosis of EA (%) . | |
|---|---|---|---|
| . | . | 20 min . | 60 min . |
| M-CSF | − | 64.8 ± 6.8 | 100 |
| + | 65.1 ± 4.7 | 100 | |
| M-CSF + IL-10 | − | 82.8 ± 8.24-150 | 100 |
| + | 84.1 ± 4.94-150 | 100 | |
Monocytes were incubated with rhM-CSF (50 ng/mL) plus rhIL-10 (25 ng/mL), or rhM-CSF alone for 1 week. The resulting macrophages were incubated with EA in the presence or absence of IL-10 for 20 or 60 minutes at 37°C. Data are expressed as the means ± SD of three experiments.
P < .05 versus M-CSF alone (20 minutes).
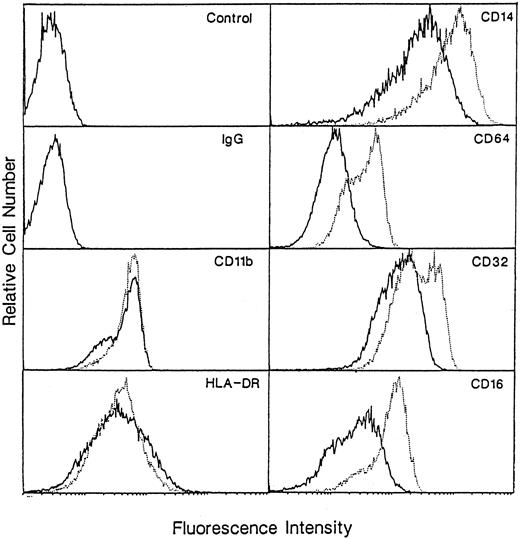
To evaluate whether or not enhanced phagocytosis of macrophages cultured in M-CSF + IL-10 is because of the upregulation of Fcγ receptor expression, we examined the expression of Fcγ I, II, and III receptor (CD16, CD32, and CD64) on both macrophages using flow cytometry. The level of expression of these three Fcγ receptors on macrophages cultured in M-CSF + IL-10 are significantly higher than that on macrophages cultured in M-CSF alone (Fig 5). These results indicate that at least one of the reason of enhanced Fcγ receptor-mediated phagocytosis in macrophages cultured in M-CSF + IL-10 may be caused by the upregulation of Fcγ receptors.
Flow cytometry of surface-marker expression on macrophages generated from monocytes cultured with M-CSF or M-CSF + IL-10. Monocytes were incubated with M-CSF (50 ng/mL) + IL-10 (25 ng/mL) or M-CSF alone for 7 days. These cells were then stained for HLA-DR, CD14, CD16, CD32, CD64, CD11b Ag as described in Materials and Methods. Macrophages cultured in M-CSF alone are represented by a solid line, and those cultured in M-CSF + IL-10 are shown as dotted lines. Data are from representative experiment of three performed.
Flow cytometry of surface-marker expression on macrophages generated from monocytes cultured with M-CSF or M-CSF + IL-10. Monocytes were incubated with M-CSF (50 ng/mL) + IL-10 (25 ng/mL) or M-CSF alone for 7 days. These cells were then stained for HLA-DR, CD14, CD16, CD32, CD64, CD11b Ag as described in Materials and Methods. Macrophages cultured in M-CSF alone are represented by a solid line, and those cultured in M-CSF + IL-10 are shown as dotted lines. Data are from representative experiment of three performed.
Effect of IL-10 on the expression of other cell surface antigens.We also examined the expression of other cell surface antigens such as CD14, CD11b, and HLA-DR, which related to function of monocyte/macrophage. The surface expression of CD14 on macrophage cultured in M-CSF + IL-10 was higher than that on macrophages cultured in M-CSF alone (Fig 5). In contrast, the expression of HLA-DR and CD11b were not different significantly between these two macrophages.
Macrophages cultured in M-CSF + IL-10 show enhanced IL-6 production.Monocyte-derived macrophages cultured in M-CSF + IL-10 or M-CSF alone were washed, and then stimulated with LPS (100 ng/mL) for 24 hours. The culture supernatants were assayed for the amount of IL-6 by ELISA. Without LPS stimulation, the production of IL-6 was marginal, but after LPS stimulation, both macrophages produced significant amount of IL-6. Macrophages cultured in M-CSF + IL-10 produced higher level of IL-6 than those cultured in M-CSF alone (Table 5). However, when macrophages cultured in M-CSF + IL-10 or M-CSF alone were stimulated with LPS in the presence of IL-10, the production of IL-6 was suppressed.
IL-6 Production by Macrophage Generated From Monocytes Cultured With M-CSF or M-CSF + IL-10
| Stimulated With . | IL-6 (ng/105 cells) . | |
|---|---|---|
| . | M-CSF . | M-CSF + IL-10 . |
| — | 0.2 ± 0.3 | 0.3 ± 0.4 |
| LPS | 13.0 ± 6.9 | 39.0 ± 11.55-150 |
| LPS + IL-10 | 3.2 ± 0.5 | 8.1 ± 1.4 |
| Stimulated With . | IL-6 (ng/105 cells) . | |
|---|---|---|
| . | M-CSF . | M-CSF + IL-10 . |
| — | 0.2 ± 0.3 | 0.3 ± 0.4 |
| LPS | 13.0 ± 6.9 | 39.0 ± 11.55-150 |
| LPS + IL-10 | 3.2 ± 0.5 | 8.1 ± 1.4 |
Monocyte were incubated with rhM-CSF (50 ng/mL) or rhM-CSF + IL-10 (25 ng/mL) for 1 week, then recovered macrophages were stimulated with LPS (100 ng/mL) in the presence or absence of IL-10 for 24 hours. IL-6 in the culture supernatant was measured using an ELISA.
P < .05 versus M-CSF alone (LPS stimulation).
DISCUSSION
Although IL-10 generally plays roles as a negative regulator of cytokine network, it also promotes the proliferation and differentiation of B cells7,24 and mast cells.8
In the present work, we showed that IL-10 acts as positive cytokine on the growth and differentiation of human monocytes cultured in M-CSF, although IL-10 alone is ineffective. Furthermore our present study showed that macrophages generated from monocytes cultured with M-CSF + IL-10 showed enhanced activities in ROI and IL-6 production and FcγR mediated phagocytosis compared to macrophage generated from monocytes cultured with M-CSF alone. These findings add a new aspects to our knowledge of IL-10 function, and provide evidence that IL-10 acts not only as a negative factor but also as a positive factor on monocyte/macrophage.
We asked then why IL-10 augments the growth and differentiation of M-CSF stimulated monocytes, and suggested that this may be partly due to the upregulation of M-CSF receptor, c-fms, at both the mRNA and protein levels. However, our results showed that the actual enhancement of c-fms expression is modest (1.7-fold enhancement by 125I-M-CSF binding assay). At present, it is not certain that the enhancement of c-fms expression level would in any way affect the cells' response to M-CSF, because the number of occupied receptors necessary to activate the cell is not known. Further studies to clarify the relationship between the upregulation of c-fms and the augmented growth and differentiation of monocytes cultured in M-CSF + IL-10 are underway. Alternatively, the augmenting effect of IL-10 may be partly caused by the common signaling event with M-CSF. Many cytokines activate JAK or Tyk kinases leading to STAT proteins phosphorylation.25,26 Novak et al27 have reported that M-CSF activates STAT 1 and STAT 3 transcription factors by phosphorylation of Tyk 2 and JAK 1. As well as M-CSF, IL-10 also activates Tyk 2 and JAK 1 and phosphorylation of STAT 1 and STAT 3.28 These results suggest that IL-10 and M-CSF partly share a pathway of signaling. Therefore, the enhancing effect of IL-10 on the action of M-CSF may be mediated partly through these common signaling events.
Horiguch et al29 have reported that GM-CSF stimulate the downregulation of c-fms in murine macrophages. If cytokines like GM-CSF are produced from monocytes by M-CSF stimulation, those cytokines may downregulate c-fms expression, and suppression of the cytokine production by IL-10 may apparently stimulate the upregulation of c-fms. However, other possibility exist that c-fms is IL-10 inducible gene. At present, we don't know whether IL-10 acts directly on the upregulation of c-fms or acts indirectly through suppressing the production of cytokines that downregulate c-fms expression. The study to clarify this point is underway.
As for the effect of IL-10 on macrophage functions, we showed that IL-10 augmented the FcγR-mediated phagocytosis of the M-CSF–stimulated macrophages. This is probably caused by the upregulated expression of FcγR by IL-10, because we found that expression of all three FcγR(CD16, CD32, and CD64, respectively) is enhanced in macrophages cultured in M-CSF + IL-10 compare to macrophage cultured in M-CSF. Velde et al3 have reported that IL-10 alone enhance FcγR I expression in human monocytes. However, in their study the increase of FcγR II or FcγR III expression has not been observed. This might be owing to the difference between their and our culture systems; they examined the effect of IL-10 in the absence of M-CSF but we examined in the presence of M-CSF. Thus, it is likely that IL-10 upregulates the FcγR II and FcγR III only in the presence of M-CSF. Recent work30 indicates an important role for protein tyrosine kinase in distal activation events that follow FcγR-mediated phagocytosis in monocytes and macrophages, and both physical and functional association between FcγR and Src family of tyrosine kinases such as fgr, hck, lyn, or syk have been found.31-33 Therefore, enhanced phagocytosis by IL-10 could be due to not only the upregulated expression of FcγR but also the change of some event such as the interaction between the tyrosine kinase and the FcγR in the cells.
IL-10 reportedly inhibits the production of ROIs such as H2O2 and monokines such as IL-1, IL-6, IL-8, and tumor necrosis factor-α from human monocytes or macrophages.4,5,34,35 However, macrophages cultured in M-CSF + IL-10 had an enhanced capacity to produce IL-6 or ROI on subsequent stimulation of the cells by LPS or zymosan. This discrepancy might be due to a difference in the experimental system; we added both M-CSF and IL-10 to the culture of monocytes and then examined the LPS-stimulated IL-6 production or zymosan-stimulated ROI production by recovered macrophages in the absence of IL-10, whereas, in other studies, IL-10 was added simultaneously with LPS or other stimulant.4,5,34,35 Indeed, when macrophages cultured in M-CSF + IL-10 or macrophages cultured in M-CSF alone were stimulated with LPS in the presence of IL-10, the production of IL-6 was suppressed. In contrast to IL-6 production by LPS stimulation. ROI production by zymosan was not suppressed by addition of IL-10 simultaneously with the stimulant. This result is different from previous study.5 This reason might be caused by a difference in macrophage and stimulant; we used human monocyte-derived macrophages and zymosan as stimulant but other studies used murine peritoneal macrophages and PMA as stimulant.
In summary, the present study showed that IL-10 functions not only as a negative regulator of a cytokine networks but also as a positive regulator of the proliferation and differentiation of monocytes by cooperating with M-CSF.
Supported by Grant 4-1-4-A and Grant 5101 from the Japan Health Science Foundation.
Address reprint requests to Shin-ichi Hashimoto, Department of Immunology, National Institute of Health, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal