Key Points
Identify a novel oncogenic WDR5/ATAD2 signaling by affecting cell cycle progress in T-ALL.
Targeting WDR5/ATAD2 signaling through the CK2/IKAROS axis demonstrates the synergistic antileukemic efficacy in T-ALL.
Visual Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological malignancy with a poor prognosis and limited options for targeted therapies. Identifying new molecular targets to develop novel therapeutic strategies is the pressing immediate issue in T-ALL. Here, we observed high expression of WD repeat-containing protein 5 (WDR5) in T-ALL. With in vitro and in vivo models, we demonstrated the oncogenic role of WDR5 in T-ALL by activating cell cycle signaling through its new downstream effector, ATPase family AAA domain-containing 2 (ATAD2). Moreover, the function of a zinc finger transcription factor of the Kruppel family (IKAROS) is often impaired by genetic alteration and casein kinase II (CK2) which is overexpressed in T-ALL. We found that IKAROS directly regulates WDR5 transcription; CK2 inhibitor, CX-4945, strongly suppresses WDR5 expression by restoring IKAROS function. Last, combining CX-4945 with WDR5 inhibitor demonstrates synergistic efficacy in the patient-derived xenograft mouse models. In conclusion, our results demonstrated that WDR5/ATAD2 is a new oncogenic signaling pathway in T-ALL, and simultaneous targeting of WRD5 and CK2/IKAROS has synergistic antileukemic efficacy and represents a promising potential strategy for T-ALL therapy.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological malignancy that originates from immature T lymphocytes or lymphoid progenitors.1,2 T-ALL accounts for 10% to 15% of pediatric and up to 25% of adult ALL cases.3 An overall survival has been achieved at 80% in the pediatric setting by using a risk-based stratification toward intensive multiagent combination chemotherapeutic protocols.4 The overall survival for adult patients are <50%,3 particularly the outcome remains poor for those patients who do experience disease relapse or are refractory to induction therapy. The rapid progress in targeted therapies and immunotherapies such as the recently approved blinatumomab, inotuzumab, ozogamicin, and chimeric antigen receptor T-cell therapy has dramatically improved the outcome of B-cell acute lymphoblastic leukemia (B-ALL).5,6 However, there have been no new agents specifically approved for relapsed/refractory T-ALL since nelarabine was approved in 2005.7 The targeted therapies benefiting T-ALL are still limited owing to the biological heterogeneity of T-ALL.8,9 Therefore, identifying novel “druggable” molecular markers and illustrating the underlying mechanisms are immediate pressing issues in T-ALL.10
WD repeat-containing protein 5 (WDR5) is the core subunit of the histone 3 lysine 4(H3K4) methyltransferase complex.11,12 WDR5 directly interacts with mixed lineage leukemia (MLL) and increases H3K4 trimethylation (H3K4me3) at the target genes’ promoters, leading to the target gene’s transcriptional activation and resulting in tumorigenesis.11,13-15 We reported that WDR5 is highly expressed and associated with poor survival in B-ALL.16 Recently, it has been reported that ubiquitin-specific peptidase 44 enhanced the growth of T-ALL by interacting with WDR5 and repressing its ubiquitination.17 In addition, WDR5 inhibitors such as OICR-9429 have been reported to exhibit antitumor activities in many human cancers.15,18-21 However, little is known about the WDR5 oncogenic roles in T-ALL.
Casein kinase II (CK2) is a ubiquitous and highly conserved eukaryotic serine/threonine-protein kinase,22 which plays a vital role in cell cycle progression, cell differentiation, and transcription regulation.23-25IKZF1 gene-encoding protein IKAROS is an important tumor suppressor in ALL and the direct target of CK2.26 CK2 is an essential pro-oncogene that phosphorylates IKAROS, thus reducing its DNA-binding and leukemia-suppressor function in high-risk B-ALL.25,27,28 CK2 is overexpressed in T-ALL.27,29 CX-4945 is a small molecule inhibitor of CK2 that exhibits potent and highly selective anti-CK2 activity; we and others have demonstrated that CX-4945 has therapeutic efficacy in high-risk B-ALL.26,29-33
Here, we reported the association of WDR5 high expression with activation of cell cycle signaling in T-ALL and revealed the antileukemia effect of targeting WDR5 by inducing cell cycle arrest. Interestingly, we identified ATPase family AAA domain-containing protein 2 (ATAD2) as a core mediator of cell cycle signaling and the downstream target of WDR5 in T-ALL. Targeting the CK2/IKAROS axis downregulates WDR5 expression by restoring IKAROS function and exhibits a strong synergistic antileukemic efficacy with WDR5 inhibition, either by short hairpin RNA (shRNA) ablation or pharmacological means in human leukemia cells and patient-derived xenograft (PDX) mouse models of T-ALL. Our results demonstrate the role of WDR5/ATAD2 signaling in the oncogenesis of T-ALL, revealing the potential clinical relevance of targeting this new oncogenic signaling pathway directly using WDR5 inhibitors or upstream through CK2/IKAROS axis and providing strong preclinical evidence for combining these 2 agents for T-ALL.
Methods
Clinical samples
A total of 38 T-ALL patients’ bone marrow (BM) samples and 32 normal control BM mononuclear cells from healthy volunteers were obtained from Zhongda Hospital Southeast University. Measurable residual disease negative was defined as blasts <0.01% by multiparameter flow cytometry according to the 2024 European LeukemiaNet (ELN) recommendations.34 High-risk T-ALL was defined as patients with either age >35 years old, white blood cell >100 × 109/L, early T-cell precursor ALL (ETP-ALL), RAS/PTEN mutation, or NOTCH1/FBXW7 wild type according to the National Comprehensive Cancer Network (NCCN) guidelines version 2.2024. For drug treatment, primary cells were isolated with lymphocyte separation medium (MP Biomedicals LLC, Irvine, CA) in a sterile environment and cultured in RPMI-1640 (Gibco, Beijing, China) supplemented with 10% fetal bovine serum (HyClone, Shanghai, China) immediately to do further experiments as previously reported.35,36 This study was approved by the Ethics Committee of Zhongda Hospital Southeast University, and all patients provided written informed consent.
Plasmid construction, lentiviral transduction, and target gene knockdown
Global transcriptome analysis by RNA-seq
Briefly, CEM cells were treated with 5 μM CX-4945, 20 μM OICR-9429, or dimethyl sulfoxide (DMSO) control for 72 hours. Total RNA was extracted from the cells with a Qiagen RNA isolation kit (Qiagen, Shanghai, China). All samples were studied in triplicate. RNA sequencing (RNA-seq) was performed as previously reported.26
T-ALL PDX mouse models
Three patient samples for the PDX mouse model were obtained from Zhongda Hospital Southeast University, and 5 × 105 leukemia cells per mouse were transplanted intravenously (IV) into NOD/ShiLtJGpt-Prkdcem26Cd52Il2rgem26Cd22/Gpt (NCG) mice (GemPharmatech Co., Ltd, Nanjing, China).
Following engraftment, mice (n = 20 per group per patient sample × 3 patients) received the vehicle, CX-4945, OICR-9429, and combination (combo) for 25 days. Then, when the vehicle mice met the early removal criteria due to the excessive leukemia burden, 10 mice per group were euthanized to observe the drug efficacy on leukemia burden. The single-cell suspension of euthanized mice BM or spleen cells was collected, and the red blood cells were removed with red blood cell lysis buffer (Biosharp, Hefei, China). The resulting cells were used for living cell counts, quantitative chromatin immunoprecipitation–quantitative polymerase chain reaction assay, quantitative reverse transcription–polymerase chain reaction, and flow cytometry analysis of leukemia burden. The remaining 10 mice per group were followed until the mice died or met early removal criteria for survival analysis. The dead mice were counted daily, and the Kaplan-Meier method was used to generate the survival curves and analyze the survival difference.
All experimental operations were performed with the consent of the Animal Care Committee of Southeast University and complied with the Regulations for the Administration of Affairs Concerning Experimental Animals of China.
Details and additional experimental methods can be found in the supplemental Materials, available on the Blood website.
Ethical approval and informed consent
These written informed consents were provided by all the patients with the Declaration of Helsinki before enrollment in the study. The institutional review board of Zhongda Hospital Southeast University, Nanjing, China, approved the study.
Results
Association of WDR5 expression with cell cycle signaling in T-ALL
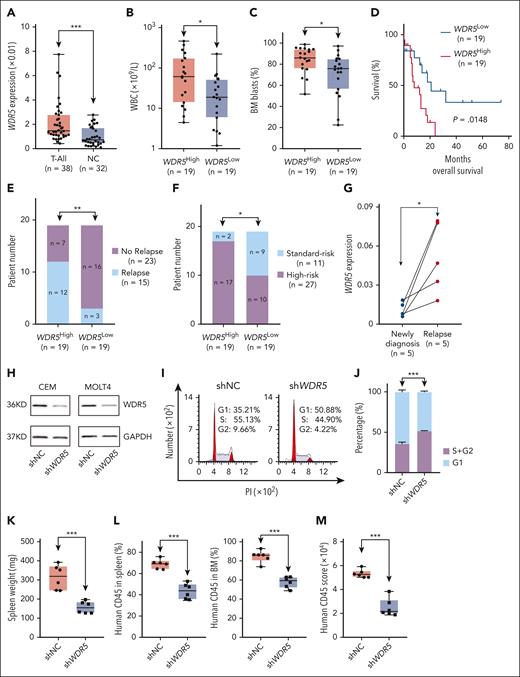
WDR5 is significantly overexpressed in our T-ALL cohorts vs normal controls (Figure 1A; supplemental Table 1). High WDR5 expression is associated with significantly higher white blood cell count (Figure 1B), higher BM blasts (Figure 1C), poorer survival (Figure 1D), higher relapse rate (Figure 1E), and more high-risk patients (Figure 1F) in our cohorts (supplemental Table 2). WDR5 messenger RNA (mRNA) level is significantly elevated in 5 pairs of the relapsed vs newly diagnosed T-ALL samples (Figure 1G). Also, WDR5 is significantly overexpressed in 3 public T-ALL cohorts and a B-ALL cohort (supplemental Figure 1A-D). In addition, WDR5 is highly expressed, and no statistical difference was observed in different immune phenotypes, ETP-ALL vs non-ETP-ALL, and the various molecular subtypes of the T-ALL cohorts (supplemental Figure 1E-G). Moreover, the patients were divided into WDR5-high and WDR5-low groups based on the WDR5 mRNA level in the 3 public T-ALL cohorts, and the enrichment of cell cycle genes and activation of cell cycle signaling was significantly associated with WDR5-high vs WDR5-low expression; the representative data can be found in supplemental Figure 1H-J. Taken together, these data revealed the oncogenic roles of WDR5 high expression which is possibly mediated by activating cell cycle signaling in T-ALL.
Oncogenic role of WDR5 through cell cycle regulation in T-ALL. (A) Comparison of WDR5 mRNA level in T-ALL cohort vs normal BM controls. (B-E) Association of WDR5 expression with WBC (B), BM blasts (C), survival (D), and relapse (E). The cohort was divided into WDR5high and WDR5low groups based on the median value of WDR5 mRNA level as the cutoff value. (F) The association of WDR5 expression with risk stratification in the T-ALL cohort. (G) Comparison of WDR5 mRNA level in 5 paired samples of newly diagnosed vs the relapsed ones. (H) Effect of WDR5 KD by shRNA on its protein level in CEM (left) and MOLT4 (right) cells. (I-J) Effect of WDR5 KD on cell cycle progress in CEM cells (I, representative images; J, bar graph). (K-L) Effect of WDR5 silence by shRNA on spleen weights (K) and percentage of human CD45+ cells in the spleen and BM of the xenograft mouse model (L). The mice were IV injected with the CEM-shNC or CEM-shWDR5 cells, respectively, for 28 days, and the single cells were prepared and analyzed. (M) Quantitation data of immunohistological images for human CD45+ cells in the spleen from CEM-shNC and CEM-shWDR5 mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PI, propidium iodine; WBC, white blood cell.
Oncogenic role of WDR5 through cell cycle regulation in T-ALL. (A) Comparison of WDR5 mRNA level in T-ALL cohort vs normal BM controls. (B-E) Association of WDR5 expression with WBC (B), BM blasts (C), survival (D), and relapse (E). The cohort was divided into WDR5high and WDR5low groups based on the median value of WDR5 mRNA level as the cutoff value. (F) The association of WDR5 expression with risk stratification in the T-ALL cohort. (G) Comparison of WDR5 mRNA level in 5 paired samples of newly diagnosed vs the relapsed ones. (H) Effect of WDR5 KD by shRNA on its protein level in CEM (left) and MOLT4 (right) cells. (I-J) Effect of WDR5 KD on cell cycle progress in CEM cells (I, representative images; J, bar graph). (K-L) Effect of WDR5 silence by shRNA on spleen weights (K) and percentage of human CD45+ cells in the spleen and BM of the xenograft mouse model (L). The mice were IV injected with the CEM-shNC or CEM-shWDR5 cells, respectively, for 28 days, and the single cells were prepared and analyzed. (M) Quantitation data of immunohistological images for human CD45+ cells in the spleen from CEM-shNC and CEM-shWDR5 mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PI, propidium iodine; WBC, white blood cell.
Targeting WDR5 induces cell cycle arrest and delays leukemia engraftment
WDR5 is genetically silenced with WDR5 shRNA in CEM and MOLT4 T-ALL cells (Figure 1H). WDR5 knockdown (KD) significantly inhibited cell proliferation (supplemental Figure 2A), induced the G1 phase arrest in CEM (Figure 1I-J) and MOLT4 (supplemental Figure 2B-C) cells, and downregulated the G1/S phase transition regulation genes of CCNE2 and CDK2 (supplemental Figure 2D-G) compared with that of the scramble shRNA (shNC) controls.
WDR5 KD cells (CEM-shWDR5) and control cells (CEM-shNC) were respectively injected IV into immunodeficient NCG mice for 28 days, which were then euthanized for analysis afterward. Results revealed that WDR5 KD significantly reduced the spleen sizes (supplemental Figure 3A), spleen weights (Figure 1K), and the percentage of human CD45+ cells in the spleen and BM (Figure 1L; supplemental Figure 3B) compared with that of CEM-shNC control. Immunohistochemistry staining displayed that WDR5 KD significantly decreased infiltration of human CD45+ cells in the spleen compared with the control (Figure 1M; supplemental Figure 3C). The WDR5 KD in the engrafts was confirmed at the mRNA level (supplemental Figure 3D).
Similarly, we observed that OICR-9429, a small molecule inhibitor of WDR515,18,37 inhibited T-ALL cell proliferation in a dose-dependent manner (supplemental Figure 4A) and significantly caused the cell cycle arrest in the G1 phase in CEM (supplemental Figure 4B-C), MOLT4 (supplemental Figure 4D-E), and Jurkat (supplemental Figure 4F-G) cells compared with that of the vehicle control.
Whole transcriptome analysis in CEM cells on OICR-9429 treatment vs the DMSO vehicle control identified a total of 3161 significantly differentially expressed genes (DEGs) (584 upregulated, 2577 downregulated) (supplemental Figure 4H). Kyoto Encyclopedia of Genes and Genomes (KEGG) (supplemental Figure 4I) and Gene Ontology (GO) (supplemental Figure 4J) analyses revealed that the genes involved in the cell cycle progression were significantly enriched in the DEGs, and OICR-9429 treatment significantly altered the activity of the cell cycle pathway.
Taken together, these results reveal that genetic and therapeutic targeting of WDR5 possesses antileukemic efficacy in T-ALL by inducing G1 phase arrest.
CK2 inhibition restores IKAROS-mediated transcriptional repression of WDR5
CK2 inhibitor CX-494529,30 was found to have a dose-dependent inhibition of cell proliferation in 3 (CEM, MOLT4, Jurkat) T-ALL cell lines (supplemental Figure 5A). RNA-seq identified 9484 DEGs (6366 upregulated, 3118 downregulated) in CEM cells on CX-4945 treatment vs DMSO vehicle control (supplemental Figure 5B). The cell cycle pathway was also significantly enriched in the DEGs by KEGG (supplemental Figure 5C) analysis. CX-4945 treatment vs the DMSO control significantly induced G1 phase arrest in the 3 T-ALL cell lines (supplemental Figure 5D-I).
CX-4945 treatment downregulated WDR5 transcription (supplemental Figure 5B), which was further validated by quantitative polymerase chain reaction (supplemental Figure 6A) and western blot (supplemental Figure 6B) in the 3 T-ALL cell lines.
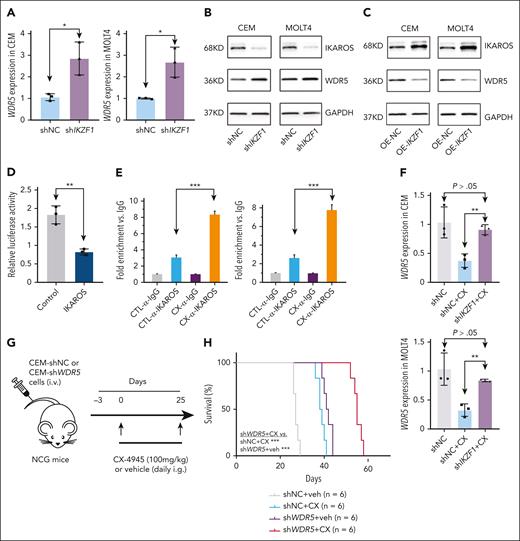
We then evaluated whether CX-4945 suppresses WDR5 by restoring the IKAROS function as we observed in B-ALL.26,29,30 KD of IKAROS-encoding gene IKZF1 significantly increased WDR5 expression at both mRNA (Figure 2A) and protein (Figure 2B) levels. IKAROS overexpression reduced WDR5 protein expression in CEM and MOLT4 cells (Figure 2C). To determine whether IKAROS is regulating the activity of the WDR5 promoter, we performed luciferase reporter assay in the IKAROS-expressed HEK293, which does not normally express endogenous IKAROS. IKAROS expression significantly represses the activity of the WDR5 promoter (Figure 2D). Next, we reveal that although IKAROS binds weakly to the promoter of WDR5 in untreated T-ALL, treatment with CX-4945 significantly increased IKAROS binding at the WDR5 promoter in the T-ALL cells (Figure 2E). IKZF1 KD also significantly blocks CX-4945–mediated repression of WDR5 transcription in the cells (Figure 2F).
IKAROS represses WDR5 transcription in T-ALL. (A-B) Effect of IKZF1 KD on WDR5 expression in mRNA level by quantitative polymerase chain reaction (qPCR; A) and protein level in CEM and MOLT4 cells (B). (C) Effect of IKZF1 overexpression on WDR5 protein level in CEM (left) and MOLT4 (right) cells. (D) Effect of IKAROS on the activity of the WDR5 promoter assessed by luciferase reporter assay in 293T cells. (E) Effect of CX-4945 on IKAROS binding at the WDR5 promoter as measured by quantitative chromatin immunoprecipitation (qChIP) in CEM (left) and MOLT4 (right) cells. (F) The effect of IKZF1 KD on CX-4945–induced WDR5 expression change in mRNA level by qPCR in CEM (upper) and MOLT4 (lower) cells. (G) Schematic representation of the xenograft mice model. CEM-shNC or CEM-shWDR5 cells were IV injected into NCG mice and the following 4 groups of mice were established: group CEM-shNC plus vehicle (receive vehicle daily through gavage for 25 days); group CEM-shNC plus CX-4945 (receive CX-4945 daily through gavage at 100 mg/kg for 25 days); group CEM-shWDR5 plus vehicle (receive vehicle daily through gavage for 25 days); and group CEM-shWDR5 plus CX-4945 (receive CX-4945 daily through gavage at 100 mg/kg for 25 days). (H) Kaplan-Meier survival curves of 4 groups of mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
IKAROS represses WDR5 transcription in T-ALL. (A-B) Effect of IKZF1 KD on WDR5 expression in mRNA level by quantitative polymerase chain reaction (qPCR; A) and protein level in CEM and MOLT4 cells (B). (C) Effect of IKZF1 overexpression on WDR5 protein level in CEM (left) and MOLT4 (right) cells. (D) Effect of IKAROS on the activity of the WDR5 promoter assessed by luciferase reporter assay in 293T cells. (E) Effect of CX-4945 on IKAROS binding at the WDR5 promoter as measured by quantitative chromatin immunoprecipitation (qChIP) in CEM (left) and MOLT4 (right) cells. (F) The effect of IKZF1 KD on CX-4945–induced WDR5 expression change in mRNA level by qPCR in CEM (upper) and MOLT4 (lower) cells. (G) Schematic representation of the xenograft mice model. CEM-shNC or CEM-shWDR5 cells were IV injected into NCG mice and the following 4 groups of mice were established: group CEM-shNC plus vehicle (receive vehicle daily through gavage for 25 days); group CEM-shNC plus CX-4945 (receive CX-4945 daily through gavage at 100 mg/kg for 25 days); group CEM-shWDR5 plus vehicle (receive vehicle daily through gavage for 25 days); and group CEM-shWDR5 plus CX-4945 (receive CX-4945 daily through gavage at 100 mg/kg for 25 days). (H) Kaplan-Meier survival curves of 4 groups of mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Taken together, these data indicate that expression of WDR5 is regulated by the CK2/IKAROS axis in T-ALL, and inhibition of CK2 through CX-4945 suppresses WDR5 expression in an IKAROS-dependent manner.
Targeting the CK2/IKAROS axis enhances the WDR5 KD-induced suppression of leukemia engraftment
We observed that WDR5 KD remarkably increased the CX-4945–mediated cell proliferation arrest in CEM and MOLT4 cells (supplemental Figure 7) compared with shNC control. Then, CEM cells with WDR5 stable KD (CEM-shWDR5) and scramble shRNA control (CEM-shNC) were IV injected into NCG mice, respectively. Mice from each engraftment type were divided into 2 cohorts and treated with either vehicle or CX-4945 to obtain the following groups: CEM-shNC plus vehicle, CEM-shNC plus CX-4945, CEM-shWDR5 plus vehicle, and CEM-shWDR5 plus CX-4945 (Figure 2G). Results revealed that mice in the CEM-shWDR5 plus CX-4945 cohort presented significantly prolonged survival (Figure 2H), reduced spleen sizes (supplemental Figure 8A) and weights (supplemental Figure 8B), and decreased percentage of human CD45+ T-ALL cells in the spleen (supplemental Figure 8C,E) and BM (supplemental Figure 8D,F) compared with CEM-shNC plus CX-4945 or CEM-shWDR5 plus vehicle. Histological analysis also revealed fewer human CD45+ T-ALL cells in the spleen of the CEM-shWDR5 plus CX-4945 mice vs the controls (supplemental Figure 8G-H). Moreover, expression of WDR5 in mRNA level and protein levels was significantly downregulated in the spleen (supplemental Figure 9A-B) of these mice compared with either single treatment control. Together, these results demonstrate that WDR5 KD-mediated suppression of leukemia engraftment in T-ALL is enhanced by CX-4945 through targeting of the CK2/IKAROS axis.
Synergistic effect of CX-4945 with OICR-9429 on cell proliferation inhibition and cell cycle arrest in T-ALL
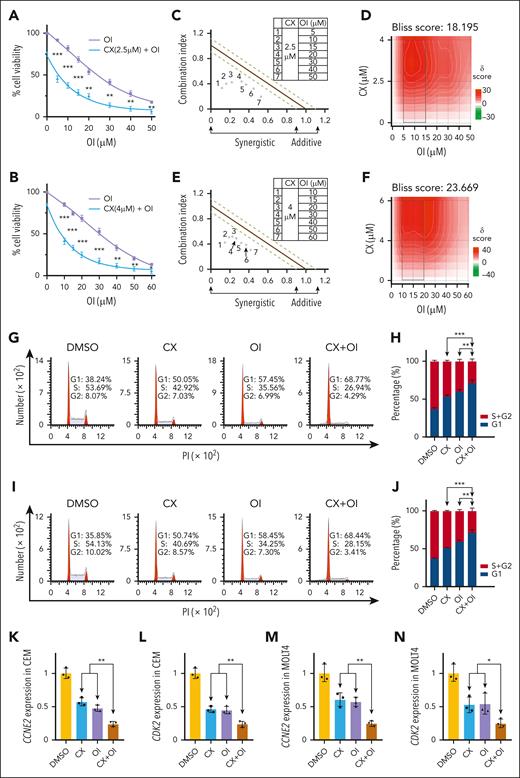
Next, we evaluated the synergistic effect of CX-4945 with the WDR5 inhibitor OICR-9429 on cell proliferation of T-ALL cells. Results revealed that OICR-9429 exhibited a significant cell proliferation arrest in a dose-dependent manner and that CX-4945 significantly increased the effect compared with the single drug control in CEM (Figure 3A), MOLT4 (Figure 3B), and Jurkat (supplemental Figure 10A) T-ALL cells. CalcuSyn synergy analysis and Bliss model revealed that the combination has a synergistic effect in CEM (Figure 3C-D), MOLT4 (Figure 3E-F), and Jurkat (supplemental Figure 10B-C) T-ALL cells. The combination produced a more G1 phase arrest vs either single drug in the 3 (CEM, MOLT4, Jurkat) T-ALL cells (Figure 3G,I; supplemental Figure 10D), and quantification data revealed that the combination significantly induced G1 phase arrest compared with either single drug in the cells (Figure 3H,J; supplemental Figure 10E). Consistently, the combination significantly induced the downregulation of the key G1 phase regulatory genes, CCNE2 and CDK2, in CEM (Figure 3K-L) and MOLT4 (Figure 3M-N) T-ALL cells compared with either single drug.
Synergistic effect of CX-4945 and WDR5 inhibitor on arresting cell proliferation and cell cycle in T-ALL. (A-B) Effect of the combination of CX-4945 and WDR5 inhibitor vs single drug on cell proliferation in CEM (A) and MOLT4 cells (B). (C-F) Synergistic analysis of the 2 inhibitors on proliferation arrest in CEM (C-D) and MOLT4 cells (E-F) with combination index (C,E) and Bliss models (D,F). Cells were treated with the indicated doses of drugs for 72 hours. (G-J) Effect of the combination of CX-4945 and WDR5 inhibitor vs single drug controls on cell cycle arrest in CEM (G, representative images; H, bar graph) and MOLT4 (I, representative images; J, bar graph) cells. Cells were treated with indicated doses of the drugs for 72 hours. (K-N) Effect of the combination of CX-4945 and WDR5 inhibitor vs the single drug control on mRNA levels of CCNE2 (K,M) and CDK2 (L,N) in CEM and MOLT4 cells. The cells were treated with indicated doses of the drugs for 72 hours, and the mRNA levels were quantitated with qPCR. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PI, propidium iodine; OI, OICR-9429.
Synergistic effect of CX-4945 and WDR5 inhibitor on arresting cell proliferation and cell cycle in T-ALL. (A-B) Effect of the combination of CX-4945 and WDR5 inhibitor vs single drug on cell proliferation in CEM (A) and MOLT4 cells (B). (C-F) Synergistic analysis of the 2 inhibitors on proliferation arrest in CEM (C-D) and MOLT4 cells (E-F) with combination index (C,E) and Bliss models (D,F). Cells were treated with the indicated doses of drugs for 72 hours. (G-J) Effect of the combination of CX-4945 and WDR5 inhibitor vs single drug controls on cell cycle arrest in CEM (G, representative images; H, bar graph) and MOLT4 (I, representative images; J, bar graph) cells. Cells were treated with indicated doses of the drugs for 72 hours. (K-N) Effect of the combination of CX-4945 and WDR5 inhibitor vs the single drug control on mRNA levels of CCNE2 (K,M) and CDK2 (L,N) in CEM and MOLT4 cells. The cells were treated with indicated doses of the drugs for 72 hours, and the mRNA levels were quantitated with qPCR. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PI, propidium iodine; OI, OICR-9429.
Furthermore, combining CX-4945 with OICR-9429 significantly increased the apoptosis of CEM (supplemental Figure 11A-B) and MOLT4 (supplemental Figure 11C-D) T-ALL cells compared with either single drug.
To evaluate whether the single drugs and combination-induced cell proliferation arrest is reversible, cells were treated with vehicle control, OICR-9429, CX-4945, and combo for 3 and 7 days, and then the drugs were washed out and cells were recultured with fresh media for 3 days and 7 days. Cell proliferation, apoptosis, and cell cycle analysis were measured before washout and 3 and 7 days after washout. Results revealed that both short-term (3 days) and long-term (7 days) continuous treatment with single drugs (CX-4945 or OICR-9429) and/or combination significantly inhibited cell proliferation compared with the nontreatment control, and this trend was maintained even after drug washout (supplemental Figure 12A-B). Consistently, the % apoptotic cells (supplemental Figure 12C-E) and % G1 arrest cells (supplemental Figure 12F-H) had significant increases on day 3 and day 7 after the drug washout.
These data indicated the in vitro synergistic effect of CX-4945 with WDR5 inhibitor OICR-9429 on the proliferation of T-ALL cell lines by inducing apoptosis in addition to cell cycle arrest.
Moreover, we confirmed that combining CX-4945 with WDR5 inhibitor OICR-9429 induced the synergistic effect on the cytotoxicity of primary cells from 4 patients with T-ALL (supplemental Figure 13); and this is comparable to results from genetic inhibition. The clinical characteristics for 1 newly diagnosed and 2 relapsed patients can be found in supplemental Table 3, and those for another newly diagnosed patient in supplemental Table 1 (Patient 2). These results indicated the in vitro synergistic effect of CX-4945 with WDR5 inhibitor OICR-9429 on the proliferation of T-ALL primary cells from patients with T-ALL.
Combination of CX-4945 with OICR-9429 suppresses leukemia development of T-ALL in vivo
One human leukemia xenograft (CEM xenograft) and 3 PDX models were used to evaluate the synergy of the 2 drugs in vivo.
For CEM xenograft, human CEM T-ALL cells (2 × 105 per mouse) were IV injected into 4 groups of NCG mice, respectively. The mouse model received the following treatments: group 1 (vehicle control), group 2 (CX-4945 alone), group 3 (WDR5 inhibitor OICR-9429 alone), and group 4 (CX-4945 + OICR-9429 combo) (Figure 4A). Results revealed that the combination of CX-4945 with OICR-9429 significantly prolonged survival of the mouse models (Figure 4B), reduced spleen sizes (Figure 4C) and weights (Figure 4D), and decreased percentage of human CD45+CD7+ T-ALL cells in the spleen and BM (Figure 4E; supplemental Figure 14) compared with that of single treatment controls. Histological analysis revealed that fewer human CD45+ T-ALL cells in the spleen of the mice were detected on the combo treatment vs the single treatment controls (Figure 4F-G). Tumors from mice in the combination group had significant downregulation of WDR5 and ATAD2 in both protein and mRNA levels (Figure 4H-I) and cell cycle regulation genes CCNE2 and CDK2 (Figure 4I) at the mRNA level compared with single treatment controls.
The combination of CX-4945 and WDR5 disruption has a synergistic antileukemic effect in the T-ALL mice model. (A) Schematic representation of the human T-ALL CEM cell-xenograft mouse model (CEM xenograft). CEM (2 × 105 per mouse) were IV injected into NCG mice, and the following 4 groups of mice were established: group 1 (vehicle control); group 2 (CX-4945 daily through gavage at 100 mg/kg for 25 days); group 3 (WDR5 inhibitor OICR-9429 every 2 days through intraperitoneal injection at 60 mg/kg for 25 days); and group 4 (CX-4945 and OICR-9429 combination treatment using the same doses as provided previously). (B) Comparison of Kaplan-Meier survival curves in the combination of CX-4945 and WDR5 inhibitor OICR-9429 compared with either single drug control of CEM-xenograft mouse models. The mice were treated with the indicated drugs for 25 days. (C-D) Spleen images (C) and weights (D) of 4 groups of mice posttreatment. (E) The percentage of human CD45+ and CD7+ cells in the spleen and BM from 4 groups of mice post-treatment. (F-G) Representative immunohistological images (F) and quantitation data (G) of human CD45 in the spleen from 4 groups of mice post-treatment. (H) The protein level of WDR5 and ATAD2 and the internal control of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the spleen tissues of the mice after the treatment. (I) mRNA level of WDR5, ATAD2, CCNE2, and CDK2 in the spleen tissues of the mice after the treatment. Scale bar, 50 μm. ∗∗∗P < .001. i.g., intragastic gavage; i.p., intraperitoneal injection; OI, OICR-9429.
The combination of CX-4945 and WDR5 disruption has a synergistic antileukemic effect in the T-ALL mice model. (A) Schematic representation of the human T-ALL CEM cell-xenograft mouse model (CEM xenograft). CEM (2 × 105 per mouse) were IV injected into NCG mice, and the following 4 groups of mice were established: group 1 (vehicle control); group 2 (CX-4945 daily through gavage at 100 mg/kg for 25 days); group 3 (WDR5 inhibitor OICR-9429 every 2 days through intraperitoneal injection at 60 mg/kg for 25 days); and group 4 (CX-4945 and OICR-9429 combination treatment using the same doses as provided previously). (B) Comparison of Kaplan-Meier survival curves in the combination of CX-4945 and WDR5 inhibitor OICR-9429 compared with either single drug control of CEM-xenograft mouse models. The mice were treated with the indicated drugs for 25 days. (C-D) Spleen images (C) and weights (D) of 4 groups of mice posttreatment. (E) The percentage of human CD45+ and CD7+ cells in the spleen and BM from 4 groups of mice post-treatment. (F-G) Representative immunohistological images (F) and quantitation data (G) of human CD45 in the spleen from 4 groups of mice post-treatment. (H) The protein level of WDR5 and ATAD2 and the internal control of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the spleen tissues of the mice after the treatment. (I) mRNA level of WDR5, ATAD2, CCNE2, and CDK2 in the spleen tissues of the mice after the treatment. Scale bar, 50 μm. ∗∗∗P < .001. i.g., intragastic gavage; i.p., intraperitoneal injection; OI, OICR-9429.
For the PDX mouse model, all 3 PDX mouse models were from high-risk patients with T-ALL, including T-ALL-P1 (new diagnosis), T-ALL-P2 (relapse/refractory), and T-ALL-P3 (relapse/refractory, ETP-ALL). The clinical features of the patients for 3 PDX mouse models (PDX-P1, -P2, -P3) can be found in supplemental Table 3. The primary cells (5 × 105 per mouse) from the 3 patients were IV injected into 4 groups of NCG mice, respectively, as described in the CEM-xenograft mouse model. The leukemia engraftment in the PDX model was monitored before the treatment, the change of % leukemia blast cells in the peripheral blood of the PDX mice was evaluated for all 3 PDX models (supplemental Figures 15A-B, 17A-B, and 18A-B), and the leukemia engraftment (% leukemia blast cells) was confirmed to be ≥5% in the peripheral blood of the mice for all 3 PDX models when the treatment started (supplemental Figures 15D, 17D, and 18D). The treatment schemes for each PDX can be found in supplemental Figures 15C, 17C, and 18C, respectively. Results revealed that the combination of CX-4945 with OICR-9429 significantly prolonged survival of the PDX models (Figure 5A), reduced spleen sizes (supplemental Figures 16A, 17E, and 19A) and weights (supplemental Figures 16B, 17F, and 19B), and decreased percentage of human CD45+CD7+ T-ALL cells in the spleen (Figures 4, 5B; supplemental Figures 16C, 17G, and 19C) and BM (supplemental Figures 16C-D, 17G-H, and 19C-D) compared with that of single treatment controls. Histological analysis revealed that fewer human CD45+ T-ALL cells in the spleen of the mice were detected on the combo treatment vs the single treatment controls (supplemental Figures 16E-F, 17I-J, and 19E-F). Tumors from mice in the combination group had significant downregulation of WDR5 and ATAD2 in both mRNA and protein levels (Figure 5C-E; supplemental Figures 16G, 17K, and 19G) and cell cycle regulation genes CCNE2 and CDK2 (Figure 5C-E) at the mRNA level compared with single treatment controls.
Therapeutic efficacy of the combination of CX-4945 and WDR5 inhibitor in patient-derived T-ALL leukemia-xenograft (PDX) mouse model. (A) Comparison of Kaplan-Meier survival curves in the combination of CX-4945 and WDR5 inhibitor OICR-9429 compared with either single drug controls in 3 different PDX mouse models: PDX-P1 (Patient, [Pt.] T-ALL-P1 in supplemental Table 3), PDX-P2 (Pt. T-ALL-P2), and PDX-P3 (Pt. T-ALL-P3). (B) Comparison of percentage of human CD45+CD7+ cells in the spleen of the mice in combo group vs either single drug controls. (C-E) Comparison of mRNA level of WDR5, ATAD2, CCNE2, and CDK2 in combo group vs either single drug controls in the spleen of the mouse models. The mice in panels A-E were treated with the indicated drugs for 25 days, and then, when the vehicle mice met the early removal criteria due to the excessive leukemia burden, the mice were euthanized and the single cells were isolated from the spleen for flow cytometry analysis; the mRNA of the cells was prepared for qPCR analysis. ∗∗∗P < .001. OI, OICR-9429.
Therapeutic efficacy of the combination of CX-4945 and WDR5 inhibitor in patient-derived T-ALL leukemia-xenograft (PDX) mouse model. (A) Comparison of Kaplan-Meier survival curves in the combination of CX-4945 and WDR5 inhibitor OICR-9429 compared with either single drug controls in 3 different PDX mouse models: PDX-P1 (Patient, [Pt.] T-ALL-P1 in supplemental Table 3), PDX-P2 (Pt. T-ALL-P2), and PDX-P3 (Pt. T-ALL-P3). (B) Comparison of percentage of human CD45+CD7+ cells in the spleen of the mice in combo group vs either single drug controls. (C-E) Comparison of mRNA level of WDR5, ATAD2, CCNE2, and CDK2 in combo group vs either single drug controls in the spleen of the mouse models. The mice in panels A-E were treated with the indicated drugs for 25 days, and then, when the vehicle mice met the early removal criteria due to the excessive leukemia burden, the mice were euthanized and the single cells were isolated from the spleen for flow cytometry analysis; the mRNA of the cells was prepared for qPCR analysis. ∗∗∗P < .001. OI, OICR-9429.
Furthermore, no significant toxicities of the combination in the liver, kidney, heart, and peripheral blood were observed in the mouse model (supplemental Table 4). These results reveal the therapeutic efficacy of the combination of CX-4945 with OICR-9429 in curbing leukemia development in both human T-ALL leukemia xenograft and PDX mouse models, particularly for high-risk patients including ETP-ALL.
Identification of the direct downstream target of WDR5 in T-ALL
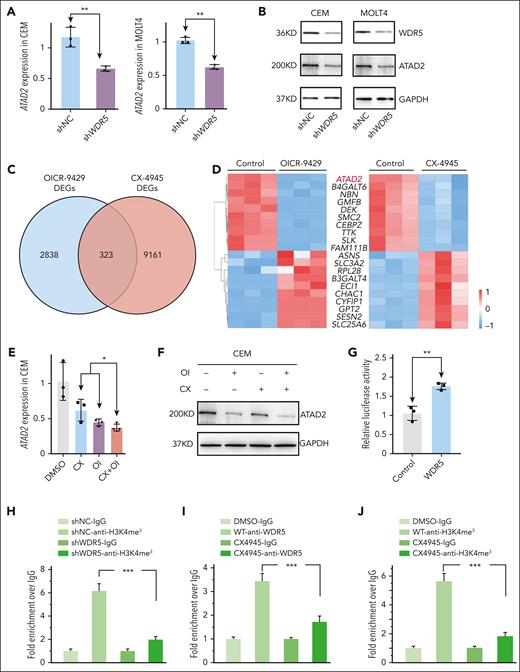
WDR5 KD significantly repressed ATAD2 expression both in mRNA (Figure 6A) and protein (Figure 6B) levels in T-ALL cells. Next, we overlapped the DEGs of RNA-seq data in T-ALL cells treated with CX-4945 with that of OICR-9429 (Figure 6C) and obtained 323 frequently altered DEGs with ATAD2 as one of the top downregulated DEGs (Figure 6D). Treatment with CX-4945, OICR-9429, or in combination significantly downregulated expression of ATAD2 at the mRNA level (Figure 6E; supplemental Figure 20A-B) and protein level (Figure 6F; supplemental Figure 20C-D) in 3 T-ALL cell lines in vitro and in 4 mice models in vivo (Figures 4H-I and 5C-E; supplemental Figures 16G, 17K, and 19G). Notably, luciferase reporter assay revealed that overexpressed WDR5 significantly increased the activity of the ATAD2 promoter (Figure 6G) which was consistent with the enrichment of H3K4me3 and WDR5 (Figure 6H-J) at this region. WDR5 KD significantly reduced the enrichment of H3K4me3 (Figure 6H), and CX-4945 treatment reduced the enrichment of both WDR5 and H3K4me3 at the promoter region of ATAD2 (Figure 6I-J). These data indicated that ATAD2 is the direct downstream target of WDR5 in T-ALL.
Identifying ATAD2 as the downstream target on CX-4945–mediated transcriptional repression of WDR5 in T-ALL. (A-B) Effect of WDR5 KD on ATAD2 expression in mRNA level (A) and protein level (B) of CEM (left) and MOLT4 (right) cells. (C) Overlapping DEGs from the RNA-seq data in CEM cells treated with CX-4945 or WDR5 inhibitor. (D) Heat map of the top overlapped DEGs in the 2 RNA-seq analyses. (E-F) Effect of combination of CX-4945 (CX) and WDR5 inhibitor OICR-9429 (OI) on the expression of ATAD2 compared with a single drug in mRNA level (E) and protein level (F) in CEM cells. (G) Effect of WDR5 on the activity of the ATAD2 promoter assessed by luciferase reporter assay in 293T cells. (H) Effect of WDR5 KD on the H3K4me3 enrichment in the promoter region of ATAD2 in CEM cells by qChIP assay. (I-J) Effect of CX-4945 on WDR5 binding and H3K4me3 enrichment at the ATAD2 promoter in CEM cells by qChIP assay. Panels H-J are representative of 3 qChIP assays. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. IgG, immunoglobulin G; OI, OICR-9429.
Identifying ATAD2 as the downstream target on CX-4945–mediated transcriptional repression of WDR5 in T-ALL. (A-B) Effect of WDR5 KD on ATAD2 expression in mRNA level (A) and protein level (B) of CEM (left) and MOLT4 (right) cells. (C) Overlapping DEGs from the RNA-seq data in CEM cells treated with CX-4945 or WDR5 inhibitor. (D) Heat map of the top overlapped DEGs in the 2 RNA-seq analyses. (E-F) Effect of combination of CX-4945 (CX) and WDR5 inhibitor OICR-9429 (OI) on the expression of ATAD2 compared with a single drug in mRNA level (E) and protein level (F) in CEM cells. (G) Effect of WDR5 on the activity of the ATAD2 promoter assessed by luciferase reporter assay in 293T cells. (H) Effect of WDR5 KD on the H3K4me3 enrichment in the promoter region of ATAD2 in CEM cells by qChIP assay. (I-J) Effect of CX-4945 on WDR5 binding and H3K4me3 enrichment at the ATAD2 promoter in CEM cells by qChIP assay. Panels H-J are representative of 3 qChIP assays. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. IgG, immunoglobulin G; OI, OICR-9429.
Oncogenic role of ATAD2 by promoting cell cycle progress in T-ALL
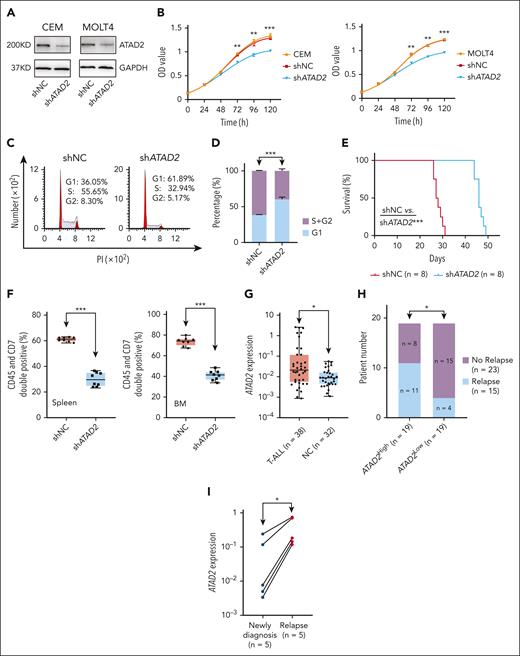
We further evaluated whether ATAD2 drives the oncogenic effects by promoting cell cycle progress in T-ALL. ATAD2 is efficiently knocked down in T-ALL cell lines (Figure 7A), and its KD significantly inhibited T-ALL cell proliferation (Figure 7B), induced G1 phase arrest (Figure 7C-D; supplemental Figure 21A-B), and downregulated the expression of CCNE2 and CDK2 (supplemental Figure 21C-F) in the T-ALL cells compared with the scramble shRNA controls. These data illustrate that ATAD2 plays important oncogenic roles by activating cell cycle signaling in T-ALL.
Oncogenic roles of ATAD2 by regulating cell cycle progression in T-ALL. (A) The efficiency of ATAD2 KD by shRNA (shATAD2) vs scramble shRNA control (shNC) in CEM (left) and MOLT4 (right) cells by western blot. (B) Effect of ATAD2 KD vs shNC on cell proliferation in CEM (left) and MOLT4 (right) cells. (C-D) Effect of ATAD2 KD vs shNC on cell cycle progression in CEM cells (C, representative images; D, bar graph). (E-F) Effect of ATAD2 silence by shRNA vs shNC on Kaplan-Meier survival curves (E), and percentage of human CD45+CD7+ cells (F) in the spleen and BM of the xenograft mouse model. The mice were IV injected with the CEM-shNC or CEM-shATAD2 cells, respectively, for 28 days, and the single cells were prepared and analyzed. (G) Comparison of ATAD2 mRNA level in our T-ALL cohorts vs normal BM controls. The mRNA levels were determined by qPCR assay. (H) Association of ATAD2 expression with relapse in our cohort. (I) Comparison of ATAD2 mRNA levels in 5 pairs of T-ALL newly diagnosed samples vs relapsed ones. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Oncogenic roles of ATAD2 by regulating cell cycle progression in T-ALL. (A) The efficiency of ATAD2 KD by shRNA (shATAD2) vs scramble shRNA control (shNC) in CEM (left) and MOLT4 (right) cells by western blot. (B) Effect of ATAD2 KD vs shNC on cell proliferation in CEM (left) and MOLT4 (right) cells. (C-D) Effect of ATAD2 KD vs shNC on cell cycle progression in CEM cells (C, representative images; D, bar graph). (E-F) Effect of ATAD2 silence by shRNA vs shNC on Kaplan-Meier survival curves (E), and percentage of human CD45+CD7+ cells (F) in the spleen and BM of the xenograft mouse model. The mice were IV injected with the CEM-shNC or CEM-shATAD2 cells, respectively, for 28 days, and the single cells were prepared and analyzed. (G) Comparison of ATAD2 mRNA level in our T-ALL cohorts vs normal BM controls. The mRNA levels were determined by qPCR assay. (H) Association of ATAD2 expression with relapse in our cohort. (I) Comparison of ATAD2 mRNA levels in 5 pairs of T-ALL newly diagnosed samples vs relapsed ones. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Next, engraftment of ATAD2 KD cells (CEM-shATAD2) vs control cells (CEM-shNC) was observed by IV injecting them into immunodeficient NCG mice for 28 days. Results revealed that ATAD2 KD significantly extended survival (Figure 7E) and reduced the spleen sizes (supplemental Figure 22A), spleen weights (supplemental Figure 22B), and the percentage of human CD45+ CD7+ cells in the spleen and BM (Figure 7F; supplemental Figure 22C-D) compared with that of CEM-shNC control. Immunohistochemistry staining displayed that ATAD2 KD significantly decreased infiltration of human CD45+ cells in the spleen compared with the control (supplemental Figure 22E-F). The ATAD2 KD in the engrafts was confirmed at the mRNA level (supplemental Figure 22G).
Moreover, higher ATAD2 expression was detected in our T-ALL cohort vs normal BM controls (Figure 7G) and the public cohorts (supplemental Figure 23A). In addition, high ATAD2 expression was associated with a significantly higher relapse rate in our cohort (Figure 7H). ATAD2 mRNA levels were significantly elevated in 5 pairs of T-ALL relapsed vs newly diagnosed samples (Figure 7I). Bioinformatic pathway analysis exhibited that upregulated ATAD2 expression is strongly associated with cell cycle activation in the T-ALL patient cohort (supplemental Figure 23B-C). These data further support that ATAD2 plays an oncogenic role in T-ALL, and WDR5/ATAD2 is a novel oncogenic signaling pathway regulating cell cycle progress in T-ALL.
The oncogenic role of ATAD2 by promoting cell cycle progress in T-ALL and the synergistic efficacy of targeting WDR5 with CK2/IKAROS axis in the disease can be found in supplemental Figure 24.
Discussion
In this study, we found that high expression of WDR5 and ATAD2 is associated with activation of cell cycle signaling in T-ALL and that silencing either WDR5 or ATAD2 induces cell cycle arrest and inhibition of tumor engraftments. CK2 inhibitor induces the cell cycle arrest by the transcriptional repression of WDR5 through IKAROS in T-ALL. We identified novel oncogenic signaling of WDR5/ATAD2 regulated by the CK2/IKAROS axis in T-ALL. Inhibition of this signaling pathway by targeting the CK2/IKAROS axis reveals therapeutic efficacy in T-ALL (supplemental Figure 24). Our results have uncovered a new oncogenic mechanism and provided evidence for the combination of WDR5 inhibitor with CK2 inhibitor as a potential strategy for clinical T-ALL therapy.
Elevated WDR5 expression has been detected in many solid tumors and is linked with adverse prognosis.18,37,38 We also reported that WDR5 high expression in B-ALL and WDR5 KD induces the target gene suppression by decreasing the enrichment of H3K4me3 in its promoter region.16 Here, our data revealed that WDR5 is overexpressed in patients with T-ALL and aberrant-high WDR5 expression activated the cell cycle pathway, whereas genetic and pharmaceutic targeting of WDR5 exhibits antileukemic efficacy by suppression of cell proliferation and induction of cell cycle arrest. Our results indicated that WDR5 is involved in the oncogenic mechanisms of the leukemogenesis of T-ALL. CK2-mediated dysfunction of IKZF1-coding protein IKAROS is critical for leukemogenesis in high-risk B-ALL.29,30 CK2 inhibitor CX-4945 significantly restored IKAROS function by decreasing IKAROS phosphorylation levels in T-ALL cells,25,27 and CX-4945 mediates its antileukemic activity by restoring IKAROS function and suppressing downstream oncogene transcription.29,30 In this study, we found that the expression of WDR5 in T-ALL is directly regulated by IKAROS. CX-4945 treatment inhibits WDR5 expression in an IKAROS-dependent manner in T-ALL cells. These results revealed that the expression of WDR5 in T-ALL is regulated by the CK2/IKAROS axis and targeting WDR5 by CX-4945 has antileukemic activity.
The cell cycle is a classic pathway that has been widely demonstrated to engage in physiology and tumor biology processes. Patients with cancer with increased activation of cell cycle signaling have a poor prognosis.39,40 We found that patients with high WDR5 expression are associated with activating cell cycle signaling and poor outcomes. Genetic and pharmaceutical targeting of WDR5 induces cell cycle arrest at the G1 phase in T-ALL. We further identified ATAD2 as the downstream target gene of WDR5, which plays a vital role in the regulation of cell cycle progress in T-ALL. ATAD2 is a chromatin-modifying enzyme and has been recognized as a promising oncoprotein in recent years.41-43ATAD2 high expression is also linked with cell cycle pathway activation and poor outcomes in solid tumors.42,44-46 Our data revealed that high ATAD2 expression is not only associated with abnormal activation of the cell cycle pathway but also associated with a higher relapse rate in T-ALL. Particularly, ATAD2 KD significantly induced the cell cycle arrest in the G1 phase. These data indicate that there exists a novel oncogenic signaling of WDR5/ATAD2, which plays a critical role in cell cycle progress in T-ALL. In this study, we observed that CX-4945 also induces the G1 phase arrest in T-ALL as we previously reported in B-ALL.26 Both CX-4945 and WDR5 inhibitors suppress ATAD2 expression, and the combination of CX-4945 with WDR5 inhibitor revealed a synergistic effect on cell proliferation inhibition, cell cycle arrest, and eventually the apoptosis of the T-ALL cells, including the potent synergistic antileukemic efficacy in the human leukemia xenograft mouse models. These data further indicated the oncogenic role of the WDR5/ATAD2 signaling and the regulatory roles of the CK2/IKAROS axis in this novel oncogenic signaling in T-ALL.
The combination of CX-4945 and WDR5 inhibitor OICR-9429 has a synergistic antileukemic effect in primary samples from high-risk patients with T-ALL. We observed that the combination of CX-4945 with OICR-9429 exhibited synergistic antileukemic effects on patient samples from high-risk T-ALL. Consistently, the combination of CX-4945 with WDR5 inhibitor OICR-9429 produced significant therapeutic efficacy in the 3 high-risk T-ALL PDX mouse models (1 newly diagnosed, 1 relapsed, and 1 relapsed ETP-ALL). These data further demonstrated that targeting WDR5/ATAD2 signaling by the combination of CX-4945 with WDR5 inhibitor has synergistic antileukemic efficacy in T-ALL, especially the high-risk T-ALL, including ETP-ALL.
We did not observe the significant difference of WDR5 high expression in different immune phenotypes, ETP-ALL vs non-ETP-ALL, and the various molecular subtypes of T-ALL, revealing the oncogenic roles of WDR5 high expression is not solely associated with some molecular subtypes. Targeting WDR5 may exhibit the antitumor roles for a wider spectrum of patients with T-ALL. Indeed, our data revealed targeting WDR5/ATAD2 signaling induces cell cycle arrest and has therapeutic efficacy for both newly diagnosed and relapsed high-risk T-ALL. These data are consistent with the cell cycle inhibitors; epigenomic drugs have more potential to become broad-spectrum antitumor agents and may be effective in T-ALL.47-49 Moreover, patients with relapsed T-ALL, particularly ETP-ALL, have extremely poor prognosis and limited therapeutic options.47,50-53 The 2 patients with relapse including 1 ETP-ALL used in our PDX models have very aggressive diseases with a very short remission time and died in a short period after relapse (supplemental Table 3). Our data revealed the strong therapeutic efficacy for the relapsed T-ALL in the PDX mouse models, which highlights the treatment potential for those high-risk T-ALL.
WDR5 performs a variety of critical chromatin-centric processes in the nucleus by serving as a core scaffolding component of many multiprotein complexes.19,54 WDR5 uses 2 major binding interfaces, the WDR5 interaction motif (WIN) and the WDR5-binding motif, to assemble multiprotein complexes.11,12 With the WIN sites, WDR5 interacts with the mixed lineage leukemia/SET-domain) (MLL_SET; MLL1-4, SETd1A, and SETd1B) family of histone methyltransferase complexes, which catalyze histone H3K4 di- and tri-methylation.19,54,55 WDR5 has been identified as an essential cofactor for MYC-promoted tumorigenesis by direct interaction with the WDR5-binding motif site and harbors the association of MYC to chromatin at tumor-critical target genes through the WIN site.56-58 Small-molecule inhibitor OICR-9429 is a WDR5 WIN-site inhibitor.59,60 Here, we found that the combination of OICR-9429 with CX-4945 demonstrated a strong therapeutic efficacy on T-ALL PDX mouse models. As MYC is also a potent oncogene in T-ALL, the question is whether MYC is involved in the mechanism underlying the antitumor efficacy of the combination. Although MYC is also the IKAROS target gene, CX-4945 restores the IKAROS function and significantly suppresses the expression of MYC.61 Thus, we considered that the synergistic effect of CX-4945 combined with WDR5 inhibitor is mainly through CX-4945 restoring IKAROS transcriptional repressor function to suppress WDR5/ATAD2 signaling (supplemental Figure 21) rather than the posttranscriptional blocking the WDR5/MYC interaction, although we identified the 9 overlapped gene targets (ABCE1, DDX21, DEK, PSMA1, SSB, USP1, MPHOSPH10, PLK4, and CNX3) between the OICR-9429 DEGs and MYC target genes.62
In summary, we identified new WDR5/ATAD2 oncogenic signaling in T-ALL and the regulatory roles of the CK2/IKAROS axis within the signaling. Targeting the oncogenic signaling through the CK2/IKAROS axis induces cell proliferation inhibition and cell cycle arrest. Our data have demonstrated that the combination of CX-4945 with WDR5 inhibitor has a potent synergistic antileukemic efficacy in T-ALL and provides preclinical evidence for dual targeting WDR5/ATAD2 through CK2/IKAROS as a potential option of T-ALL therapy.
Acknowledgments
The authors are grateful to the staff at the Public Scientific Research Platform of Zhongda Hospital Southeast University for providing the technical assistance in flow cytometry.
This work was supported, in part, by the National Natural Science Foundation of China (grant 82070166, Z.G.); Medical Research Key Projects, Jiangsu Commission of Health (grant ZD2021003, Z.G.); Jiangsu Provincial “333” project (grant BRA2019103, Z.G.); Jiangsu Provincial Medical Key Discipline (Laboratory) Cultivation Unit (grant JSDW202212, Z.G.); and the Milstein Medical Asian American Partnership Foundation Research Project Award in Hematology (2017, Z.G.).
Authorship
Contribution: Z.G. and Q.H. designed the project; Q.H., Y.G., H.X., L.Z., Y.W., C.Y., and J.L. performed the experiments; Q.H. and Y.G. analyzed the data; Z.G. supervised the project and performed data analysis; C. Song, R.L., J.W., Y.Y., and S.D. provided constructive suggestions for the revision of the manuscript; and Z.G., C. Song, C. Steiner., R.L., J.W., Y.Y., Q.H., and S.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zheng Ge, Department of Hematology, Zhongda Hospital, School of Medicine, Southeast University, Institute of Hematology Southeast University, 87 Dingjiaqiao St, Nanjing 210009, China; email: zhengge@seu.edu.cn.
References
Author notes
Q.H. and Y.G. contributed equally to this work.
The patient data sets for this study are not publicly accessible because of local health research ethics protocols; however, the data sets are available on request from the corresponding author, Zheng Ge (zhengge@seu.edu.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Therapeutic efficacy of the combination of CX-4945 and WDR5 inhibitor in patient-derived T-ALL leukemia-xenograft (PDX) mouse model. (A) Comparison of Kaplan-Meier survival curves in the combination of CX-4945 and WDR5 inhibitor OICR-9429 compared with either single drug controls in 3 different PDX mouse models: PDX-P1 (Patient, [Pt.] T-ALL-P1 in supplemental Table 3), PDX-P2 (Pt. T-ALL-P2), and PDX-P3 (Pt. T-ALL-P3). (B) Comparison of percentage of human CD45+CD7+ cells in the spleen of the mice in combo group vs either single drug controls. (C-E) Comparison of mRNA level of WDR5, ATAD2, CCNE2, and CDK2 in combo group vs either single drug controls in the spleen of the mouse models. The mice in panels A-E were treated with the indicated drugs for 25 days, and then, when the vehicle mice met the early removal criteria due to the excessive leukemia burden, the mice were euthanized and the single cells were isolated from the spleen for flow cytometry analysis; the mRNA of the cells was prepared for qPCR analysis. ∗∗∗P < .001. OI, OICR-9429.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/13/10.1182_blood.2024024130/3/m_blood_bld-2024-024130-gr5.jpeg?Expires=1765899727&Signature=SNfcvpzokXz7WOQRucdYrYM0l-~J4fBm6-okp4y1WWp6YBbZkW9QCTfAsKai7nCYj7qlAqYFbC51hpToocX4c~uwj2EJ95XapbQZY-QbZWGmNySTgNdx0QD8I~bPB6MOVRS-3oC0oGpi7vZvUlb3-jFPDLkTVGE2mAcUWbsRPOGkC9pvwxylJty4qU-NUQR7mwZErBHncTdjQuMJHXUoyD8mUwXz5zJP1N1hIFbkQzqRxT4-745P3Fw1bLicuSVYJxxjIulx6BCllZIrvzxaqRPnGZg6oyJqt96ySxvGNtY-1E2tQvQThSrik9ApQbf6PlZQxAIAokvidsfmdTo6pA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal