Visual Abstract
Artificial intelligence (AI) and its subdiscipline, machine learning (ML), have the potential to revolutionize health care, including hematology. The diagnosis and treatment of hematologic disorders depend on the integration of diverse data sources, such as imaging, pathology, omics, and laboratory parameters. The increasing volume and complexity of patient data have made clinical decision-making more challenging. AI/ML hold significant potential for enhancing diagnostic accuracy, risk stratification, and treatment response prediction through advanced modeling techniques. Generative AI, a recent advancement within the broader field of AI, is poised to have a profound impact on health care and hematology. Generative AI can enhance the development of novel therapeutic strategies, improve diagnostic workflows by generating high-fidelity images or pathology reports, and facilitate more personalized approaches to patient management. Its ability to augment clinical decision-making and streamline research represents a significant leap forward in the field. However, despite this potential, few AI/ML tools have been fully implemented in clinical practice due to challenges related to data quality, equity, advanced infrastructure, and the establishment of robust evaluation metrics. Despite its promise, AI implementation in hematology faces critical challenges, including bias, data quality issues, and a lack of regulatory frameworks and safety standards that keep pace with rapid technological advancements. In this review, we provide an overview of the current state of AI/ML in hematology as of 2025, identify existing gaps, and offer insights into future developments.
Introduction
Artificial intelligence (AI) and machine learning (ML) have emerged as transformative technologies in various fields, including health care. With the ability to analyze vast amounts of data and extract meaningful insights, AI and ML hold promise for revolutionizing medical practice, improving diagnostic accuracy, and enhancing patient outcomes.1 AI refers to the development of computer systems capable of performing tasks that typically require human intelligence, such as reasoning, learning, and problem-solving. ML, a subset of AI, describes algorithms that enable computers to learn from data and make predictions without explicit programming.
Historically, AI and ML in medicine focused on 3 major areas: natural language processing of text, visual processing of images,2 and predictive algorithms for clinical decision support.3 AI and ML have been particularly impactful in hematology, in which pathology is paramount and the amount of laboratory and genomic data on our patients grows every day. Recent advancements have enabled accurate and rapid analysis of blood smears, bone marrow aspirates, and flow cytometry data by AI tools, facilitating the detection and classification of hematologic disorders.4-8 AI tools have shown promise in predicting patient outcomes, identifying treatment response patterns, and guiding therapeutic decisions in leukemia, lymphoma, sickle cell disease, and thrombosis.9-12 In recent years as large datasets and computational power have become increasingly accessible,13 AI and ML have become more sophisticated. The emergence of generative AI, a type of AI technology that can create novel content, has expanded the opportunities for application to health care.14,15 AI and ML are now being leveraged for more creative endeavors including writing responses to patient messages, modeling for mechanistic studies, drug discovery, and the generation of synthetic patients for clinical trials.16-19
AI and ML are advancing rapidly, presenting both opportunities and challenges for health care, particularly in hematology. Researchers, clinicians, and policymakers are striving to keep pace with these technological developments. One key challenge is determining how to effectively evaluate the performance and safety of AI/ML tools in a clinical setting, ensuring they meet the rigorous standards required for patient care. Additionally, integrating these technologies into clinical workflows remains complex because it requires addressing issues related to data quality, regulatory compliance, and ethical considerations.
Furthermore, the application of AI in rare hematologic diseases represents a promising yet underexplored opportunity. Given the diagnostic complexity, limited data availability, and lack of standardized treatment pathways often associated with rare, nonneoplastic hematologic disorders, AI-driven tools (such as natural language processing, pattern recognition, and federated learning) could play a transformative role. These technologies have the potential to identify hidden patterns in fragmented datasets, support earlier diagnosis, and facilitate crossborder harmonization of care standards, ultimately improving outcomes for patients with rare hematologic conditions.
In this article, we explore the current state of AI/ML in hematology, focusing on applications in diagnosis, risk stratification, and treatment response prediction. We examine the road to clinical implementation and highlight barriers that still need to be overcome. Finally, we discuss gaps in the current development of AI/ML for hematology and potential future innovations, such as the incorporation of generative AI.
An introduction to AI history and terminology
AI traces its origins to the mid-20th century, with the advent of computing technologies that enabled machines to execute tasks traditionally requiring human cognition. The term "artificial intelligence" was first introduced at the Dartmouth conference in 1956, marking the official genesis of AI as a distinct field of study. Initial AI development centered around rule-based systems, also known as expert systems, which operated on predefined "if-then" rules to make decisions. These systems were inherently limited by their inability to adapt to the complexity and variability characteristic of real-world problems.
Contemporary AI refers to the development of computer systems capable of performing tasks that typically require human intelligence, such as reasoning, learning, problem-solving, perception, language understanding, and creativity. Modern AI systems are designed to mimic cognitive functions, enabling machines to recognize patterns, make decisions, and learn from experiences. This broader and more dynamic definition allows AI to address a wider range of applications and challenges, particularly in complex fields such as health care.1
ML and DL
ML, a subset of AI, signifies a paradigm shift from rule-based systems to data-driven algorithms. Emerging in the 1980s, ML involves the development of algorithms that enable computers to learn from data and iteratively enhance their performance. These algorithms are adept at identifying patterns and making predictions without explicit programming for specific tasks. ML is broadly classified into 3 categories: supervised learning, unsupervised learning, and reinforcement learning2,20-22 (Table 1; Figure 1).
AI terminology
| Term . | Definition . |
|---|---|
| Algorithm bias | Systematic errors in AI models that result in unfair treatment of certain patient populations due to imbalanced training data |
| AI | The simulation of human intelligence in machines that can perform tasks such as learning, reasoning, and problem-solving |
| Data drift | Changes in data patterns over time that can reduce AI model performance, requiring continuous monitoring and updates |
| DL | A specialized ML approach using artificial neural networks with multiple layers to analyze complex data |
| Digital twin | A virtual representation of a patient using AI to simulate disease progression and treatment responses |
| Generative adversarial networks | A class of ML frameworks in which 2 neural networks, a generator and a discriminator, compete in a game-theoretic setup. The generator creates data samples (eg, images, text) that resemble real data, whereas the discriminator evaluates their authenticity. Over time, the generator improves at producing realistic outputs. |
| Generative AI | AI systems that create new content, such as text, images, or synthetic data, using models such as generative adversarial networks and transformer-based language models |
| GPT-3 | Generative pretrained transformer 3 is a multimodal large language model trained and created by OpenAI and the third in its series of GPT foundation models |
| ML | A subset of AI that enables computers to learn patterns from data and make predictions without explicit programming |
| MLDevOps | The integration of ML development and operational processes to ensure the reliable deployment of AI models in health care |
| Natural language processing | A field of AI that enables computers to understand, interpret, and generate human language |
| Neural networks | AI structures modeled after the human brain, composed of interconnected nodes (neurons) for data processing |
| Reinforcement learning | An AI approach in which an agent learns by interacting with an environment and receiving rewards or penalties |
| Supervised learning | An ML technique in which algorithms are trained on labeled datasets, mapping inputs to known outputs |
| Transformer-based large language models | A type of deep learning model based on the transformer architecture, which uses self-attention mechanisms to process and generate human-like text |
| Unsupervised learning | An ML technique that identifies patterns and structures in unlabeled data without predefined outcomes |
| Term . | Definition . |
|---|---|
| Algorithm bias | Systematic errors in AI models that result in unfair treatment of certain patient populations due to imbalanced training data |
| AI | The simulation of human intelligence in machines that can perform tasks such as learning, reasoning, and problem-solving |
| Data drift | Changes in data patterns over time that can reduce AI model performance, requiring continuous monitoring and updates |
| DL | A specialized ML approach using artificial neural networks with multiple layers to analyze complex data |
| Digital twin | A virtual representation of a patient using AI to simulate disease progression and treatment responses |
| Generative adversarial networks | A class of ML frameworks in which 2 neural networks, a generator and a discriminator, compete in a game-theoretic setup. The generator creates data samples (eg, images, text) that resemble real data, whereas the discriminator evaluates their authenticity. Over time, the generator improves at producing realistic outputs. |
| Generative AI | AI systems that create new content, such as text, images, or synthetic data, using models such as generative adversarial networks and transformer-based language models |
| GPT-3 | Generative pretrained transformer 3 is a multimodal large language model trained and created by OpenAI and the third in its series of GPT foundation models |
| ML | A subset of AI that enables computers to learn patterns from data and make predictions without explicit programming |
| MLDevOps | The integration of ML development and operational processes to ensure the reliable deployment of AI models in health care |
| Natural language processing | A field of AI that enables computers to understand, interpret, and generate human language |
| Neural networks | AI structures modeled after the human brain, composed of interconnected nodes (neurons) for data processing |
| Reinforcement learning | An AI approach in which an agent learns by interacting with an environment and receiving rewards or penalties |
| Supervised learning | An ML technique in which algorithms are trained on labeled datasets, mapping inputs to known outputs |
| Transformer-based large language models | A type of deep learning model based on the transformer architecture, which uses self-attention mechanisms to process and generate human-like text |
| Unsupervised learning | An ML technique that identifies patterns and structures in unlabeled data without predefined outcomes |
GPT-3, generative pretrained transformer 3.
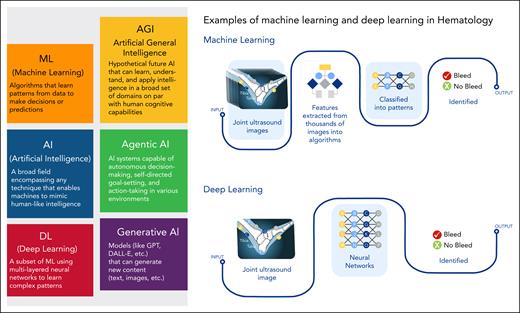
Definitions of AI subtypes and examples of ML and DL applications in hematology. Various definitions of AI subtypes are illustrated and examples of ML and DL techniques in the field of hematology are shown.
Definitions of AI subtypes and examples of ML and DL applications in hematology. Various definitions of AI subtypes are illustrated and examples of ML and DL techniques in the field of hematology are shown.
In supervised learning, algorithms are trained on labeled datasets in which input data are paired with the correct output. Conversely, unsupervised learning algorithms operate on unlabeled data, aiming to uncover hidden structures within the dataset. Reinforcement learning involves training algorithms through trial and error, in which they receive feedback from their actions to optimize future performance.
Deep learning (DL), a specialized subfield of ML, emerged in the early 2000s and has significantly transformed AI.21 DL algorithms are based on artificial neural networks, inspired by the structure and function of the human brain. These networks consist of multiple layers of interconnected nodes (neurons) that process and transform data hierarchically (Figure 1). DL excels in managing large, complex datasets and has been particularly successful in applications such as image and speech recognition, natural language processing, and autonomous driving. The advent of DL has greatly enhanced AI’s capabilities in health care, including hematology, by enabling more accurate and efficient data analysis.
Next-generation AI methods
The evolution of AI continues with the development of next-generation methods that further augment and diversify the applications of AI and ML. Generative AI, for instance, involves models capable of creating new content, such as images, text, and music. These models, including generative adversarial networks and transformer-based large language models such as generative pretrained transformer, have unlocked new possibilities for innovation in various domains, including medical research and clinical practice22 (Table 1; Figures 1 and 2). Generative AI can be used to simulate patient data, develop novel therapeutic compounds, and generate realistic synthetic datasets for training and validating AI models.23
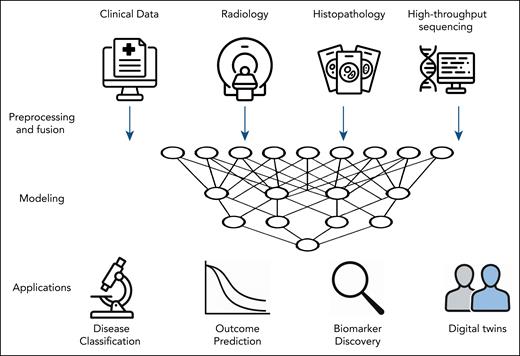
Multimodal ML framework for integrative analysis in hematology. The integration of heterogeneous data types (clinical variables, radiologic imaging, histopathology, and high-throughput sequencing) using a multimodal ML pipeline is depicted. After preprocessing and data fusion, ML models are trained to capture relationships across modalities. These models support key clinical applications including the classification of hematologic malignancies (eg, lymphoma subtyping), prediction of clinical outcomes (eg, survival or progression after CAR T-cell therapy), discovery of prognostic and predictive biomarkers, and development of digital twins to simulate individualized treatment responses. Icons in this figure were generated using ChatGPT (OpenAI).
Multimodal ML framework for integrative analysis in hematology. The integration of heterogeneous data types (clinical variables, radiologic imaging, histopathology, and high-throughput sequencing) using a multimodal ML pipeline is depicted. After preprocessing and data fusion, ML models are trained to capture relationships across modalities. These models support key clinical applications including the classification of hematologic malignancies (eg, lymphoma subtyping), prediction of clinical outcomes (eg, survival or progression after CAR T-cell therapy), discovery of prognostic and predictive biomarkers, and development of digital twins to simulate individualized treatment responses. Icons in this figure were generated using ChatGPT (OpenAI).
AI application in hematology
AI in diagnostic hematopathology
The diagnosis of hematologic disorders requires the interpretation and integration of multiple test results. Many of these test methods rely on manual evaluation of phenotypic results, which can be subjective with limited reproducibility. Diagnostic ambiguity remains a regular occurrence and is one of the greatest challenges in hematology24 (Figure 3).
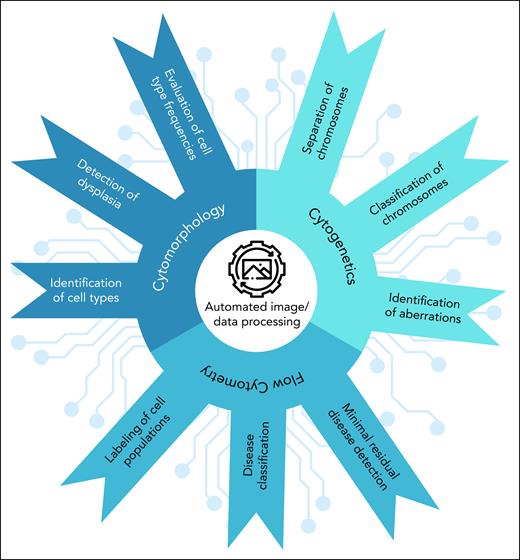
Application of AI in diagnostic hematology. The use of AI technologies in various aspects of diagnostic hematology, including cell type identification, detection of dysplasia, chromosomal analysis, and disease classification are highlighted. AI-driven techniques such as automated image/data processing and others are shown to improve diagnostic accuracy and enable minimal residual disease detection.
Application of AI in diagnostic hematology. The use of AI technologies in various aspects of diagnostic hematology, including cell type identification, detection of dysplasia, chromosomal analysis, and disease classification are highlighted. AI-driven techniques such as automated image/data processing and others are shown to improve diagnostic accuracy and enable minimal residual disease detection.
For example, cytomorphology, chromosome banding analysis, and multiparameter flow cytometry all rely on the analysis and interpretation of images of different objects, mainly cell populations and chromosomes. Although a trained professional can quickly segment an image and identify the relevant objects, effectively distinguishing between artifact and informative material, this process is difficult for a computer algorithm to accomplish. Rule-based algorithms quickly reach their limits because of the high biological diversity, the smooth transitions between cell types and maturation states, and the partially overlapping properties (Figure 3). More modern AI approaches, however, have made automated image processing and object differentiation feasible. Additionally, the use of AI can speed up diagnosis. Finally, there can be variation between trained professionals in the image analysis. There is potential for AI to be able to mitigate these differences.
In cytomorphology, AI methods have been applied to stained peripheral blood and bone marrow samples for the automated identification and classification of individual cells of different types, lineages, and maturation states.5,25 Models trained on images from peripheral blood can detect dysmorphic cell features,6 a basic requirement for the identification of malignant cells and the classification of hematologic neoplasms. AI methods can also assess red blood cell morphology, assisting in the diagnosis of classical hematology disorders such as thrombotic microangiopathies that have historically been difficult to tease apart.7 Due to higher cell density, the interpretation of bone marrow smears is more challenging and requires a higher degree of human intervention.8,26 DL-based methods have also been applied to stained slides of tissue biopsies and resections to correlate distinct morphological features with genetic aberrations,27 differentiate between leukemia subtypes,28 and predict prognosis.29 These AI-based cell differentiation systems may allow hematologists and hematopathologists to focus on cases that require unique human skills and expertise.
Cytogenetic abnormalities provide important information for patient stratification and prognostication and can be detected through the classification of chromosomes, a time-consuming and tedious process. AI methods can detect and label chromosomes and assign them to their respective positions in the karyogram.30-32 These methods work well on normal chromosomes but can have difficulty automatically detecting chromosomal abnormalities, with structural variations in particular being difficult to classify due to the variety of possibilities and often limited available training material. However, a significant portion of patients diagnosed with hematologic disorders have a normal karyotype or numerical abnormalities, both of which can be readily identified by AI methods, again leaving complex cases for human experts.
Multiparameter flow cytometry data can be analyzed computationally in 2 ways: based on the matrix of raw expression values of all measured fluorescent dyes, or through image analysis of bidimensional expression plots with sequential gating applied to identify and label cell populations of interest. The transformation of raw expression values into images as input into algorithms has traditionally been the preferred strategy for automated evaluation of these data.24,33 However, to avoid potential human bias, newer approaches are using the raw data with promising results for disease and subtype classification34,35 and minimal residual disease detection,36 paving the way for future integration of these methods not just at the point of diagnosis, but also for ongoing care.
AI in genomics
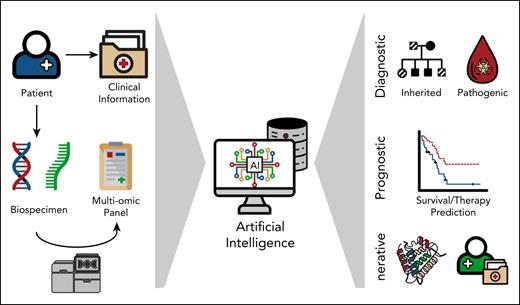
Determining and interpreting a patient’s genetic profile is critical for developing individualized treatment plans that increase the likelihood of durable efficacy and reduce side effects. Over the past decade, molecular profiling in hematology has evolved from single-gene analysis to increasingly larger genetic panels, culminating in comprehensive assays such as whole-genome and whole-transcriptome sequencing (Figure 4).
Application of AI in genomic/precision medicine. Schematic overview of AI integration in precision medicine. Patient-derived clinical data and biospecimens undergo multiomic profiling and sequencing analysis. These heterogeneous datasets are processed using AI algorithms to extract diagnostic (eg, identification of inherited and pathogenic variants), prognostic (eg, survival and therapy response prediction), and generative insights (eg, protein structure modeling and digital twin simulations), enabling advanced biomedical decision-making.
Application of AI in genomic/precision medicine. Schematic overview of AI integration in precision medicine. Patient-derived clinical data and biospecimens undergo multiomic profiling and sequencing analysis. These heterogeneous datasets are processed using AI algorithms to extract diagnostic (eg, identification of inherited and pathogenic variants), prognostic (eg, survival and therapy response prediction), and generative insights (eg, protein structure modeling and digital twin simulations), enabling advanced biomedical decision-making.
The increasing amount and complexity of genomic data has made the application of AI inevitable. An important aspect of this field is using AI for the clinical interpretation of genetic variants and the functional annotation of genomic sequences. AI has been used to classify variants across hereditary cancer genes,37 differentiate between benign and pathogenic variants,38 and identify genetic driver lesions in various cancers including a broad range of hematologic malignancies.39 Many disease-associated variants fall in noncoding regions, the effects of which can be predicted by AI models. These approaches are the first step to supporting variant interpretation in a clinical setting and generating patient-specific cancer genome profiles.
The manifestation of hematologic disorders is often polygenic, and an important part of genomics looks beyond the analysis of individual genes or genetic abnormalities toward gene-gene interactions and their effects on measures such as overall survival. AI methods, particularly neural networks, are a powerful method for modeling these intricate, often nonlinear relationships without having to enumerate all interaction possibilities. AI models can be developed to integrate and analyze gene mutation profiles, transcription profiles, and clinical data to estimate survival curves and predict personalized outcomes40,41 (Figure 4).
AI models can also be used to learn disease patterns associated with different genetic abnormalities. Supervised ML classification methods have been developed to stratify patients into established subtypes by evaluating a combination of genomic features and gene expression profiles.42,43 The development of such methods has been facilitated by the increasing availability of large sequencing studies and the pooling of interinstitutional cohorts, which has the added benefit of producing robust methods that demonstrate comparable performance regardless of data origin. Similar methods may also be used to identify new biomarkers and disease subtypes.
To best use the information provided by a patient’s genetic profile, suitable treatment options must also be available. In the future, generative AI technology can be used to develop novel agents, screen thousands of potential agents in a matter of hours, and predict synergistic effects, thus saving researchers the tedious and time-consuming process of traditional experimental testing. Genetic profiles also play an important role in the development of detailed “digital twins,” a synthetic replica of your patient that can be used to trial different therapeutic strategies in silico and model disease trajectory, minimizing real-life side effects and complications.
AI in clinical treatment selection and response prediction
One of the most challenging clinical scenarios in hematology is when multiple effective therapeutic options are available for a patient. For example, deciding between targeted therapy, chimeric antigen receptor (CAR) T-cell treatment, or hematopoietic stem cell transplant can be a difficult and nuanced deliberation for any clinician. AI methods have the opportunity to add personalized estimates to these conversations to help clinicians and their patients better navigate these challenging situations.
AI has been used to predict the performance of hypomethylating agents for myelodysplastic syndrome. Hypomethylating agents are the current standard of care for patients with intermediate- and high-risk myelodysplastic syndrome, yet only 30% to 40% of treated patients respond. One group developed a model that effectively predicted a patient’s response to therapy 90 days after initiation by assessing serial blood counts.44 Allogeneic hematopoietic stem cell transplantation is another therapy that, although potentially curative for acute myeloid leukemia, carries considerable risk of failure and death. An AI method to predict mortality 100 days after transplantation was able to outperform the European Group for Blood and Marrow Transplantation risk score.45 Researchers are also working on expanding the use of AI to predict response to CAR T-cell therapy.46 Although a validated CAR T-cell survival model is yet to be stablished, a growing list of variables have been linked to therapy response, laying the groundwork for future predictive models.47,48
Cellular therapies such as CAR T cells require complex manufacturing processes, and AI methods can be used to optimize different steps. An AI model created to predict response to granulocyte colony-stimulating factor–mediated stem cell mobilization can be used to identify patients who may have less than optimal collections after a single pheresis session and preemptively plan for additional sessions or alternate stimulation protocols.49 Another model leveraging multi–time point, multiomic profiling during early stages of CAR T-cell manufacturing is able to predict end-of-production product quality, again allowing for clinicians to make better treatment plans for their patients.50
AI is also at the forefront of clinical diagnosis and treatment in classical hematology. Image analysis has been used to diagnose venous thromboembolism (VTE) in radiology studies, and natural language processing has been used to identify the incidence of VTE by reviewing medical charts.51,52 AI is being leveraged to identify and incorporate numerous variables to better predict risk in patients, such as the risk of hospital-acquired VTE or heparin-induced thrombocytopenia.53,54 One group has worked to integrate their VTE model into the local electronic health record (EHR) so that variables are automatically updated and the risk score is regularly recalculated, providing real-time risk stratification of admitted patients.55 In transfusion medicine, AI methods have been used to optimize matching of blood products, predict response to infusion, and anticipate transfusion reactions.56
Despite these promising applications, AI models are still infrequently used in routine clinical practice due to challenges in acquiring high quality data that represent the full diversity of our patient populations for training and subsequent validation. Molecular data in particular are not equally available across populations, leading to data underrepresentation of specific diseases and patients. EHR software lags behind in integrating omics data as standardized, structured variables, and even institutions that are able to provide sequencing to their patients may not be able to readily extract those results for widescale analysis. Although this is changing, most AI models do not automatically pull data from the EHR and are not integrated into the user interface for easy access during the clinical workflow.
AI challenges in clinical implementation and regulatory approvals
Implementing predictive algorithms in clinical care proved challenging even before the development of more advanced AI models. The proprietary Epic Sepsis Model was widely adopted by institutions before an external validation study suggested the model had poor discrimination and calibration in predicting the onset of sepsis, highlighting the critical need for testing on different datasets before models are implemented.57 This experience also served as a flag that there were no established best practices for evaluating commercially developed algorithms. The US Food and Drug Administration (FDA) is tasked with ensuring the safety and effectiveness of many AI-driven medical products. When the Epic Sepsis Model was released, risk-scoring EHR models were not FDA regulated. That has changed since then, and the FDA has expanded its guidance for clinical decision support software.
Even FDA-approved AI/ML “Software as a Medical Devices” have experienced performance issues however. A comprehensive analysis of these devices in 2021 highlighted numerous limitations of the FDA evaluation process.58 The FDA continues to work on updating guidance documents with emerging best practices, but it can be difficult to keep pace with the rapid evolution of AI technology. A key regulatory consideration is determining what evidence is needed to demonstrate that a model’s performance can be generalized to the full intended population. Issues of bias, overfitting to a specific set of data, and the impact of data changing (“data drift”) over time need to be defined and quantified with clear evaluation metrics. Data can change over time due to shifts in diagnostic criteria, treatment protocols, changing patient demographics, and billing codes. New methods are being developed for tracking model performance over time, but more work is needed for identifying metrics that can provide reliable and timely monitoring of shifts in clinical AI models.59
A recent US Department of Health and Human Services recommendation focused on initial guidance for institutional review boards.60 However, given the numerous ethical issues that can arise with AI, there have been increasing calls for separate algorithm review boards at academic medical centers.61 In direct response to growing AI research at their institution, Stanford developed an ethics and society review board that stipulated researchers could not receive grant funding until they wrote a statement describing their proposed research’s risks to society and a commitment to potential mitigation strategies.62 A thoughtful review of potential biases and risks before conducting and implementing AI research is a critical first step in ensuring the safety of AI use in health care. Unequal behavior of algorithms toward different population subgroups can violate core principles of bioethics.63
Given the various issues for different clinical domains, data types, and patient populations, ensuring fairness and equity in AI is highly nuanced and contextual, and making a generalized strategy of evaluation is challenging if not impossible.64 Substantial investment must be made in AI governance so that local leaders can integrate national standards with the unique needs of their own institution. MLDevOps, a set of practices and principles that combine the disciplines of ML and DevOps (Development and Operations), can play an important role in improving the process of developing, deploying, and maintaining AI models and applications.65 This ongoing stewardship can allow institutions to leverage the power of AI technology while ensuring ongoing safety and effectiveness in the face of dynamic clinical data. In parallel with evolving regulatory frameworks, global efforts are underway to establish principles and guidelines for the responsible integration of AI in clinical practice. These include initiatives to ensure the protection of patient privacy, promote transparent validation of models across diverse populations, and enhance algorithm explainability for both clinicians and patients. Institutions and regulatory bodies are increasingly recognizing the importance of making AI models not only accurate, but also interpretable and trustworthy, to support clinical decision-making and foster user confidence. This multistakeholder effort is critical to building a safe and equitable AI ecosystem in health care.
Patient perspectives on AI application in health care
Because AI is rapidly transforming hematology, its acceptance among patients is shaped by a complex interplay of trust, perceived accuracy, and concerns about equity. A systematic review synthesized data from multiple studies and found that patients generally express cautious optimism about AI in medical diagnostics. Although patients recognize the potential for AI to improve accuracy and efficiency, many remain concerned about the loss of human interaction and the risk of misdiagnosis.66
Furthermore, in a study that explored the patient perspective on the use of AI for skin cancer screening, the most commonly perceived benefits were faster diagnosis and improved health care access (60% each), whereas the main concern was increased patient anxiety (40%). AI’s diagnostic accuracy was viewed as both its greatest strength (69%) and weakness (85%).67 Most patients emphasized the need for human-AI collaboration (94%). In cases of disagreement between AI and clinicians, 67% preferred seeking a biopsy. Overall, 75% would recommend AI use in health care to others.67
In another study, patient perceptions and attitudes toward AI in oncology demonstrated considerable variability, with education level emerging as a key influencing factor.68 Approximately half of the participants anticipated that AI would improve health care outcomes, reflecting a moderate degree of optimism about its integration into clinical oncology. However, a substantial proportion expressed discomfort, particularly regarding AI's role in diagnostic processes. Notably, 33.1% of patients reported being very uncomfortable with AI diagnosing cancer, a sentiment significantly more common among individuals with lower educational attainment.68
A systematic review of multiple studies examining breast cancer patients’ attitudes toward AI in diagnostic imaging reveals a consistent preference for AI to function as a supportive tool rather than a replacement for human clinicians.69 Across the reviewed literature, patients expressed greater trust in AI-assisted diagnostics when human oversight, particularly from radiologists, was maintained. This underscores the perceived value of clinical expertise and the reassurance it provides in emotionally sensitive contexts such as breast cancer diagnosis.69
Overall, patients generally view AI in health care with cautious optimism, recognizing its potential to improve diagnostic accuracy and efficiency, but expressing concerns about diagnostic errors, data privacy, and the loss of human interaction. Trust in AI is closely tied to education level and increases when AI is used to support, rather than replace, clinical decision-making. Future research should focus on longitudinal studies to assess how patient attitudes evolve with increased exposure to AI, and on developing targeted educational interventions that address specific concerns. Incorporating patient-centered design and transparent communication will be key to enhancing trust and acceptance of AI in hematology. In addition, integrating patient-reported outcomes into AI-driven clinical workflows, particularly through tools such as large language models, can further align care with patient values and foster a more holistic and personalized approach to clinical decision-making.
Conclusion
AI and ML continue to evolve, and researchers, providers, and policy makers are racing to keep up. Many practical challenges lie ahead, including understanding how AI and ML tools should be evaluated and integrated into clinical practice. Tools that affect how we care for our patients must be held to the highest standard but defining that standard remains elusive. Future hematologists cannot avoid AI and will need to work with these tools to enhance their diagnostic capabilities, streamline workflow efficiency, and deliver more personalized care to patients. AI models will continue to proliferate despite their limitations, and hematologists must be involved in these conversations so that AI is ultimately integrated into clinical care in a provider- and patient-centered approach.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author(s) used ChatGPT (OpenAI) to create icons for a figure. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Acknowledgment
R.S. has received research support from the National Cancer Institute, National Institutes of Health grants K08CA282987 and P30 CA008748.
Authorship
Contribution: A.N., O.E., S.A., B.L., R.S., S.M., and T.H. wrote sections and reviewed and approved the final manuscript; M.M. reviewed and approved the final manuscript; S.S. developed tables and reviewed and approved the final manuscript; and A.S. developed images and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: A.N. is an employee at, and owns stock in, Incyte. O.E. is cofounder of, and holds equity in, Volastra Therapeutics; is a member of the scientific advisory board of, and holds equity in, Owkin, Freenome, AcuamarkDx, and Harmonic Discovery; and reports funding from Johnson and Johnson/Janssen, Sanofi, and AstraZeneca. S.A. serves as an advisory board member for HEMA Biologics, Novo Nordisk, Genentech, Sanofi, CSL Behring, and BioMarin; serves as a scientific advisor for XaTek, Inc; and is a member of the blood products advisory committee for the US Food and Drug Administration. R.S. reports speaker honorarium from Incyte and Sanofi. S.S. reports consulting for GlaxoSmithKline and Novartis. A.S. reports advisory board role with Sobi; and honorarium from Incyte. T.H. is the owner of MLL Munich Leukemia Laboratory. The remaining authors declare no competing financial interests.
The American Society of Hematology Subcommittee on Artificial Intelligence consists of the authors of this article and advisory member Ilana Goldberg.
Correspondence: Aziz Nazha, Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Chapel Hill Rd, Chery Hill, NJ 44195; email: azizn38@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal