Key Points
Area-level lower education and higher poverty were associated with both increased mortality before and decreased access to transplants.
For patients who receive allo-HCT, socioeconomic barriers have a less pronounced impact on mortality.
Visual Abstract
Whether allogeneic hematopoietic cell transplant (allo-HCT) to treat acute myeloid leukemia (AML) is equitably accessible regardless of social determinants of health (SDOH) remains unknown. We examined associations of SDOH with access to allo-HCT and other outcomes. Patients presenting for treatment (n = 692) at 13 AML treatment centers were prospectively recruited to a registered clinical trial (number NCT01929408). Various patient-, AML-, and SDOH-specific variables were collected. Outcomes included mortality without allo-HCT, receipt of allo-HCT, and mortality after allo-HCT. Individual multivariable models (Fine-Gray for the first 2 outcomes, Cox regression for the third) were fit for each SDOH variable, adjusting for relevant patient- and AML-specific variables. Allo-HCT was used to treat 46% of patients. A 10% increase in the proportion with less than a high school education, in households receiving Supplemental Nutrition Assistance Program, receiving Supplemental Security Income, or in poverty led to modeled adjusted hazard ratios (aHRs) of 1.21 (0.99-1.46), 1.13 (0.97-1.31), 1.41 (1.01-1.97), and 1.16 (0.96-1.39) for death without allo-HCT. The aHRs were 0.67 (0.55-0.83), 0.88 (0.76-1.01), 0.71 (0.48-1.05), and 0.91 (0.75-1.09) for lessened receipt of allo-HCT. Among those who received allo-HCT, aHRs for mortality were 1.18 (0.87-1.60), 1.13 (0.92-1.38), 1.21 (0.81-1.82), and 1.04 (0.79-1.36). Results highlight increased mortality without allo-HCT and decreased access to allo-HCT, but lesser magnitude of increased mortality after allo-HCT, among patients from lower resourced areas due to limited education and/or increased poverty. Targeted interventions and policy changes are needed to ensure that marginalized patient populations have equitable chances for AML cure compared with others.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3061.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe social determinants of health (SDOH) factors associated with increased mortality before and decreased access to transplants, based on a prospective study of patient-, acute myeloid leukemia (AML)–, and SDOH-specific variables among 692 patients presenting for treatment at 13 AML treatment centers
Determine the impact of SDOH factors on patients who receive allogeneic hematopoietic cell transplantation (allo-HCT) for AML, based on a prospective study of patient-, AML-, and SDOH-specific variables among 692 patients presenting for treatment at 13 AML treatment centers
Identify clinical implications of associations of SDOH with access to allo-HCT, mortality without access to allo-HCT, and mortality with allo-HCT, based on a prospective study of patient-, AML-, and SDOH-specific variables among 692 patients presenting for treatment at 13 AML treatment centers
Release date: June 19, 2025; Expiration date: June 19, 2026
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is potentially curative for many patients with acute myeloid leukemia (AML), which is the most common indication for that procedure. The extent to which social determinants of health (SDOH) affect access to allo-HCT is not clearly known. SDOH are defined as the conditions in which people live and work that affect their health1,2 and are one mechanism through which health inequities could occur.3 SDOH can occur at the individual, area (neighborhood, city, or county), or societal level.1 At the individual and area levels, SDOH are often called social risks or needs and include low income, low education level, food insecurity, and housing instability.4-8 In general, SDOH are associated with both mortality5,9-13 and access to health care.14,15
Several SDOH could potentially reduce access to allo-HCT. The high cost and limited number of specialized centers in the United States that provide allo-HCT16 represent potential obstacles to this curative therapy, especially for patients from diverse or low socioeconomic background.17 Providers’ biases and broader system-level challenges, including racial inequities, might also be a barrier to care.18,19 For example, physician referral patterns to specialized transplant centers can differentially impact patients from underrepresented minority or low socioeconomic status groups.20
Housing insecurity is another potential limitation,19 as nearly half of referred patients must relocate to stay near the transplant center.21 Moreover, allo-HCT requires intensive time commitment and prolonged hospitalization that can lead to reductions in income from unpaid time off for both patients and their caregivers.22 These financial burdens could be critical, as wealth disparities among groups from different races and ethnicities are well documented.23
Although income and education at the individual level have been studied for general medical care, area-level SDOH are less explored, particularly with respect to allo-HCT, with past studies limited by their retrospective nature.15,24,25 The current study is unique in its prospective, longitudinal, observational, multicenter design that allowed detailed collection of patient-reported outcomes as well as less frequently examined variables, including reliance on programs, such as the Supplemental Nutrition Assistance Program (SNAP) and Supplemental Security Income (SSI), housing affordability, and commuting behaviors (defined as driving to work alone). These factors allow for a more nuanced understanding of the complex socioeconomic barriers that might impede access to allo-HCT, allowing for a more comprehensive approach to addressing these disparities. This study was conducted in a sample of patients who were being treated for AML and examined how SDOH factors could impede selection for allo-HCT. We also examined the impact of these factors on outcomes after allo-HCT to better characterize whether patients with socioeconomic barriers derive similar benefit from allo-HCT.
Materials and methods
Study design, setting, and participants
This was a multicenter, prospective, observational, non–population-based, longitudinal clinical trial conducted within the United States (NCT01929408). Centers included both academic medical centers and community oncology clinics and hospitals across 11 states. The following centers participated in the study: Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium (coordinating site), Cleveland Clinic, Stanford University, University of Utah, Roswell Park Cancer Institute, Northwestern University, University of Pennsylvania, University of Maryland, Duke University, Washington University in St Louis, Hackensack University, Confluence Health/Wenatchee Valley Hospital and Clinic, and Skagit Valley Hospital.
Patients were enrolled between July 2013 and December 2017 after presenting to receive AML therapy at 1 of 13 US centers. Inclusion criteria were as follows: (1) aged 18 to 80 years; (2) newly diagnosed AML, relapsed/refractory AML, or higher-risk myelodysplastic syndromes (10%-19% bone marrow blasts); (3) receipt of AML-like therapy, either lower or higher intensity; (4) ability to speak and read English; and (5) ability to provide informed consent. Exclusion criteria were as follows: (1) <6 months projected overall survival due to active second malignancy or other medical problem; and (2) receipt of purely palliative/supportive care treatment for AML. All patients meeting eligibility criteria were approached, unless prevented for medical reasons. All sites followed similar steps to screen patients for study eligibility. European Leukemia Network (ELN) classification 2017 was used to categorize patient risks.28
Timing of study evaluations
Questionnaires were collected at the time of enrollment and then approximately at months +1, +3, +6, +9, +12, +18, and +24 after study enrollment. Administration of all surveys was event driven and corresponded approximately with the time points illustrated in supplemental Table 1 (available on the Blood website) and Figure 1 and were adjusted to correspond with recovery of peripheral blood counts (defined as absolute neutrophil count >1500/mm3 and platelet count >100 000/mm3). Furthermore, patients who proceeded to allo-HCT had their timing of surveys to align with the transplant procedure and following recovery.
Study schema. QOL, quality of life. KPS, Karnofsky Performance Scale; SA, specific aim.
Study schema. QOL, quality of life. KPS, Karnofsky Performance Scale; SA, specific aim.
The trial had 3 primary aims, 2 of which focused on comparing clinical outcomes by conditioning intensity and efficacy based on receipt of allo-HCT vs not.29,30 The third aim, addressed herein, focused on examining the impacts of SDOH on the likelihood of receiving allo-HCT, as well as the risk of death without allo-HCT and death after allo-HCT.
The study was approved by the institutional review boards of each collaborating site, and participants provided written informed consent. Timing of study evaluations, study time frame, data collection and sources, and bias management are described in supplemental Materials and methods.
Variables, data sources, and measurement
Study outcomes
(1) Mortality without allo-HCT; (2) receipt of allo-HCT (access to allo-HCT); and (3) overall mortality among those who received allo-HCT.
Exposure/predictors
SDOH at the zip-code level in which patients lived: median household income; percentage of adults aged ≥25 years with less than a high school education or equivalent (ie, General Education Diploma); percentage of households below the federal poverty level (FPL); percentage of households receiving SNAP/food stamps; percentage of occupied housing units (rented and owner occupied) where ≥30% of income is spent on housing; percentage of owner-occupied housing units with a mortgage that have a second mortgage, home equity loan, or both; percentage of households receiving SSI (disability); and percentage of people working for pay who drive to work alone. Data for each area measure was drawn from the American Community Survey conducted by the US Census Bureau. We drew American Community Survey data for each zip code reported in 2017, the most recent completed survey, and the average of data from the previous 5 years. Higher values on median household income and percentage driving to work alone indicate an area with less social risk. For all other area measures, higher values indicate an area with more social risk. Measures were chosen to reflect both general social risk (income, education, poverty level, debt level, and SSI) and specific social risks (food insecurity, housing instability, and transportation). A subset of the measures also indicates the capacity to navigate administrative burdens, including administrative literacy (education) and other administrative burdens (SNAP, SSI). Area measures may reflect more barriers to care even if someone has high individual socioeconomic status as the individual may not have needed community supports or may have to support others in their community. Separate SDOH measures, rather than summary measures, were chosen as these can help target patient- and policy-level intervention.
We also included patient-reported race as another SDOH predictor serving as a proxy for exposure to the SDOH of discrimination.
Other numerous AML- and patient-specific covariates are described in supplemental Material but notably include patient age, the HCT-Comorbidity Index (HCT-CI),31,32 diagnoses, the Karnofsky performance status,33 the Patient Health Questionnaire 9,34 activities of daily living,35 the 4-minute walk test,36 the Functional Assessment of Cancer Treatment–General,37 and the ELN classification 2017.28 Other variables, study size, and missing data are described in supplemental Material and supplemental Table 2.
Statistical analysis
Outcomes examined were receipt of allo-HCT and overall mortality, both without allo-HCT and after allo-HCT. Each of these outcomes was treated as a time-to-event end point, with time 0 corresponding to study enrollment date, and associations of SDOH factors with the cause-specific hazard of each outcome were assessed using Cox regression. For the outcome mortality among allo-HCT recipients, patients were left-truncated at time of allo-HCT to account for the differing lengths of time from start of treatment to allo-HCT. Consistency of the results from Cox regression with those using Fine-Gray regression was also assessed for the outcomes receipt of allo-HCT and death without HCT, where one is considered a competing risk for the other. All SDOH factors were modeled as continuous linear variables, with the corresponding hazard ratio (HR) presented in terms of an increase in income of $25 000 and an increase in other factors of 10%. The assumption of linearity was checked for each factor and model, and all factors and models sufficiently met the assumption of linearity. Models were adjusted for patient age (continuous variable), HCT-CI (continuous variable), disease status, ELN risk, and intensity of induction therapy. Additional adjustment factors included Karnofsky Performance Scale, Patient Health Questionnaire 9, activities of daily living, 4-minute walk test, and Functional Assessment of Cancer Treatment, with each of these measured longitudinally and modeled as a continuous linear time-varying covariate. The model for mortality after allo-HCT was adjusted for only age, HCT-CI, diagnosis, ELN, and disease status after HCT to eliminate the potential impact of allo-HCT on frailty and quality-of-life measures. Each SDOH factor as well as race was modeled separately from the other SDOH factors because of the collinearity between SDOH factors.
Results
Descriptive data
Participant characteristics and exposure
Patient characteristics are reported in Table 1. As reported previously, 816 eligible patients were approached, 702 were enrolled, and 692 ultimately participated.29 Data from 692 patients were analyzed. Patients aged ≥65 years constituted 43% of the cohort, and those with augmented HCT-CI scores of ≥4, 60%; whereas 68% of patients met either or both of these criteria. Diagnoses were mostly newly diagnosed AML (77%). Most patients had intermediate or higher (79%) cytogenetic/molecular risk per the ELN 2017.
Patient characteristics (n = 692)
| Characteristics . | Value . |
|---|---|
| Patient and AML variables | |
| Patient age, 18-64 y, % | 57 |
| Patient age, ≥65 y, % | 43 |
| Patient age, median (range), y | 62 (18-80) |
| Patient female gender, % | 43 |
| Augmented HCT-CI score ≥4 at study enrollment, % | 60 |
| Newly diagnosed AML, % | 77 |
| Relapsed or refractory AML, % | 14 |
| High-risk MDS (at least 10% marrow blasts), % | 9 |
| Intermediate risk per ELN for AML subset, % | 43 |
| Adverse risk per ELN for AML subset, % | 36 |
| Karnofsky performance status >70, % | 83 |
| Complete remission after initial treatment, % | 56 |
| Received allo-HCT, % | 46 |
| Did not receive allo-HCT, % | 54 |
| Unrelated donor, % | 68 |
| SDOH factors per zip code (n = 646) | |
| Income, median (range), $ | 65 837 (0-160 618) |
| Those with less than a high school education, median (range), % | 7.3 (0.6-46.4) |
| Those below federal poverty level, median (range), % | 9.5 (1.7-52.6) |
| Those on SNAP, median (range), % | 10.2 (0-63.4) |
| Those spending ≥30% of their income on housing, median (range), % | 30.0 (9.1-52.1) |
| Those with a second mortgage or a home equity loan, median (range), % | 16.0 (0-33.3) |
| Those on SSI, median (range), % | 4.2 (0-48.8) |
| Those driving to work alone, median (range), % | 79.2 (2.0-98.7) |
| White, % | 86 |
| Asian, % | 3 |
| Black, % | 6 |
| Other race, % | 4 |
| Hispanic, % | 4 |
| Characteristics . | Value . |
|---|---|
| Patient and AML variables | |
| Patient age, 18-64 y, % | 57 |
| Patient age, ≥65 y, % | 43 |
| Patient age, median (range), y | 62 (18-80) |
| Patient female gender, % | 43 |
| Augmented HCT-CI score ≥4 at study enrollment, % | 60 |
| Newly diagnosed AML, % | 77 |
| Relapsed or refractory AML, % | 14 |
| High-risk MDS (at least 10% marrow blasts), % | 9 |
| Intermediate risk per ELN for AML subset, % | 43 |
| Adverse risk per ELN for AML subset, % | 36 |
| Karnofsky performance status >70, % | 83 |
| Complete remission after initial treatment, % | 56 |
| Received allo-HCT, % | 46 |
| Did not receive allo-HCT, % | 54 |
| Unrelated donor, % | 68 |
| SDOH factors per zip code (n = 646) | |
| Income, median (range), $ | 65 837 (0-160 618) |
| Those with less than a high school education, median (range), % | 7.3 (0.6-46.4) |
| Those below federal poverty level, median (range), % | 9.5 (1.7-52.6) |
| Those on SNAP, median (range), % | 10.2 (0-63.4) |
| Those spending ≥30% of their income on housing, median (range), % | 30.0 (9.1-52.1) |
| Those with a second mortgage or a home equity loan, median (range), % | 16.0 (0-33.3) |
| Those on SSI, median (range), % | 4.2 (0-48.8) |
| Those driving to work alone, median (range), % | 79.2 (2.0-98.7) |
| White, % | 86 |
| Asian, % | 3 |
| Black, % | 6 |
| Other race, % | 4 |
| Hispanic, % | 4 |
MDS, myelodysplastic syndrome.
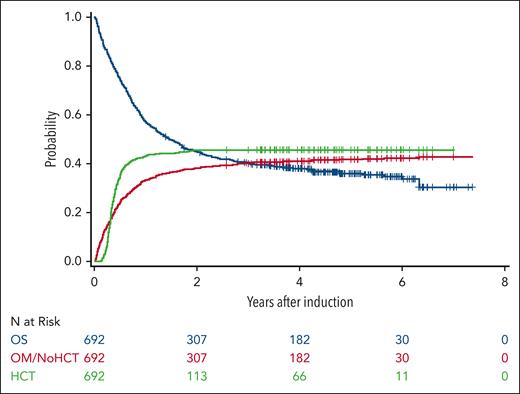
Overall, 46% patients received an allo-HCT, and 77% of those underwent allo-HCT in first complete remission (CR1) (Figure 2). Among older and/or comorbid patients, 36% received an allo-HCT and 80% were in CR1. Of patients aged ≥65 years, 31% received an allo-HCT, with 84% of these in CR1. Of the ELN intermediate-risk patients, 53% received an allo-HCT, with 79% in CR1; and 43% of the ELN adverse-risk patients received an allo-HCT, with 77% in CR1.
Survival curves for overall survival (OS), overall mortality (OM) without transplant, and receipt of transplant.
Survival curves for overall survival (OS), overall mortality (OM) without transplant, and receipt of transplant.
Details of study flow are in Figure 1 as well as supplemental Materials.
Median income per zip code was $65 837 (range, $0-$160 618), whereas median percentage with less than high school education was 7.3% (range, 0.6%-46.4%). Median percentage below FPL was 9.5% (range, 1.7%-52.6%); those on SNAP, 10.2% (range, 0%-63.4%); those spending ≥30% of their income on housing, 30.0% (range, 9.1%-52.1%); those with a second mortgage or home equity loan, 16.0% (range, 0%-33.3%); those on SSI, 4.2% (range, 0%-48.8%); and those driving to work alone, 79.2% (range, 2.0%-98.7%). Most patients were White (86%); Asian patients constituted 3%; Black patients, 6%; and other races, 4%.
Length of follow-up and missing data
Median follow-up among 253 survivors was 54 (range, 1-88) months after start of treatment to allow capturing post allo-HCT outcomes. supplemental Table 1 shows low rates of missing data in general.
Primary outcomes
Among patients who received HCT, the median time from initial treatment to allo-HCT was 4.2 months; 75% of transplants were performed within 180 days, and 95% within 1 year.
Factors associated with death without allo-HCT
A total of 291 patients died without receiving allo-HCT. Initially, we investigated the impact of patient- and AML-specific variables on mortality without allo-HCT, and those with notable associations were chosen as the variables to adjust for when analyzing SDOH variables (details in supplement Material). The associations of SDOH factors, as well as race, with death without HCT are summarized in Table 2. In particular, as the proportion in the area with less than a high school education increased by 10%, the adjusted cause-specific hazard of mortality without allo-HCT increased by 21% (95% confidence interval [CI], −1% to 46%). The adjusted cause-specific hazard also increased by 41% (95% CI, 1% to 97%) for each 10% increase in the proportion on SSI, by 16% (95% CI, −4% to 39%) for each 10% increase in FPL, and by 13% (95% CI, −3% to 31%) for each 10% increase in SNAP. Risk was increased by 38% (95% CI, −16% to 127%) among patients from other races compared with White patients. Conclusions from Fine-Gray regression were qualitatively similar.
Association of SDOH with mortality without transplant
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 0.89 | 0.78-1.02 | .101 | |
| Proportion with less than a high school education | 1.21 | 0.99-1.46 | .058 | |
| Proportion below federal poverty level | 1.16 | 0.96-1.39 | .119 | |
| Proportion on SNAP | 1.13 | 0.97-1.31 | .105 | |
| Proportion spending ≥30% of their income on housing | 0.99 | 0.82-1.19 | .889 | |
| Proportion with a second mortgage or a home equity loan | 0.93 | 0.73-1.18 | .538 | |
| Proportion on SSI | 1.41 | 1.01-1.97 | .045 | |
| Proportion driving to work alone | 1.10 | 0.98-1.24 | .114 | |
| Race | ||||
| White | 1 | --- | .051 | |
| Asian | 0.62 | 0.27-1.43 | ||
| Black | 0.59 | 0.36-0.97 | ||
| Other | 1.38 | 0.84-2.27 | ||
| Race∗ | ||||
| White | 1 | --- | .024 | |
| Asian | 0.59 | 0.26-1.33 | ||
| Black | 1.11 | 0.69-1.76 | ||
| Other | 1.92 | 1.20-3.06 |
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 0.89 | 0.78-1.02 | .101 | |
| Proportion with less than a high school education | 1.21 | 0.99-1.46 | .058 | |
| Proportion below federal poverty level | 1.16 | 0.96-1.39 | .119 | |
| Proportion on SNAP | 1.13 | 0.97-1.31 | .105 | |
| Proportion spending ≥30% of their income on housing | 0.99 | 0.82-1.19 | .889 | |
| Proportion with a second mortgage or a home equity loan | 0.93 | 0.73-1.18 | .538 | |
| Proportion on SSI | 1.41 | 1.01-1.97 | .045 | |
| Proportion driving to work alone | 1.10 | 0.98-1.24 | .114 | |
| Race | ||||
| White | 1 | --- | .051 | |
| Asian | 0.62 | 0.27-1.43 | ||
| Black | 0.59 | 0.36-0.97 | ||
| Other | 1.38 | 0.84-2.27 | ||
| Race∗ | ||||
| White | 1 | --- | .024 | |
| Asian | 0.59 | 0.26-1.33 | ||
| Black | 1.11 | 0.69-1.76 | ||
| Other | 1.92 | 1.20-3.06 |
Each row represents 1 model for 1 SDOH variable adjusted for age and HCT-CI; each as a continuous linear variable; Karnofsky performance status, Patient Health Questionnaire 9 scale for depression, activities of daily living scale, 4-meter walk speed, quality of life per Functional Assessment of Cancer Treatment; each as continuous linear time-varying covariates; and diagnosis type, ELN risk, intensity of induction therapy.
Unadjusted model (univariate analysis).
Factors affecting receipt of allo-HCT
Patient- and AML-specific variables associated with receiving allo-HCT are described in supplemental Material.
There was a decrease in the adjusted cause-specific hazard of receiving allo-HCT by 33% (95% CI, 17% to 45%) as the percentage of residents with less than a high school education in a neighborhood increased by 10%. The decrease was 29% (95% CI, −5% to 52%) for each 10% increase in households receiving SSI, 9% (95% CI, −9% to 25%) for each 10% increase in percentage of households below FPL, and 12% (95% CI, −1% to 24%) for each 10% increase in households receiving SNAP. The decrease in the adjusted cause-specific hazard of receiving allo-HCT was 16% (95% CI, −40% to 50%) and 3% (95% CI, −91% to 50%), respectively, among Black patients and those from other races compared with White patients, whereas Asian patients had a 32% increase (95% CI, −25% to 131%) in the adjusted cause-specific hazard of receiving allo-HCT. Details are summarized in Table 3. Conclusions from Fine-Gray regression were qualitatively similar.
Association of SDOH with receipt of allo-HCT (access to transplant)
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 1.03 | 0.92-1.17 | .588 | |
| Proportion with less than a high school education | 0.67 | 0.55-0.83 | <.001 | |
| Proportion below federal poverty level | 0.91 | 0.75-1.09 | .298 | |
| Proportion on SNAP | 0.88 | 0.76-1.01 | .074 | |
| Proportion spending ≥30% of their income on housing | 1.08 | 0.90-1.30 | .388 | |
| Proportion with a second mortgage or a home equity loan | 1.16 | 0.94-1.42 | .332 | |
| Proportion on SSI | 0.71 | 0.48-1.05 | .082 | |
| Proportion driving to work alone | 0.97 | 0.89-1.06 | .540 | |
| Race | ||||
| White | 1 | --- | .676 | |
| Asian | 1.32 | 0.75-2.31 | ||
| Black | 0.84 | 0.50-1.40 | ||
| Other | 0.97 | 0.50-1.91 | ||
| Race∗ | ||||
| White | 1 | --- | .282 | |
| Asian | 1.54 | 0.90-2.64 | ||
| Black | 0.78 | 0.48-1.28 | ||
| Other | 0.87 | 0.46-1.63 |
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 1.03 | 0.92-1.17 | .588 | |
| Proportion with less than a high school education | 0.67 | 0.55-0.83 | <.001 | |
| Proportion below federal poverty level | 0.91 | 0.75-1.09 | .298 | |
| Proportion on SNAP | 0.88 | 0.76-1.01 | .074 | |
| Proportion spending ≥30% of their income on housing | 1.08 | 0.90-1.30 | .388 | |
| Proportion with a second mortgage or a home equity loan | 1.16 | 0.94-1.42 | .332 | |
| Proportion on SSI | 0.71 | 0.48-1.05 | .082 | |
| Proportion driving to work alone | 0.97 | 0.89-1.06 | .540 | |
| Race | ||||
| White | 1 | --- | .676 | |
| Asian | 1.32 | 0.75-2.31 | ||
| Black | 0.84 | 0.50-1.40 | ||
| Other | 0.97 | 0.50-1.91 | ||
| Race∗ | ||||
| White | 1 | --- | .282 | |
| Asian | 1.54 | 0.90-2.64 | ||
| Black | 0.78 | 0.48-1.28 | ||
| Other | 0.87 | 0.46-1.63 |
Each row represents 1 model for 1 SDOH variable adjusted for age and HCT-CI; each as a continuous linear variable; Karnofsky performance status, Patient Health Questionnaire 9 scale for depression, activities of daily living scale, 4-meter walk speed, and quality of life per Functional Assessment of Cancer Treatment; each as continuous linear time-varying covariates; and diagnosis type, ELN risk, and intensity of induction therapy.
Unadjusted model (univariate analysis).
We also investigated potential statistical interactions between certain SDOH variables and select patient- and AML-specific variables (supplemental Table 3). Although a few pairs showed some evidence of an interaction, the level of evidence was not considered strong enough to warrant subgroup analyses, as no such analyses were prespecified.
Secondary outcomes
The associations between SDOH variables and mortality after allo-HCT are summarized in Table 4. Among these results, as the percentage of residents with less than a high school education in a neighborhood increased by 10%, the adjusted hazard of death after allo-HCT increased by 18% (95% CI, −13% to 60%). The adjusted risk of death increased by 21% (95% CI, −19% to 82%) as the percentage of households receiving SSI increased by 10% and by 13% (95% CI, −8% to 38%) as the percentage receiving SNAP increased by 10%. As percentage of households below FPL increased by 10%, the adjusted risk of death increased by only 4% (95% CI, −21% to 36%). The adjusted hazard of mortality after allo-HCT in Black patients was similar to that in White patients (HR = 0.98; 95% CI, 0.45 to 2.17), whereas the HR for Asian vs White patients was 0.91 (95% CI, 0.40 to 2.09).
Association of SDOH with mortality after allo-HCT (n = 315)
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 1.00 | 1.00-1.00 | .558 | |
| Proportion with less than a high school education | 1.18 | 0.87-1.60 | .283 | |
| Proportion below federal poverty level | 1.04 | 0.79-1.36 | .804 | |
| Proportion on SNAP | 1.13 | 0.92-1.38 | .240 | |
| Proportion spending ≥30% of their income on housing | 1.03 | 0.78-1.35 | .845 | |
| Proportion with a second mortgage or a home equity loan | 0.99 | 0.73-1.36 | .970 | |
| Proportion on SSI | 1.21 | 0.81-1.82 | .345 | |
| Proportion driving to work alone | 0.96 | 0.85-1.10 | .570 | |
| Race | ||||
| White | 1 | --- | .722 | |
| Asian | 0.91 | 0.40-2.09 | ||
| Black | 0.98 | 0.45-2.17 | ||
| Other | 0.44 | 0.11-1.80 | ||
| Race∗ | ||||
| White | 1 | --- | .528 | |
| Asian | 0.80 | 0.35-1.81 | ||
| Black | 0.77 | 0.36-1.65 | ||
| Other | 0.41 | 0.10-1.65 |
| Variable . | Hazard ratio . | 95% CI . | P value . | Global P value . |
|---|---|---|---|---|
| Median income | 1.00 | 1.00-1.00 | .558 | |
| Proportion with less than a high school education | 1.18 | 0.87-1.60 | .283 | |
| Proportion below federal poverty level | 1.04 | 0.79-1.36 | .804 | |
| Proportion on SNAP | 1.13 | 0.92-1.38 | .240 | |
| Proportion spending ≥30% of their income on housing | 1.03 | 0.78-1.35 | .845 | |
| Proportion with a second mortgage or a home equity loan | 0.99 | 0.73-1.36 | .970 | |
| Proportion on SSI | 1.21 | 0.81-1.82 | .345 | |
| Proportion driving to work alone | 0.96 | 0.85-1.10 | .570 | |
| Race | ||||
| White | 1 | --- | .722 | |
| Asian | 0.91 | 0.40-2.09 | ||
| Black | 0.98 | 0.45-2.17 | ||
| Other | 0.44 | 0.11-1.80 | ||
| Race∗ | ||||
| White | 1 | --- | .528 | |
| Asian | 0.80 | 0.35-1.81 | ||
| Black | 0.77 | 0.36-1.65 | ||
| Other | 0.41 | 0.10-1.65 |
Each row represents 1 model for 1 SDOH variable adjusted for age and HCT-CI; each as a continuous linear variable; relapsed/refractory AML vs newly diagnosed, intermediate vs unfavorable cytogenetics, status at HCT (CR1 vs other), and intensity of induction therapy.
Unadjusted model (univariate analysis).
Discussion
To the best of our knowledge, this study is the first to prospectively evaluate the impact of numerous SDOH variables on access to allo-HCT, the only potentially curative therapy for many patients with AML or higher-risk myelodysplastic syndrome, while considering various patient- and AML-specific variables. Our results highlight the potential negative influence of area-level educational attainment and benefit use (SNAP, SSI) on both mortality without allo-HCT and the likelihood of receiving allo-HCT, an indicator of access to allo-HCT. In particular, patients from areas with lower education or needing SSI benefits experienced the highest risks of dying before receiving allo-HCT, as well as being less likely to receive allo-HCT, compared with patients with less social risk as measured by these factors; this is indicative of potential problems accessing transplant. This study highlights potential areas of limitations in caring for patients from underserved areas and sets the stage for future efforts to improve their access to allo-HCT and AML outcomes in general.
Associations between race and mortality or receiving allo-HCT had relatively small numbers. That said, we observed encouraging overall outcomes among Asian patients compared with White patients before and after adjustment for patient and AML risk factors. The modest unadjusted increased risk of mortality without allo-HCT in Black patients compared with White patients was reversed after adjustment for patient- and AML-specific factors, suggesting a higher burden of these factors among Black patients. However, surviving Black patients had a numerically decreased hazard of allo-HCT receipt, although those who received allo-HCT had a similar outcome to White patients. A recent large US-based retrospective study, not including SDOH variables we examined, showed increased use of allo-HCT across all races, but significant disparities in HCT rates per race persisted, particularly for adult non-Hispanic Black patients,38 which is overall consistent with our results. The sample of non-White patients (14%) in the current study was limited but in general similar to overall rates reported by the Center for International Blood and Marrow Transplantation Research,39 which itself suggests inequitable access among different races to allo-HCT and AML treatment.
Consistent with the results from our prospective study, retrospective studies accounting for different socioeconomic variables have shown that patients with AML with lower educational attainment15 and those living in high-poverty areas40 are less likely to receive allo-HCT. Another single-center retrospective study suggested lack of associations of socioeconomic factors but association of race with post allo-HCT mortality.41 Although most SDOH factors had a numerically increased risk of mortality after allo-HCT as social risk increased, none of the increased risks was large enough to definitively conclude such an association. However, the possibility that these increases might be real should not be discounted, and this deserves further attention in future studies. The impact of SDOH factors on access to allo-HCT could result from both referral and selection decisions. Clinics initially treating patients with AML with exposure to SDOH might decline to refer them for allo-HCT, but transplant centers may also decline to select this group of patients for allo-HCT because of the impact of SDOH. We hypothesized that SDOH would reduce the selection of patients for allo-HCT, and future research is needed to identify the best interventions to address the impact of SDOH on selection for transplant.
Increased access to allo-HCT may increase cure rates of patients with AML who reside in lower education and higher poverty areas. These results highlight the need for targeted interventions to improve access to allo-HCT for patients from lower socioeconomic backgrounds. Efforts should focus on addressing financial barriers, improving health literacy, and enhancing support systems to ensure equitable access to life-saving treatments. These are some of the key focuses of the ACCESS initiative.42 One potential option in the overall field of cancer care is unconditional cash payments to help offset nonmedical costs associated with intensive treatments for patients from lower socioeconomic backgrounds.43 Applicability of such an intervention to allo-HCT remains to be investigated.
A lower area-level educational attainment and more receipt of SSI in the area were the 2 factors that showed the most evidence of negatively impacting risks of mortality without allo-HCT, and as the proportion of patients in areas with less than a high school education level increase, receipt of allo-HCT similarly decreased. In addition, mortality after allo-HCT was numerically increased with an increasing proportion of lower education in the area. This could be related to administrative burden, or the additional steps a person must take when interacting with an institution for items such as government benefits and health insurance.37 Administrative burden is distinct from financial hardship, but ability to cope with administrative burden tends to be lower for people from disadvantaged areas and backgrounds.44 Areas with lower educational attainment could have fewer resources to understand and navigate these burdens. A role for administrative burden is also consistent with the modest association of percentage receiving SSI in the area and higher risk of mortality without allo-HCT. SSI can be a burdensome program,45 and people who have received SSI and their neighbors may be more averse to engaging in health care with high administrative burden (prior authorizations, appealing denials of coverage). SSI benefits may also be an important surrogate for poverty and disability or comorbidity burden to target if we want to improve access to allo-HCT.
These results have implications for both clinical care and policy. Hematologists and health care personnel should be aware of the potential overwhelming nature of the transplant process, particularly for patients with lower education levels. Patient navigators may be important partners to help patients and their families navigate the administrative process and access financial aid when needed.46 Health care policy makers may choose to provide financial aid for patients undergoing allo-HCT and provide resources to reduce administrative burdens or fund supportive personnel, such as patient navigators.
The results of the study should be considered within its limitations. Although we were able to examine several socioeconomic markers, we were unable to examine area measures at the census block or at the individual level as these data were unavailable for this sample and results could differ particularly for individual-level markers. The lack of racial and ethnic diversity in the study population is another limitation. The sample was also too small to thoroughly examine the differences in the impact of SDOH between White, Black, and Asian patients or to assess potential mechanisms for inequities in access to allo-HCT. This is evidenced by the relatively large CIs for racial groups. Future research will focus on recruiting patients from diverse racial and ethnic backgrounds, including work to increase recruitment of Spanish-speaking patients in the United States. This study also examined indicators separately to better identify factors that could impede allo-HCT access, but summary or aggregate measures, such as the Area Deprivation Index,40 were not examined. However, although summary measures can be useful for assessing the total burden, identifying the specific intervention targets can be more challenging with summary measures. Future studies with a larger sample could look at the specific contribution of each factor when tested simultaneously and provide more precise estimates of the contribution from each SDOH. Information on family and community involvement for each participant was not available. This study was also not able to assess the role of donor availability on access to allo-HCT. However, recent findings highlight that alternative donor types can achieve good outcomes for patients from underrepresented minority groups.47 This trial did not allow non–English-speaking participants and was done among those who had already been referred to AML-treating centers; hence, we might have underestimated the total impact of SDOH on access to care. It is also possible that participants from especially disadvantaged areas did not participate. However, the distributions of the SDOHs show that a full range of experiences were represented in the sample. Finally, we did not study associations between SDOH and the availability of a caregiver, another potential barrier to care to be addressed in the future.
Allo-HCT is a potentially curative treatment for patients with AML, and SDOH may impede access to this crucial treatment among marginalized patients and those who come from areas with low education and/or poverty. This impediment was due either to increased mortality before allo-HCT could be arranged and/or to a lower likelihood of receiving allo-HCT among survivors. Although health care personnel can provide assistance through linkage to financial aid and other resources, long-term solutions at the societal level are needed, such as Medicare’s recent decision to cover patient navigation.48 Additional research into specific solutions addressing SDOH is warranted to increase access to allo-HCT and improve AML outcomes in general. The impacts of education level and poverty on mortality risks after receiving allo-HCT may have been of a lesser magnitude than on mortality without allo-HCT, although this requires further study. Nonetheless, this is encouraging, as outcomes might be improved if barriers to allo-HCT are ameliorated.
Acknowledgments
The authors are grateful to all research nurses and data coordinators for implementation of protocols. They are grateful to the many physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff who cared for their patients, and to the patients who allowed them to care for them and who participated in their ongoing clinical research. They are also grateful to Helen Crawford and Roslyn Pierce for help in preparing the manuscript for submission.
Research reported in this article was funded, in part, by a Patient-Centered Outcome Research Institute award (CE-1304-7451), in part, by a Research Scholar Grant from the American Cancer Society (RSG-13-084-01-CPHPS), and supported, in part, by an American Society of Hematology Bridge Award.
Funding organizations had absolutely no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The statements and findings in this article are solely the responsibility of the authors and do not necessarily represent the views of any funding organization. Neither the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee, nor the American Cancer Society nor the American Society of Hematology influenced the findings in this publication.
The authors dedicate this article to the late Elihu Estey, University of Washington and Fred Hutchinson Cancer Center, who died a few years ago but was a key investigator to help initiate and execute the trial.
Authorship
Contribution: M.L.S. and T.G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; M.L.S. designed and executed the clinical trial and was principal investigator of the clinical trial; N.W., S.M.W.J., T.G., and M.L.S. conceived and designed the current study; A.T.G., B.C.M., P.J.S., J.G., K.A., S. Luger, M.R.B., D.R., T.M.W., E.S.W., M.A.S., S.M., J.S., M.G., K.K., M.-E.P., B.M.S., S. Lee, F.R.A., and M.L.S. performed acquisition of data and patient enrollment; N.W., S.M.W.J., T.G., and M.L.S. drafted the manuscript; N.W., S.M.W.J., T.G., A.T.G., B.C.M., P.J.S., S. Luger, M.R.B., D.R., T.M.W., E.S.W., M.A.S., S.M., J.A., M.-E.P., B.M.S., S. Lee, F.R.A., R.R., and M.L.S. interpreted data; B.C.M., P.J.S., S. Luger, M.R.B., T.M.W., E.S.W., M.A.S., S.M., J.A., M.-E.P., B.M.S., S. Lee, F.R.A., and R.R. performed critical revision of the manuscript for important intellectual content; T.G. performed statistical analysis; M.L.S. obtained funding; and T.G. and M.L.S. supervised the study.
Conflict-of-interest disclosure: N.W. serves on the advisory board for Mustang Bio. A.T.G. is a consultant with AbbVie. T.G. has done consulting work for REGiMMUNE. B.C.M. works for AstraZeneca and is a current equity holder in both AbbVie and AstraZeneca. P.J.S. has grants from Chimerix, Inc, Amgen, and Ancuro, Inc; is on the Board of Directors or advisor committee of Bristol Myers Squibb, Daiichi Sankyo, Inc, Gilead Sciences, Inc, Takeda Pharmaceuticals, RJH BioSciences, and JSK Therapeutics; has a leadership role at the National Cancer Network; and holds 2 US patents he collects royalties on. J.G. is a current employee and holder of stock options at Incyte Corporation. S. Luger is on the Board of Directors for Amgen, Novartis, Daiichi Sankyo, Marker Therapeutics, and AbbVie; is a paid consultant with Amgen, Novartis, and Astellas; and receives research funding from Takeda. E.S.W. is a consultant and member of the Board of Directors for AbbVie, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Kite, Novartis, Pfizer, Takeda, NuProbe, PharmaEssentia, Jazz, Amgen, Blueprint, CTI Biopharma, Daiichi Sankyo, GlaxoSmithKline, Immunogen, Janssen, Johnson and Johnson, Mana, Qiagen, Rigel, Schrodinger, Sellas, Stemline, Sumitomo Pharma, and Syndax; is on the Speakers Bureau of Kura, Astellas, and Dava Oncology; and is the Section Editor for UptoDate. M.A.S. has research funding from Bristol Myers Squibb and serves on the Board of Directors for Bristol Myers Squibb, Kurome, and Schroedinger. J.A. receives an honorarium from Incyte. M.-E.P. receives research funding from AbbVie, Ascentage, Astex, Biosight, Bristol Myers Squibb/Celegene, Cardiff Oncology, Glycomimetics, Immunogen, Nohla Therapeutics, Oscotec, Pfizer, Telios, Trillium, and VinceRx. M.L.S. receives an honorarium and is a consultant with Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Mohamed L. Sorror, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109-1024, M2-B803; email: msorror@fredhutch.org.
References
Author notes
N.W. and S.M.W.J. contributed equally to this study.
Presented in abstract form at the 66th Annual Meeting of the American Society of Hematology, San Diego, CA, 7 to 10 December 2024.
This is not an intervention clinical trial. There is no data sharing plan for the study because the requirements of the International Committee of Medical Journal Editors are not applicable for this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal