Key Points
First-line acalabrutinib-obinutuzumab resulted in significantly higher PFS and OS than chemoimmunotherapy regardless of high-risk features.
Acalabrutinib-obinutuzumab resulted in improved PFS rates vs acalabrutinib monotherapy in a post hoc analysis.
Visual Abstract
Acalabrutinib is a Bruton tyrosine kinase inhibitor approved for the treatment of chronic lymphocytic leukemia. We present results from ELEVATE-TN after a median follow-up of 74.5 months. Overall, 535 patients were randomized (acalabrutinib-obinutuzumab, n = 179; acalabrutinib, n = 179; chlorambucil-obinutuzumab, n = 177). Median age was 70 years, 63.0% had unmutated immunoglobulin heavy chain variable region gene (uIGHV), 13.6% had del(17p) and/or mutated TP53, and 17% had complex karyotype (CK; ≥3 chromosomal abnormalities). Median progression-free survival (PFS) was not reached (NR) for acalabrutinib-obinutuzumab and acalabrutinib vs 27.8 months for chlorambucil-obinutuzumab (both P < .0001); estimated 72-month overall PFS rates were 78.0%, 61.5%, and 17.2%, respectively. Acalabrutinib-obinutuzumab resulted in improved PFS vs acalabrutinib monotherapy (hazard ratio [HR], 0.58; P = .0229). Patients with uIGHV, del(17p) and/or mutated TP53, or CK had significantly improved PFS with acalabrutinib ± obinutuzumab vs chlorambucil-obinutuzumab (P < .0001, P ≤ .0009, and P < .0001 for both acalabrutinib-containing arms, respectively). Median overall survival (OS) was NR for all treatments, with significantly longer OS for acalabrutinib-obinutuzumab than chlorambucil-obinutuzumab (HR, 0.62; P = .0349). Estimated 72-month OS rates were 83.9%, 75.5%, and 74.7% for acalabrutinib-obinutuzumab, acalabrutinib, and chlorambucil-obinutuzumab, respectively. Adverse events (AEs) occurring after >4 years were mostly grade 1 to 2. Rates of AEs, serious AEs, and events of clinical interest were similar between acalabrutinib-containing arms and consistent with the known safety profiles of acalabrutinib and obinutuzumab. Efficacy and safety of acalabrutinib-containing arms were maintained, with longer PFS in both acalabrutinib arms than chlorambucil-obinutuzumab including in patients with high-risk features. This trial was registered at www.ClinicalTrials.gov as #NCT02475681.
Introduction
Targeted therapies for treatment-naive (TN) chronic lymphocytic leukemia (CLL) have displaced chemoimmunotherapy alone as first-line treatment.1-3 The first-generation, covalent Bruton tyrosine kinase inhibitor (BTKi), ibrutinib, and the B-cell lymphoma-2 (BCL2) protein inhibitor, venetoclax, were developed and tested alone4,5 or combined with anti-CD20 monoclonal antibodies,4,6,7 resulting in longer progression-free survival (PFS) and overall survival (OS) for patients with CLL compared with chemoimmunotherapy.8 Subsequently, acalabrutinib, a second-generation, covalent BTKi, was approved for relapsed/refractory mantle cell lymphoma and CLL/small lymphocytic lymphoma.9 High rates of durable responses and a well-tolerated safety profile were reported for acalabrutinib, including in patients with high-risk genomic features and in a head-to-head randomized trial vs ibrutinib in patients with relapsed/refractory CLL.10-13
Nevertheless, CLL remains a heterogeneous disease in which patients with high-risk genomic features, such as del(17p)(p13.1) [del(17p)], mutated TP53 (TP53m), unmutated immunoglobulin heavy chain variable region gene (uIGHV), and complex karyotype (CK), have inferior outcomes compared with other patients with CLL.14-18 These high-risk genomic features and other prognostic factors should be considered by clinicians in selecting frontline treatment for CLL.
ELEVATE-TN is a randomized trial evaluating the efficacy and safety of acalabrutinib ± obinutuzumab compared with chemoimmunotherapy in adult patients with TN CLL.19 Previously published results consistently showed longer PFS for patients treated with acalabrutinib ± obinutuzumab than chlorambucil-obinutuzumab, including patients with high-risk features.19,20 With longer follow-up, this ongoing trial also provides insight into the impact of adding an anti-CD20 antibody to a covalent BTKi.
We present efficacy and safety results of ELEVATE-TN after 6 years of follow-up and post hoc analyses comparing the acalabrutinib-containing arms, with a special focus on the benefit of adding obinutuzumab to acalabrutinib.
Methods
Study design and participants
ELEVATE-TN is an ongoing phase 3 randomized open-label trial conducted at 142 centers in 18 countries. Patients aged ≥65 years or 18 to 65 years with comorbidities (Cumulative Illness Rating Scale–Geriatric score of >6; creatinine clearance, 30-69 mL/min by Cockcroft-Gault) with TN CD20+ CLL requiring treatment per International Workshop on CLL 2008 criteria for active disease were enrolled.21 Detailed descriptions of inclusion and exclusion criteria and baseline screening procedures were previously published.19 Data cutoff date for this analysis was 3 March 2023.
Randomization and treatment arms
Participants were randomized 1:1:1 via interactive web response system into 3 treatment arms (arm A, acalabrutinib-obinutuzumab; arm B, acalabrutinib monotherapy; arm C, chlorambucil-obinutuzumab) and stratified by presence or absence of del(17p), Eastern Cooperative Oncology Group performance status (ECOG PS; 0-1 vs 2), and geographic region of origin (North America, western Europe, or other).
Treatments were administered in 28-day cycles. In the acalabrutinib-containing arms, beginning with cycle 1, acalabrutinib 100 mg was taken orally twice a day as monotherapy (arm B) or in combination with intravenous obinutuzumab (arm A), with obinutuzumab beginning cycle 2 with a dose of 1000 mg split over days 1 (100 mg) and 2 (900 mg) and then 1000 mg on days 8 and 15 of cycle 2 and day 1 of cycle 3 for a maximum of 6 cycles. Treatment with acalabrutinib continued until disease progression or unacceptable toxicity.
In the chemoimmunotherapy arm (arm C), beginning cycle 1, the same regimen of obinutuzumab was combined with chlorambucil (0.5 mg/kg orally) and given on days 1 and 15 for 6 cycles. Patients progressing on chlorambucil-obinutuzumab could crossover to acalabrutinib monotherapy. Patients in the crossover arm received acalabrutinib twice a day until disease progression or unacceptable toxicity.
The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of good clinical practice guidelines. The study protocol was approved by local institutional review boards and independent ethics committees at each site; all patients provided written informed consent.
Outcomes and assessments
Efficacy
The primary end point was independent review committee (IRC) assessment of PFS for acalabrutinib-obinutuzumab vs chlorambucil-obinutuzumab. PFS was defined as time from randomization to disease progression (per the International Workshop on CLL 2008 criteria21) or death. A key secondary end point was IRC-assessed PFS with acalabrutinib monotherapy vs chlorambucil-obinutuzumab.
Secondary end points were IRC-assessed overall response rate (ORR), time-to-next treatment, and OS. Exploratory end points included investigator-assessed PFS, investigator-assessed ORR, and minimal residual disease (MRD) negativity. Because the study crossed the boundary for efficacy at interim analysis (data cutoff date: 8 February 2019), all IRC assessments were discontinued; all efficacy results presented here are investigator assessed. All analyses are post hoc and P values are descriptive.
Although not powered to examine the difference in PFS rates between acalabrutinib-obinutuzumab vs acalabrutinib monotherapy, a post hoc analysis was conducted.
All efficacy analyses were performed on the intent-to-treat population, defined as all patients analyzed in the arm to which they were randomly assigned. Except for OS, all efficacy end points included data only before crossover from chlorambucil-obinutuzumab to acalabrutinib monotherapy.
Efficacy assessment
To assess disease response and progression, patients were evaluated with contrasted computed tomography or magnetic resonance imaging scans at baseline, every 12 weeks to cycle 25, and every 24 weeks thereafter until disease progression. Best investigator-assessed response could be determined at any scheduled, per-protocol follow-up visit. ORR was defined as achieving a complete response (CR), CR with incomplete blood count recovery (CRi), nodular partial remission, or partial remission at or before initiation of subsequent anticancer therapy.
Bone marrow biopsy and aspirate from patients with CR suggested by physical examination, laboratory evaluations, and imaging studies were sent for central laboratory analysis to confirm CR and peripheral blood samples from patients with CR were used to evaluate for MRD via flow cytometry with a 10–4 threshold.
Subgroup analyses
Preplanned subgroup analyses were performed to investigate the consistency and robustness of investigator-assessed PFS for acalabrutinib ± obinutuzumab vs chlorambucil-obinutuzumab. Potential prognostic variable subgroups included patient features (age, race, sex, and region of origin), disease characteristics (staging, lymph node size, and levels of β2-microglobulin), and genomic markers of poor prognosis15 including del(17p), TP53m, uIGHV, and CK (defined as ≥3 cytogenetic abnormalities with ≥1 structural abnormality, excluding inversion of chromosome 9). Data for patients with CK defined as ≥5 cytogenetic abnormalities with ≥1 structural abnormality, excluding inversion of chromosome 9, are included in supplemental Figure 1D and 4D (available on the Blood website). CK was determined by metaphase karyotype analysis by central laboratory testing. After interleukin-2/DSP30–stimulated cultures for 72 hours, chromosomes were banded using Giemsa staining. Karyotypes were analyzed and reported per the International System for Human Cytogenetic Nomenclature 2013.
Safety
The frequency, severity, and relatedness of adverse events (AEs; as graded by the National Cancer Institute Common Terminology Criteria for AEs version 4.03) and the frequency of AEs requiring treatment discontinuation or dose reduction were assessed. Patients who were randomized but did not receive study treatment were excluded. AE reporting ended 30 days after the last dose of study drugs or the start of new anticancer therapy (whichever occurred first).
Statistical analysis
The sample size was calculated to achieve ∼90% power for detecting a PFS hazard ratio (HR) of 0.60 between the acalabrutinib-obinutuzumab and chlorambucil-obinutuzumab arms. Kaplan-Meier estimates with log-rank test were used to analyze time-to-event end points, and the Cox proportional hazards model was used to calculate HRs and corresponding 95% confidence intervals (CIs).19
The multivariate analyses, including patients from the 2 acalabrutinib-containing arms, used the Cox proportional hazards model with a backward elimination method to select predictors of PFS and OS. Five baseline variables, including del(17p) status, IGHV status, sex, ECOG PS (either ≥2 or 0-1), and age (either ≥70 or <70 years of age), were included in the model for predictor selection.
Statistical analyses were conducted using the Linux SAS version 9.4 software.
Results
Patient characteristics and exposure
Overall, 535 patients were randomized to treatment with acalabrutinib-obinutuzumab (n = 179), acalabrutinib monotherapy (n = 179), or chlorambucil-obinutuzumab (n = 177). Nine patients (1.7%) were randomized but did not receive treatment, 1 in the acalabrutinib monotherapy arm and 8 in the chlorambucil-obinutuzumab arm. One patient was randomized to acalabrutinib-obinutuzumab but received acalabrutinib monotherapy.
Baseline demographics and disease characteristics were previously published and are presented in supplemental Table 1.19,20 Baseline disease characteristics for the crossover population are presented in supplemental Table 2.
At a median follow-up of 74.5 months (range, 0-89), study discontinuation rates for acalabrutinib were 45.8% with acalabrutinib-obinutuzumab and 53.1% with acalabrutinib monotherapy (supplemental Table 3). When assessing discontinuation by study drug, the most common reasons for discontinuation of acalabrutinib were AEs/serious AEs (n = 36 [20.1%] for acalabrutinib-obinutuzumab, and n = 32 [17.9%] for acalabrutinib monotherapy), followed by progressive disease in 11 patients (6.1%) in the acalabrutinib-obinutuzumab arm and 25 patients (14.0%) in the acalabrutinib monotherapy arm. Discontinuation owing to death occurred in 4 (2.2%) and 16 patients (8.9%) in the acalabrutinib-obinutuzumab arm and in the acalabrutinib monotherapy arm, respectively; most deaths (3/4) in the acalabrutinib-obinutuzumab arm and approximately one-third of the deaths (5/16) in the acalabrutinib monotherapy arm were caused by infections with no specific trend for the other causes of death leading to discontinuation. Of patients treated with acalabrutinib-obinutuzumab, 164 of 178 patients (92.1%) completed the obinutuzumab treatment regimen, whereas 10 patients (5.6%) discontinued the drug owing to AE/serious AEs.
Overall, 199 patients (37.2%) exited the study, mostly owing to death (acalabrutinib-obinutuzumab, n = 28 [15.6%]; acalabrutinib monotherapy, n = 41 [22.9%]; chlorambucil-obinutuzumab, n = 42 [23.7%]) or consent withdrawal (acalabrutinib-obinutuzumab, n = 16 [8.9%]; acalabrutinib monotherapy, n = 15 [8.4%]; chlorambucil-obinutuzumab, n = 24 [13.6%]).
Efficacy results
PFS
At a median follow-up of 74.5 months, PFS was significantly longer for patients in the acalabrutinib-containing arms, with median PFS not reached (NR) in both arms vs 27.8 months (95% CI, 22.6-33.2; both P < .0001) for chlorambucil-obinutuzumab (Figure 1). Compared with chlorambucil-obinutuzumab, acalabrutinib (either with obinutuzumab or as monotherapy) was associated with significant reduction in disease progression or death (86% risk reduction: HR, 0.14; 95% CI, 0.10-0.20 for combination; 76% risk reduction: HR, 0.24; 95% CI, 0.17-0.32 for monotherapy). The estimated 72-month PFS rate was 78.0% (95% CI, 70.6-83.7) for acalabrutinib-obinutuzumab, 61.5% (95% CI, 53.4-68.7) for acalabrutinib monotherapy, and 17.2% (95% CI, 11.2-24.3) for chlorambucil-obinutuzumab. In the crossover population, the median PFS2 (time to second disease progression or death) was NR; the estimated 72-month PFS2 rate was 53.6% (95% CI, 38.3-66.8). The post hoc comparison between the acalabrutinib-containing arms showed that there was a 42% lower risk of disease progression or death in patients treated with acalabrutinib-obinutuzumab than monotherapy (HR, 0.58; 95% CI, 0.39-0.86).
Investigator-assessed PFS overall. ∗HR based on stratified Cox proportional hazards model; †P value based on stratified log-rank test. ‡HR based on unstratified Cox proportional hazards model; §P value based on unstratified log-rank test. A, acalabrutinib; Clb, chlorambucil; O, obinutuzumab.
Investigator-assessed PFS overall. ∗HR based on stratified Cox proportional hazards model; †P value based on stratified log-rank test. ‡HR based on unstratified Cox proportional hazards model; §P value based on unstratified log-rank test. A, acalabrutinib; Clb, chlorambucil; O, obinutuzumab.
The estimated 72-month PFS rate was higher in the acalabrutinib-containing arms (acalabrutinib-obinutuzumab and acalabrutinib monotherapy) than the chlorambucil-obinutuzumab arm for patients with high-risk genomic features (uIGHV, del(17p), TP53m, and CK with ≥3 abnormalities), and post hoc comparisons between the acalabrutinib-containing arms showed no significant risk reduction in disease progression or death for patients with these features (supplemental Figure 1). Among the 114 patients with lymph nodes ≥5 cm treated with an acalabrutinib-containing regimen, the HR between the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms for PFS was 0.67 (95% CI, 0.35-1.27; P = .7007; supplemental Figure 2).
In a multivariate analysis of baseline characteristics as predictors of PFS in patients treated in the acalabrutinib-containing arms, patients aged ≥70 years had an increased risk of progression or death compared with patients aged <70 years (HR, 1.64; 95% CI, 1.10-2.46; P = .0154; supplemental Table 4). In addition, patients with ECOG PS ≥2 had more than a twofold increased risk of progression or death compared with patients with ECOG PS 0-1 (HR, 2.87; 95% CI, 1.52-5.41; P = .0012). The baseline characteristics of del(17p) status, IGHV status, and sex were not associated with increased risk of progression or death.
ORR
ORR of CLL was significantly higher for patients who received acalabrutinib-obinutuzumab (96.1%; 95% CI, 92.1-98.1; P < .0001) or as monotherapy (89.9%; 95% CI, 84.7-93.5; P = .0499) than chlorambucil-obinutuzumab (83.1%; 95% CI, 76.8-87.9). CR rates for acalabrutinib-obinutuzumab, acalabrutinib monotherapy, and chlorambucil-obinutuzumab were 36.9%, 19.0%, and 13.6%, respectively.
Patients treated with acalabrutinib ± obinutuzumab whose CLL achieved a CR or CRi had longer PFS than patients treated with acalabrutinib ± obinutuzumab whose CLL did not achieve a CR/CRi (HR, 0.23; 95% CI, 0.12-0.42; P < .0001; Table 1). For patients whose CLL did not achieve CR/CRi, the rate of death or progressive disease was lower with acalabrutinib-obinutuzumab than acalabrutinib monotherapy (30.1% vs 41.4%). In the chlorambucil-obinutuzumab arm, patients whose CLL achieved CR/CRi had longer PFS than patients whose CLL did not achieve CR/CRi (HR, 0.34; 95% CI, 0.19-0.62; P = .0004); the rate of death or disease progression for patients treated with chlorambucil-obinutuzumab whose CLL did not achieve CR/CRi was 74.5%. In the post hoc analyses between acalabrutinib-obinutuzumab and acalabrutinib monotherapy, both treatment arms demonstrated increased ORR and CR/CRi rates over time throughout the study (Figure 2).
Investigator-assessed CR/CRi as a predictor of investigator-assessed PFS
| Treatment group PFS event . | PFS event rate . | HR (95% CI)∗ . | P value∗ . | |
|---|---|---|---|---|
| CLL with CR/CRi . | CLL without CR/CRi . | |||
| A + O | n = 66 | n = 113 | ||
| Death | 3 (4.5) | 19 (16.8) | 0.29 (0.13-0.65) | .0026 |
| Disease progression | 4 (6.1) | 15 (13.3) | ||
| Death or disease progression | 7 (10.6) | 34 (30.1) | ||
| A | n = 34 | n = 145 | ||
| Death | 2 (5.9) | 26 (17.9) | 0.20 (0.07-0.55) | .0017 |
| Disease progression | 2 (5.9) | 34 (23.4) | ||
| Death or disease progression | 4 (11.8) | 60 (41.4) | ||
| A ± O | n = 100 | n = 258 | ||
| Death | 5 (5.0) | 45 (17.4) | 0.23 (0.12-0.42) | <.0001 |
| Disease progression | 6 (6.0) | 49 (19.0) | ||
| Death or disease progression | 11 (11.0) | 94 (36.4) | ||
| O + Clb | n = 24 | n = 153 | ||
| Death | 2 (8.3) | 11 (7.2) | 0.34 (0.19-0.62) | .0004 |
| Disease progression | 10 (41.7) | 103 (67.3) | ||
| Death or disease progression | 12 (50.0) | 114 (74.5) | ||
| Treatment group PFS event . | PFS event rate . | HR (95% CI)∗ . | P value∗ . | |
|---|---|---|---|---|
| CLL with CR/CRi . | CLL without CR/CRi . | |||
| A + O | n = 66 | n = 113 | ||
| Death | 3 (4.5) | 19 (16.8) | 0.29 (0.13-0.65) | .0026 |
| Disease progression | 4 (6.1) | 15 (13.3) | ||
| Death or disease progression | 7 (10.6) | 34 (30.1) | ||
| A | n = 34 | n = 145 | ||
| Death | 2 (5.9) | 26 (17.9) | 0.20 (0.07-0.55) | .0017 |
| Disease progression | 2 (5.9) | 34 (23.4) | ||
| Death or disease progression | 4 (11.8) | 60 (41.4) | ||
| A ± O | n = 100 | n = 258 | ||
| Death | 5 (5.0) | 45 (17.4) | 0.23 (0.12-0.42) | <.0001 |
| Disease progression | 6 (6.0) | 49 (19.0) | ||
| Death or disease progression | 11 (11.0) | 94 (36.4) | ||
| O + Clb | n = 24 | n = 153 | ||
| Death | 2 (8.3) | 11 (7.2) | 0.34 (0.19-0.62) | .0004 |
| Disease progression | 10 (41.7) | 103 (67.3) | ||
| Death or disease progression | 12 (50.0) | 114 (74.5) | ||
Data are presented as n (%) unless otherwise specified. PFS was defined as the time from the date of randomization until disease progression or death from any cause, whichever occurred first. Patients without adequate postbaseline disease assessment were censored on the date of randomization.
A, acalabrutinib; Clb, chlorambucil; O, obinutuzumab.
HR, 95% CI, and P value based on Cox proportional hazards analysis including patients with CR/CRi as a binary factor in the model.
ORRs∗ and CR/CRi rates over follow-up period in patients treated with A-O or A monotherapy. ∗Best investigator-assessed response could be determined at any scheduled, per-protocol follow-up visit. ORR is defined as achieving CR, CRi, nPR, or PR per the investigator per International Workshop on CLL 2008 criteria21 at or before initiation of subsequent anticancer therapy. ORR does not include partial response except for lymphocytes. A, acalabrutinib; nPR, nodular partial response; O, obinutuzumab; PR, partial response.
ORRs∗ and CR/CRi rates over follow-up period in patients treated with A-O or A monotherapy. ∗Best investigator-assessed response could be determined at any scheduled, per-protocol follow-up visit. ORR is defined as achieving CR, CRi, nPR, or PR per the investigator per International Workshop on CLL 2008 criteria21 at or before initiation of subsequent anticancer therapy. ORR does not include partial response except for lymphocytes. A, acalabrutinib; nPR, nodular partial response; O, obinutuzumab; PR, partial response.
Undetectable MRD
MRD samples were collected from patients whose CLL achieved CR/CRi. Patients with CLL who achieved CR/CRi in the acalabrutinib-obinutuzumab arm achieved higher rates of undetectable MRD in peripheral blood samples than patients with CLL who achieved CR/CRi receiving acalabrutinib monotherapy and chlorambucil-obinutuzumab (40.9% vs 8.8% and 8.3%, respectively; supplemental Figure 3).
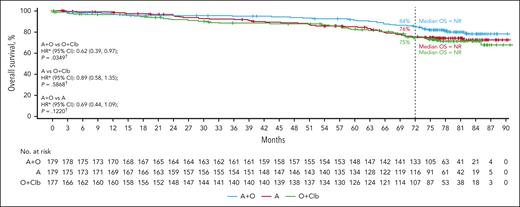
OS
The median OS was NR in any treatment arm. OS was significantly longer for acalabrutinib-obinutuzumab than chlorambucil-obinutuzumab (HR, 0.62; 95% CI, 0.39-0.97; P = .0349), but not for acalabrutinib vs chlorambucil-obinutuzumab (0.89; 95% CI, 0.58-1.35; P = .5868) or acalabrutinib-obinutuzumab vs acalabrutinib (0.69; 95% CI, 0.44-1.09; P = .1220; Figure 3). Estimated 72-month survival was 83.9% (95% CI, 77.4-88.7) for acalabrutinib-obinutuzumab, 75.5% (95% CI, 68.1-81.4) for acalabrutinib monotherapy, and 74.7% (95% CI, 67.1-80.8) for chlorambucil-obinutuzumab. The estimated 72-month OS rate was highest in the acalabrutinib-containing arms (acalabrutinib-obinutuzumab and acalabrutinib monotherapy) vs the chlorambucil-obinutuzumab arm for patients with uIGHV (83.6%, 76.4%, and 74.3%, respectively; supplemental Figure 4A), for patients with del(17p) and/or TP53m (67.5%, 71.6%, and 53.2%, respectively; supplemental Figure 4B), and for patients with CK with ≥3 abnormalities (73.5%, 71.3%, and 64.4%, respectively; supplemental Figure 4C). Estimated 72-month OS rates for patients with CK with ≥5 abnormalities were 55.6%, 62.5%, and 45.5% for the acalabrutinib-obinutuzumab, acalabrutinib monotherapy, and chlorambucil-obinutuzumab arms, respectively (supplemental Figure 4D).
OS in patients overall. ∗HR based on stratified Cox proportional hazards model; †P value based on stratified log-rank test. ‡HR based on unstratified Cox proportional hazards model; §P value based on unstratified log-rank test. Clb, chlorambucil.
OS in patients overall. ∗HR based on stratified Cox proportional hazards model; †P value based on stratified log-rank test. ‡HR based on unstratified Cox proportional hazards model; §P value based on unstratified log-rank test. Clb, chlorambucil.
In a multivariate analysis of baseline characteristics as predictors of OS in patients treated in the acalabrutinib-containing arms, patients aged ≥70 years had more than a twofold increased risk of death compared with patients aged <70 years (HR, 2.54; 95% CI, 1.53-4.22; P = .0003; supplemental Table 5). In addition, patients with an ECOG PS ≥2 had more than a twofold increased risk of death compared with patients with ECOG PS 0-1 (HR, 2.68; 95% CI, 1.33-5.42; P = .006). The baseline characteristics of del(17p) status, IGHV status, and sex were not associated with increased risk of death.
Safety
Treatment exposure
Median treatment exposure with acalabrutinib was 74.4 months (range, 0.7-88.3) in the acalabrutinib-obinutuzumab arm, 72.0 months (range, 0.3-88.7) in the acalabrutinib monotherapy arm, and 40.6 months (range, 2.7-71.7) for the 79 patients who crossed over from chlorambucil-obinutuzumab to acalabrutinib monotherapy. The mean (standard deviation) relative dose intensity of acalabrutinib was 93.9% (11.8%) and 95.7% (10.0%) for the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms, respectively. The proportions of patients in the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms with permanent dose reduction of acalabrutinib were 3.9% and 1.7%, respectively, and the proportions of patients who permanently discontinued acalabrutinib treatment were 44.4% and 53.1.%, respectively. The median exposure to obinutuzumab was 5.5 months (range, 0.9-7.1) for patients receiving acalabrutinib-obinutuzumab and 5.6 months (range, 0.9-7.4) for those receiving chlorambucil-obinutuzumab. The median duration of exposure with chlorambucil was 5.5 months (range, 0.5-7.2).
Safety outcomes
Safety outcomes for patients treated in the chlorambucil-obinutuzumab arm were previously published, and safety results reported for the acalabrutinib monotherapy arm do not include patients who crossed over from chlorambucil-obinutuzumab to acalabrutinib monotherapy.19,20 The most common AEs affecting ≥15% of patients treated in the acalabrutinib-containing arms were diarrhea, headache, arthralgia, neutropenia, and fatigue for acalabrutinib-obinutuzumab and diarrhea and headache for acalabrutinib monotherapy; AEs were mostly grade 1-2, except for neutropenia (supplemental Table 6).
The most common grade ≥3 AEs affecting ≥5% of patients in the acalabrutinib-containing arms were neutropenia (n = 55 [30.9%]), COVID-19 (n = 16 [9.0%]), thrombocytopenia (n = 15 [8.4%]), pneumonia (n = 13 [7.3%]), anemia (n = 13 [7.3%]), diarrhea (n = 11 [6.2%]), and syncope (n = 9 [5.1%]) in the acalabrutinib-obinutuzumab arm and neutropenia (n = 21 [11.7%]), anemia (n = 16 [8.9%]), COVID-19 (n = 13 [7.3%]), and pneumonia (n = 11 [6.1%]) in the acalabrutinib monotherapy arm. Neutropenia (n = 71 [42.0%]) was the most common grade ≥3 AE affecting patients in the chlorambucil-obinutuzumab arm (Table 2). Overall, patients on acalabrutinib monotherapy had fewer grade ≥3 AEs than those receiving acalabrutinib-obinutuzumab throughout the study (n = 126 [70.4%] vs n = 150 [84.3%], respectively).
Grade 3 or higher AEs affecting 5% or more of patients
| AEs (≥5% of patients), n (%) . | A + O (n = 178) . | A (n = 179) . | O + Clb (n = 169) . | |||
|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Diarrhea | 78 (43.8) | 11 (6.2) | 76 (42.5) | 1 (0.6) | 36 (21.3) | 3 (1.8) |
| Neutropenia | 61 (34.3) | 55 (30.9) | 23 (12.8) | 21 (11.7) | 77 (45.6) | 71 (42.0) |
| COVID-19 | 44 (24.7) | 16 (9.0) | 38 (21.2) | 13 (7.3) | 0 | 0 |
| Anemia | 27 (15.2) | 13 (7.3) | 31 (17.3) | 16 (8.9) | 20 (11.8) | 13 (7.7) |
| Thrombocytopenia | 26 (14.6) | 15 (8.4) | 16 (8.9) | 6 (3.4) | 23 (13.6) | 19 (11.2) |
| Pneumonia | 25 (14.0) | 13 (7.3) | 27 (15.1) | 11 (6.1) | 5 (3.0) | 3 (1.8) |
| Syncope | 12 (6.7) | 9 (5.1) | 5 (2.8) | 4 (2.2) | 1 (0.6) | 1 (0.6) |
| AEs (≥5% of patients), n (%) . | A + O (n = 178) . | A (n = 179) . | O + Clb (n = 169) . | |||
|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Diarrhea | 78 (43.8) | 11 (6.2) | 76 (42.5) | 1 (0.6) | 36 (21.3) | 3 (1.8) |
| Neutropenia | 61 (34.3) | 55 (30.9) | 23 (12.8) | 21 (11.7) | 77 (45.6) | 71 (42.0) |
| COVID-19 | 44 (24.7) | 16 (9.0) | 38 (21.2) | 13 (7.3) | 0 | 0 |
| Anemia | 27 (15.2) | 13 (7.3) | 31 (17.3) | 16 (8.9) | 20 (11.8) | 13 (7.7) |
| Thrombocytopenia | 26 (14.6) | 15 (8.4) | 16 (8.9) | 6 (3.4) | 23 (13.6) | 19 (11.2) |
| Pneumonia | 25 (14.0) | 13 (7.3) | 27 (15.1) | 11 (6.1) | 5 (3.0) | 3 (1.8) |
| Syncope | 12 (6.7) | 9 (5.1) | 5 (2.8) | 4 (2.2) | 1 (0.6) | 1 (0.6) |
Discontinuation owing to AEs
AEs led to discontinuation of acalabrutinib in 38 patients (21.2%) in the acalabrutinib-obinutuzumab arm and 32 (17.9%) in the monotherapy arm. COVID-19 was the most frequently reported cause for AE-related discontinuation among acalabrutinib-treated patients, with 3 instances in the acalabrutinib-obinutuzumab arm and 5 instances in the acalabrutinib monotherapy arm.
Events of clinical interest
Any-grade and grade ≥3 events of clinical interest for acalabrutinib had similar rates between the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms for atrial fibrillation (any grade, 7.3% and 8.9%, respectively; grade ≥3, 1.7% in each arm), hypertension (any grade, 11.2% in each arm; grade ≥3, 4.5% and 5.0%, respectively), major hemorrhage (any grade, 9.0% and 5.6%, respectively; grade ≥3, 6.7% and 4.5%, respectively), second primary malignancies (SPMs; any grade, 20.2% and 19.6%, respectively; grade ≥3, 9.6% and 5.0%, respectively), and SPMs excluding nonmelanoma skin cancer (NMSC; any grade, 13.5% and 12.3%, respectively; grade ≥3, 7.3% and 3.9%, respectively; supplemental Table 7). The rates of any-grade and grade ≥3 SPMs in the 79 patients who crossed over from chlorambucil-obinutuzumab to acalabrutinib monotherapy were 13.9% and 2.5%, respectively; the rates of any-grade and grade ≥3 SPMs excluding NMSC were 8.9% and 2.5%, respectively (only adenocarcinoma of the colon occurred in ≥2 patients [n = 2 (2.5%)]). The cumulative incidence of any-grade atrial fibrillation/flutter and any-grade hypertension remained low over time in the acalabrutinib-containing arms (supplemental Figure 5A-B). There was no difference in the cumulative incidence of infections over time in the acalabrutinib-obinutuzumab arm compared with the acalabrutinib monotherapy arm (supplemental Figure 5C).
Deaths
Death owing to any cause was reported in 32 of 178 patients (18.0%) in the acalabrutinib-obinutuzumab arm, 44 of 179 patients (24.6%) in the acalabrutinib monotherapy arm, and 43 of 169 patients (25.4%) in the chlorambucil-obinutuzumab arm including the crossover period. Deaths owing to disease progression were reported in 5 of 178 patients (2.8%) in the acalabrutinib-obinutuzumab arm, 4 of 179 patients (2.2%) in the acalabrutinib monotherapy arm, and 4 of 169 patients (2.4%) in the chlorambucil-obinutuzumab arm including the crossover period. Deaths owing to any cause, excluding the crossover period, were reported in 26 of 169 patients (15.4%) in the chlorambucil-obinutuzumab arm. Death within 30 days of the last dose occurred in 14 (7.9%), 18 (10.1%), and 9 patients (5.3%) in the acalabrutinib-obinutuzumab, acalabrutinib monotherapy, and chlorambucil-obinutuzumab arms, respectively; deaths within 30 days of the last dose, excluding the crossover period, were reported in 2 patients (1.2%) in the chlorambucil-obinutuzumab arm. All deaths within 30 days of the last dose were caused by AEs.
Discussion
When patients were first enrolled in the ELEVATE-TN trial, combinations of chemotherapy agents and anti-CD20 monoclonal antibodies were the most prescribed treatments for CLL.3 Chemotherapy agents in combination with the anti-CD20 antibody, rituximab, demonstrated long-term survival in patients with CLL; however, these survival benefits were mostly in patients without high-risk disease characteristics and were associated with toxicities that limit the tolerability of the regimen.22,23 In the past decade, real-world data analyses showed that clinical practice treatment patterns for CLL have shifted away from chemoimmunotherapy toward targeted agents following recommendations from local and international treatment guidelines.1,3,24-27 Several randomized trials for the covalent BTKi ibrutinib, with or without monoclonal antibodies, demonstrated superior PFS for patients with CLL vs chemoimmunotherapy.4,5,7 However, ibrutinib-associated toxicities may affect the long-term use of a treat-to-progression regimen and compromise the overall efficacy of ibrutinib in CLL.28-30 In addition, previous studies yielded inconsistent results for the efficacy of the addition of anti-CD20 antibodies to ibrutinib, and there is a lack of evidence that the addition of anti-CD20 antibodies improves outcomes with concomitant ibrutinib treatment in patients with CLL.31
The higher selectivity of acalabrutinib for BTK and its limited irreversible targeting to other kinases brought the expectation of a more tolerable safety profile with sustained efficacy.32 Preclinical studies in CD19+ CLL B cells also demonstrated that acalabrutinib does not impair the function of the anti-CD20 antibodies rituximab or obinutuzumab.33
Although the primary end point of ELEVATE-TN was a comparison of PFS with acalabrutinib-obinutuzumab with chlorambucil-obinutuzumab, the focus of this analysis was comparing the impact of the addition of obinutuzumab with acalabrutinib. Before this study, there was limited evidence about the impact of adding obinutuzumab to a covalent BTKi in patients with TN CLL. Treatment with obinutuzumab requires intravenous infusion administered by a health care professional, which can increase the cost and burden of treatment for the patient.34 In addition, obinutuzumab treatment is associated with infections and infusion-related reactions, which can affect overall tolerability.34 In this study, acalabrutinib-obinutuzumab improved PFS and OS outcomes compared with chlorambucil-obinutuzumab.
With longer follow-up, ELEVATE-TN demonstrated for the first time that patients with CLL that obtain CRs have significantly longer PFS, with either acalabrutinib monotherapy or acalabrutinib-obinutuzumab treatment. In post hoc analyses assessing the benefit of adding obinutuzumab to acalabrutinib, patients treated with acalabrutinib-obinutuzumab had significantly higher ORR (96% vs 90%; P = .0220) and CR rates (37% vs 19%; P = .0220) than acalabrutinib monotherapy. These ORR and CR benefits achieved with the addition of an anti-CD20 antibody to acalabrutinib in patients with CLL may have contributed to the PFS benefits observed in this study; patients treated with acalabrutinib-obinutuzumab demonstrated a 42% reduction (HR, 0.58) in the risk of CLL disease progression or death compared with those treated with acalabrutinib monotherapy.
At a median follow-up of 74.5 months, ELEVATE-TN demonstrated that patients treated with acalabrutinib ± obinutuzumab continue to achieve significantly superior outcomes compared with those on chlorambucil-obinutuzumab. OS was longer for patients in the acalabrutinib-obinutuzumab arm than the chlorambucil-obinutuzumab arm, reflecting a 38% reduction (HR, 0.62) in the risk of death for patients in the acalabrutinib-obinutuzumab arm, despite crossover from the chlorambucil-obinutuzumab arm to acalabrutinib monotherapy. The OS benefit with acalabrutinib-obinutuzumab vs chlorambucil-obinutuzumab was not observed with acalabrutinib monotherapy vs chlorambucil-obinutuzumab, possibly related to the benefit from crossover to acalabrutinib monotherapy. Median OS was not significantly different between acalabrutinib-obinutuzumab and acalabrutinib monotherapy (HR, 0.69; 95% CI, 0.44-1.09); however, 72-month OS estimates were higher with acalabrutinib-obinutuzumab than acalabrutinib monotherapy (84% vs 76%, respectively).
These results, along with those from the CLL8 study, which compared fludarabine and cyclophosphamide with and without rituximab, and results from the CLL11 study, which compared chlorambucil vs chlorambucil with either rituximab or obinutuzumab, demonstrate the benefit of anti-CD20 therapy to OS.35,36 In the CLL11 study, obinutuzumab showed survival benefit over rituximab, emphasizing the importance of this anti-CD20 treatment. Here, a lack of anti-CD20 therapy in patients receiving acalabrutinib monotherapy may have contributed to differences in 72-month OS estimates between the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms.
Patients with high-risk genomic features have worse clinical responses to chemoimmunotherapy than those without such features and have been shown to benefit from the use of BTKis vs chemoimmunotherapy regimens.37 Fixed-duration venetoclax-obinutuzumab therapy demonstrated improved PFS vs chemoimmunotherapy in patients with TN CLL and coexisting conditions, including TP53m, del(17p), and uIGHV.38 However, fixed-duration treatment with venetoclax and an anti-CD20 antibody has shown shorter responses in patients with del(17p) and/or TP53m or uIGHV than in patients without these high-risk genomic features.38 In our trial, patients with uIGHV, del(17p) and/or TP53m, and CK had significant improvement in PFS with acalabrutinib ± obinutuzumab vs chlorambucil-obinutuzumab (supplemental Figure 1). These PFS benefits for patients with high-risk genomic features were not observed in the post hoc analysis comparing acalabrutinib-obinutuzumab with acalabrutinib monotherapy, although this study was underpowered with small patient numbers in the high-risk subgroups. There remains a strong rationale for further investigation into the potential of adding obinutuzumab to acalabrutinib to improve the depth of response in patients with CLL.31
In terms of safety, the results of this study demonstrated that acalabrutinib ± obinutuzumab was safe and tolerable in patients with TN CLL. Low rates of atrial fibrillation and hypertension were observed over time in the acalabrutinib-containing arms. In addition, rates of infection were similar between the acalabrutinib monotherapy and acalabrutinib-obinutuzumab arms, despite the rate of neutropenia in the acalabrutinib-obinutuzumab arm being nearly 3 times the monotherapy arm. The rates of SPMs excluding NMSC were also similar among patients who received acalabrutinib-obinutuzumab and acalabrutinib monotherapy and who crossed over from chlorambucil-obinutuzumab to acalabrutinib monotherapy (13.5%, 12.3%, and 8.9% respectively), which are consistent with previous studies of CLL that demonstrate that the rate of SPMs is not increased with obinutuzumab plus covalent BTKi regimens vs chemoimmunotherapy alone, with ibrutinib vs placebo, or with acalabrutinib vs ibrutinib monotherapy.11,39,40 These results indicate that continuous blocking of BTK with BTKis does not increase the risk of developing SPMs. Rates of discontinuation were similar between the acalabrutinib-obinutuzumab and acalabrutinib monotherapy arms; however, progressive disease leading to discontinuation and death leading to discontinuation were much more frequent in the acalabrutinib monotherapy arm. Overall, safety outcomes of this study indicate a comparable safety profile between the acalabrutinib-containing arms.
Limitations of this analysis include that the study design was not powered to detect a statistical difference between the acalabrutinib-containing arms and the influence of geriatric function on treatment outcome was not assessed, although age and ECOG PS were predictors of worse outcomes in a multivariate analysis. In addition, the study follow-up period coincided with the COVID-19 pandemic, which may have affected overall study conduct (eg, missed patient assessment visits), similar to other studies during this period.
With a median follow-up of ∼6 years, the efficacy and safety of acalabrutinib ± obinutuzumab were maintained in patients with TN CLL including patients with high-risk genomic features. The OS benefit of acalabrutinib-obinutuzumab vs obinutuzumab-chlorambucil and the acceptable toxicity profile of acalabrutinib-obinutuzumab supports its long-term use for treatment of TN CLL. Furthermore, the addition of obinutuzumab to acalabrutinib yielded a superior median PFS and higher CR and undetectable MRD rates than acalabrutinib alone.
Acknowledgments
The authors thank Lisa Wang of AstraZeneca for her contributions to this study.
The study was funded by AstraZeneca Group. Medical writing assistance, funded by AstraZeneca, was provided by Maria Ali, Jennifer Darby, and Sarah Huh of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors. W.G.W. is supported by the National Institutes of Health, National Cancer Institute (award number P30 CA016672) and used MD Anderson Cancer Center Support Grant shared resources.
Authorship
Contribution: J.P.S. and J.C.B. designed the study; M.H., A.S., J.P.S., V.B., J.C.B., W.J., E.F., I.W.F., P.G., Y.H., A.J., M.K., T.M., K.P., W.G.W., J.A.W., R.W., P.W., G.F., L.M.F., M.E., and A.F. were study investigators; M.H., A.S., J.P.S., V.B., J.C.B., W.J., E.F., I.W.F., P.G., Y.H., A.J., M.K., T.M., K.P., W.G.W., J.A.W., R.W., P.W., G.F., L.M.F., M.E., and A.F. provided patients or study materials; M.H., J.P.S., V.B., J.C.B., W.J., A.J., T.M., K.P., and C.W.W. performed the collection and assembly of data; J.P.S., J.C.B., V.M., and G.F. analyzed the data; J.P.S., V.B., J.C.B., W.J., P.G., M.K., K.P., V.M., G.F., C.W.W., B.T.S., and P.M. interpreted the data; J.P.S., J.C.B., W.J., E.F., I.W.F., K.P., V.M., W.G.W., B.T.S., A.F., and P.M. prepared the manuscript; and all authors performed the critical review and revision of the manuscript and approval of the manuscript for submission.
Conflict-of-interest disclosure: J.P.S. reports consulting with AbbVie, AstraZeneca, BeiGene, Lilly, Newave, Merck, Genentech, TG Therapeutics, Genmab, and Regeneron. W.J. reports consultancy with AstraZeneca, AbbVie, BeiGene, Eli Lilly, Pfizer, Roche, Sobi, and Takeda; and received research funding from AstraZeneca, AbbVie, Bayer, BeiGene, Celgene, Janssen, Eli Lilly, Merck, Pfizer, Roche, Sobi, and Takeda. A.S. reports serving on the advisory board of AstraZeneca, AbbVie, Epizyme, Genentech, Kite Pharma, Bristol Myers Squibb, Novartis, Pharmacyclics, Janssen, Seagen, Lilly, and Genmab; and speaker’s bureau of AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Genentech, Genmab, Janssen, Jazz, Kite Pharma, Lilly, Pharmacyclics, Seagen, and TG Therapeutics. K.P. reports consultancy with AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech/Roche, Kite, Loxo Oncology, MEI Pharma, Merck, MorphoSys, Nurix, Pharmacyclics/Janssen, Sana Biotechnology, TG Therapeutics, Tillium Therapeutics/Pfizer, and Xencor; received research funding from Adaptive Biotechnologies, AstraZeneca, Bristol Myers Squibb, CRISPR Therapeutics, Curis, Inc, Epizyme, Fate Therapeutics, Genentech/Roche, Kite, Loxo Oncology, MEI Pharma, Merck, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuticals, Tillium Therapeutics/Pfizer, and Xencor; and reports participation in the speakers bureau of AstraZeneca, Bristol Myers Squibb, Kite, and TG Therapeutics. I.W.F. reports consultancy with AbbVie, BeiGene, Genentech, Genmab, Hutchinson MediPharma, InnoCare Pharma, Kite, Myeloid Therapeutics, Novartis, Secura Bio, Servier Pharma, TG Therapeutics, and Xencor. M.K. received research funding from Novartis; reports consultancy with AbbVie, AstraZeneca, Celgene/Bristol Myers Squibb, Adaptive Biotechnologies, ADC Therapeutics, BeiGene, Genentech, Syncopation, and Caribou Biosciences; and reports participation in the speaker’s bureau of Seagen; and serves on the data monitoring committee of Celgene and Genentech. T.M. reports serving on the advisory board of AbbVie, Janssen, AstraZeneca, Roche, Sobi, Alexion, and BeiGene; reports consultancy with AbbVie, Janssen, AstraZeneca, Roche, Sobi, Alexion, and BeiGene; received honoraria from AbbVie, Janssen, AstraZeneca, Roche, Sobi, Alexion, and BeiGene; received research funding from AbbVie and Janssen; and reports participation in the speakers bureau of AbbVie, Janssen, AstraZeneca, Roche, Sobi, Alexion, and BeiGene. R.W. reports being on the advisory board of AstraZeneca, Janssen, Secura Bio, AbbVie, and BeiGene; and speakers bureau of AbbVie, AstraZeneca, BeiGene, and Janssen; and received meeting sponsorships from AbbVie and Janssen. Y.H. received honoraria from AstraZeneca, Janssen, AbbVie, Roche, and Lilly; and research funding from Janssen. V.B. received research funding from Canada Institutes of Health Research, AstraZeneca, Leukemia and Lymphoma Society of Canada, CancerCare Manitoba Foundation, Lymphoma Canada, and Hairy Cell Leukemia Foundation; honoraria from AstraZeneca, AbbVie, BeiGene, Janssen, and Merck; and patents and royalties from Biogen and University of Manitoba. G.F. reports consultancy with, received honoraria from, and reports being in the speakers bureau of AbbVie, Roche, Takeda, AstraZeneca, Lilly, BeiGene, Janssen, and Centessa; and reports consultancy with and received honoraria from Genesis Care. P.G. reports consultancy with and received honoraria and research funding from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Galapagos, Lilly/Loxo Oncology, Janssen, Merck, Sharp & Dohme, and Roche. A.J. reports consultancy with Janssen-Cilag, MSD, AstraZeneca, Argenx, BeiGene, Takeda, Roche, AbbVie, and Gilead; and participation in the speakers bureau of Janssen-Cilag, AstraZeneca, BeiGene, Takeda, Amgen, Eli Lilly, Novartis, and Sanofi. J.C.B. received equity from Eilean Therapeutics, Vincerx Pharma, and Kurome; reports research funding from OrbiMed, Eileen, and Newave; reports consultancy with Eilean Therapeutics, OrbiMed, and Ohio State University Drug Development Institute; reports being on the advisory board of Eilean Therapeutics, Vincerx, Kurome, Newave, Orange Grove Bio, American Cancer, and Kartos; and received travel support from AstraZeneca (September 2022). A.F. reports being on the advisory board of AbbVie, AstraZeneca, BeiGene, Genentech, and Janssen; and received research support from AbbVie, AstraZeneca, BeiGene, Eli Lilly, and Genmab. W.G.W. reports consultancy with AbbVie, AstraZeneca, Bristol Myers Squibb, and Cyclacel; received research funding from AstraZeneca, Genentech, GlaxoSmithKline/Novartis, Pharmacyclics, Gilead Sciences, Bristol Myers Squibb, Kite, Oncternal Therapeutics, Cyclacel, Loxo Oncology/Lilly, GlaxoSmithKline, Janssen Biotech, Juno Therapeutics, Nurix Therapeutics, Numab Therapeutics, and Accutar Biotechnology. C.W.W. reports employment with AstraZeneca and stock and other ownership interests with Pfizer and AstraZeneca. B.T.S., P.M., and C.C.-W. report employment with AstraZeneca and stock and other ownership interests with AstraZeneca. V.M. reports employment with AstraZeneca and stock and other ownership interests with AstraZeneca and Gilead Sciences (a family member is an employee and a stock owner). J.A.W. received research funding and AbbVie, Pharmacyclics, Janssen, and Schrodinger; reports consultancy with AbbVie, AstraZeneca, BeiGene, Genentech, Loxo/Lilly, Merck, and Newave. The remaining authors declare no competing financial interests.
The current affiliation for I.W.F. is Greco-Hainsworth Tennessee Oncology Centers for Research, Nashville, TN.
The current affiliation for J.C.B. is the Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH.
Correspondence: Jeff P. Sharman, Willamette Valley Cancer Institute and Research Center, 520 Country Club Rd, Eugene, OR 97401; email: jeff.sharman@usoncology.com.
References
Author notes
Presented in part at the 65th American Society of Hematology Annual Meeting, San Diego, CA, 9 to 12 December 2023.
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal