Visual Abstract
It has been known for over half a century that throughout ontogeny, humans produce different forms of hemoglobin, a tetramer of α- and β-like hemoglobin chains. The switch from fetal to adult hemoglobin occurs around the time of birth when erythropoiesis shifts from the fetal liver to the bone marrow. Naturally, diseases caused by defective adult β-globin genes, such as sickle cell disease and β-thalassemia, manifest themselves as the production of fetal hemoglobin fades. Reversal of this developmental switch has been a major goal to treat these diseases and has been a driving force to understand its underlying molecular biology. Several review articles have illustrated the long and at times arduous paths that led to the discovery of the first transcriptional regulators involved in this process. Here, we survey recent developments spurred by the discovery of CRISPR tools that enabled for the first time high-throughput genetic screens for new molecules that impact the fetal-to-adult hemoglobin switch. Numerous opportunities for therapeutic intervention have thus come to light, offering hope for effective pharmacologic intervention for patients for whom gene therapy is out of reach.
Introduction
Sickle cell disease (SCD) and β-thalassemia are among the most common monogenic diseases and affect hemoglobin production qualitatively or quantitatively, respectively.1-4 The α- and β-type globin chains that compose the hemoglobin tetramer are each encoded by multiple genes that are arranged in clusters and activated at distinct stages of development. The human β-globin gene cluster spans an embryonic (HBE1), 2 fetal (HBG1 and HBG2), and 2 adult (HBD and HBB) genes. The transition from HBG to HBB gene expression occurs around the time of birth when erythropoiesis shifts from the fetal liver to the bone marrow.5-9 Naturally, diseases caused by defective HBB genes, such as SCD and β-thalassemia, manifest themselves as HBG production fades. SCD is caused by a mutation in the adult-expressed HBB gene, leading to aberrant physicochemical properties of hemoglobin and misshapen erythrocytes that can occlude small vessels and damage all organs.3,4 In contrast, a panoply of genetic alterations can cause β-thalassemia.1,2
Strong evidence based on human genetics, as well as therapeutic intervention, has demonstrated that the clinical course of some forms of β-thalassemia as well as SCD is improved upon the activation of fetal HBG genes (reviewed recently in Lu et al10 and Steinberg11). HBG expression can compensate for insufficient β-globin chain synthesis in β-thalassemia and counteract cell sickling in SCD. Monumental progress has been made toward curing these diseases via various forms of gene therapy, including HBB or HBG gene addition, gene modification/editing (conversion of the SCD mutation into a harmless variant, destroying binding sites for HBG repressors), and reducing the expression of the HBG repressor BCL11A (reviewed in Locatelli et al12). The current iterations of these therapies involve autologous bone marrow transplants, which entail harsh regimens to condition the host bone marrow to efficiently receive genetically modified hematopoietic stem cells. Resulting short- and long-term toxicities need to be weighed against their benefits in relation to the disease severity. The cost of gene therapy and the need for sophisticated medical infrastructure can be prohibitive in many locations worldwide. These considerations are at the heart of efforts to discover new, potentially druggable molecules that can directly or indirectly reverse the HBG-to-HBB switch.
Of course, “drugging” known regulators of the HBG-to-HBB switch brings its own challenges. For example, so far, attempts to develop small molecules against BCL11A, a DNA-binding protein that is currently the best validated HBG repressor, have not been met with success in the 17 years since the discovery of its role in hemoglobin switching.
Owing in part to the CRISPR-Cas9 revolution, substantial advances have recently been achieved in our understanding of the regulatory networks surrounding the HBG-to-HBB gene expression switch. CRISPR-Cas9 enables large-scale screens for (1) relevant cis-regulatory elements at the globin gene cluster as well as at loci implicated in HBG regulation,13-17 (2) identification of functional domains within regulatory molecules via high-resolution tiling (eg, Sher et al18), and (3) new regulators of HBG silencing,19-28 all of which revealed new insights along with potential therapeutic opportunities. Here, we focus on recent (half-decade) developments in this field, many of which have resulted from CRISPR-Cas9–based screens. For excellent reviews of the history of the field and older studies, we refer the reader to Lu et al,10 Steinberg,11 Orkin,29 and Papayannopoulou.30 Recent advances highlight the remarkable number of inputs from diverse pathways converging directly or indirectly on the HBG-to-HBB switching mechanism (Figure 1).
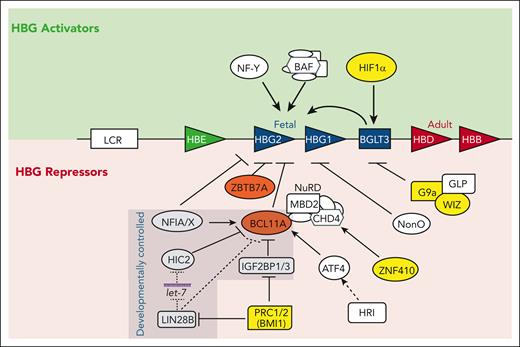
Select regulators of the fetal-to-adult hemoglobin switch. The β-globin locus on chromosome 11 is shown with the LCR and developmentally arrayed β-like globin genes. Activators are noted above and repressors (direct and indirect) are noted below. Both NF-Y and BAF also activate adult globins. Developmentally controlled factors with differential expression between fetal and adult tissues are indicated. Regulators with the most advanced targeted therapies are highlighted in yellow. Dotted lines indicate direct posttranscriptional regulation and dashed lines indicate indirect control.
Select regulators of the fetal-to-adult hemoglobin switch. The β-globin locus on chromosome 11 is shown with the LCR and developmentally arrayed β-like globin genes. Activators are noted above and repressors (direct and indirect) are noted below. Both NF-Y and BAF also activate adult globins. Developmentally controlled factors with differential expression between fetal and adult tissues are indicated. Regulators with the most advanced targeted therapies are highlighted in yellow. Dotted lines indicate direct posttranscriptional regulation and dashed lines indicate indirect control.
Most developmentally regulated tissue-specific genes are controlled by distal enhancers. In the β-globin gene cluster, there also exists a powerful distal erythroid-specific enhancer, called the locus control region (LCR), which is required for the expression of all genes in the cluster, but the developmental control of the globin genes occurs near the gene promoters. BCL11A and ZBTB7A were among the first factors identified as direct repressors of HBG genes by binding near the HBG promoters.31-34 Validating their direct function, mutations at the binding sites for these factors are found in individuals with hereditary persistence of fetal hemoglobin (HPFH), a benign condition with elevated HBG expression throughout adult life. BCL11A and ZBTB7A also occupy the LCR and the active globin genes, the biological consequences of which is unknown. BCL11A and ZBTB7A function additively and neither is sufficient to fully repress HBG genes in the absence of the other.35 This also seems to hold true for more recently identified transcriptional HBG repressors, discussed below, which are required but are by themselves insufficient for complete HBG silencing in adult erythroid cells. Lastly, repression by BCL11A and ZBTB7A is not absolute because HPFH mutations that create nearby binding sites for KLF1, TAL1, or GATA1, or forcing the proximity of the silent HBG genes with the LCR can at least partially override them.36-40
HBG repressors
BCL11A
Although the importance of BCL11A in globin switching has been known for a long time, many of the recently described pathways converge on BCL11A. The expression of BCL11A is higher in adult erythroid cells than in fetal cells, which is a mechanism by which HBG gene silencing is achieved in adult cells. The adult-enriched transcription factor KLF1 has been known for some time to activate BCL11A expression,41,42 which together with its ability to activate HBB transcription,43,44 contributes to the HBG-to-HBB switch. However, other drivers of BCL11A transcription such as GATA1 are not under developmental control. A deeper understanding of how BCL11A expression is regulated has again benefited from relatively recent advances in screening technologies. These revealed that most new regulators converge directly or indirectly on BCL11A expression and function, including the mechanisms of BCL11A developmental control.
Earlier studies have proposed that BCL11A is regulated posttranscriptionally. In the first, overexpression of the fetal-specific RNA-binding protein LIN28B, which helps suppress microRNAs (miRNAs) including let-7, was shown to reduce BCL11A mRNA levels and increase HBG production.45 Additionally, the fetal-enriched RNA-binding protein IGF2BP1 was proposed to bind BCL11A mRNA to inhibit its translation,46 establishing posttranscriptional regulation as a key contributor to BCL11A developmental regulation. Another study suggested that BCL11A mRNA levels remain mostly constant, but that BCL11A mRNA translation is repressed in fetal cells via LIN28B.47 Yet, multiple studies have demonstrated developmental differences in BCL11A mRNA synthesis, RNA polymerase II occupancy, and enhancer activity, indicating that transcriptional activation is the main mechanism for adult stage–specific BCL11A expression.23,48-50 Consistent with this model, several reports that leveraged CRISPR screening technologies identified factors that directly or indirectly converged on the transcriptional regulation of BCL11A that are discussed below.
In one of the first such screens, Grevet et al screened a protein kinase domain-targeted CRISPR library in HUDEP2 cells, an adult-type human immortalized erythroid cell line,51 for potentially druggable fetal hemoglobin (HbF) regulators. The screen identified erythroid protein kinase heme-regulated inhibitor (HRI) as a regulator of HBG transcription.22 HRI influences BCL11A mRNA production indirectly via mRNA translation of the transcription factor ATF4, which is identified as the key intermediate in a subsequent CRISPR screen. ATF4, in turn, activates BCL11A transcription by binding to an intronic (+55 kilobase [kb]) enhancer.25 Destruction of the ATF4 binding site reduced BCL11A transcription in human but not murine cells, explaining the human-specific effects of HRI or ATF4 depletion on globin gene expression. The ATF4 binding site also scored in a tiling screen for HbF induction in human erythroid cells that tiled cis-regulatory elements of the BCL11A locus.14
Another study invoked ATF4 in HBG regulation and proposed an additional mechanism of action.19 In particular, ATF4 was shown to additionally activate transcription of the c-MYB gene, a previously known HbF regulator.52 However, ATF4 expression is not specific to adult erythroid cells and hence does not explain adult-activated transcription of BCL11A.
Recently, a similar CRISPR screening approach identified the adult erythroblast-enriched bifunctional transcription factor NFIA/X, which, in addition to directly repressing the HBG1/2 genes (see below), binds to the BCL11A +55 kb, +58 kb, and +62 kb intronic enhancers.21 Loss of NFIA/X in adult erythroblasts decreased BCL11A expression by ∼30%, demonstrating that a portion of the NFIA/X effects occurs through the activation of BCL11A transcription.
Further insights into the developmental transcriptional regulation of BCL11A were provided when a CRISPR screen revealed HIC2 as a fetal-stage–specific BCL11A transcriptional repressor.23 HIC2 binds to both the +55 kb and +62 kb BCL11A erythroid enhancers and functions in part through steric competition with GATA1 and by reducing chromatin accessibility and H3K27 acetylation at the +55 kb enhancer. This naturally raised the question of how HIC2 is developmentally regulated. Subsequent experiments showed that translation of HIC2 mRNA is inhibited by adult-expressed let-7 miRNAs, providing yet another upstream rung in the ladder of BCL11A control.53 Interestingly, let-7 miRNAs have long been known to be inhibited by the fetal-expressed RNA-binding protein LIN28B.
In summary, in large part uncovered through recent CRISPR screens, BCL11A production is controlled by diverse mechanisms, involving stage-specific and nonstage-specific transcriptional activators and repressors, as well as positively and negatively acting stage-specific posttranscriptional regulators (let-7 ⊣ HIC2 ⊣ BCL11A ⊣ HBG; see shaded area in Figure 1).
NFIA/X
A CRISPR-Cas9 genetic screen targeting DNA-binding proteins revealed NFIA as a new repressor of HBG expression.21 The NFI family comprises NFIA, NFIB, NFIC, and NFIX, with NFIA and NFIX being the predominant erythroid-produced forms. Importantly, NFIA and NFIX are expressed more highly in adult erythroid cells than in their fetal counterparts. NFIX did not score in this screen, presumably because of functional redundancy with NFIA. Indeed, combined depletion raised HBG expression to levels greater than those achieved by individual depletion in HUDEP2, primary human erythroblasts, and in a human-to-mouse xenograft model. Increases in HbF levels were comparable to those achieved with BCL11A depletion and sufficient to dampen sickling in cells derived from patients with SCD. Mechanistically, NFI factors can activate or repress transcription depending on the context. Both functions seemed to matter for HBG silencing. As mentioned, NFIA/X binds to an erythroid enhancer of the BCL11A gene, and the loss of NFIA/X lowered BCL11A transcription. However, forced expression of BCL11A failed to completely restore HBG silencing in NFIA/X-depleted cells, implicating an additional mechanism. Indeed, NFIA/X also binds directly to NFI motifs at −400 base pair (bp) and +200 bp with regard to HBG start sites, and CRISPR-Cas9 mediated removal of these sites elevated HBG transcription. Hence, the bifunctionality of NFIA/X is required to achieve full HBG repression, involving the direct activation of BCL11A and repression of HBG.
A subsequent study independently identified NFIX by differential chromatin accessibility experiments, comparing differentiation stage-matched fetal and adult human erythroblasts.54 Adult stage enhanced accessibility was linked to the NFI sequence motifs. Follow-up experiments in cultured human erythroblasts, including depletion and overexpression of NFIX, confirmed its role in HBG silencing. Notably, a cis-regulatory element-focused screen for HbF induction identified intronic sequences important for NFIX expression and HbF regulation.16 Lastly, a genome-wide association study implicated a sequence variant near the NFIX gene as an HBG modulator,55 but whether the variant in question is causal remains unclear.
NonO
The transcription factor NonO (non-POU-domain-containing, octamer-binding protein), also known as p54nrb recently emerged as a potential regulator of HBG silencing56 when it was found that it can physically associate with Sox6, another protein previously implicated in the silencing of embryonic and fetal globins.57-59 NonO knockdown in HUDEP2 cells increased HBG expression. Sox6 and NonO depletion yielded additive HBG induction, and forced expression caused additive HBG repression in reporter assays, leaving open the possibility that these proteins may function independently of each other despite their ability to interact.
A mutation near the HBG promoter (175T>C) that causes the benign condition of HPFH creates an E-box, leading to the recruitment of the TAL1-LDB1 activating complex.38 It is possible, but remains to be directly tested, that this mutation simultaneously disrupts NonO binding to its putative binding site (−182 bp to −175 bp) to augment HBG derepression.
HBG activators
NF-Y
Human globin gene proximal promoters contain multiple CCAAT boxes. These conserved motifs are required for appropriate globin expression and are bound by the ubiquitous activating transcription factor NF-Y.60-63 The HBG1 and HBG2 genes contain 2 CCAAT boxes with a distal one overlapping with a BCL11A binding site. Recent studies used a comprehensive combination of screens involving Cas9 indel generation, dCas9 protein scanning to displace transcription factors, dCas9-KRAB fusions to generate regions of repressed chromatin, and base editing technology to define transcription factor function at the β-globin locus.15,64 In particular, these experiments enabled dissection of the effects of BCL11A and NF-Y on HBG activities. These efforts yielded a model in which BCL11A inhibits HBG transcription by steric inhibition of NF-Y binding, as well as the generation of a repressive chromatin environment. The latter may be the prevailing mechanism because NF-Y binding to the HBG gene was maintained in cells deficient in MBD2 (below), in which BCL11A levels are elevated.65 Further confirming a role for NF-Y is that the creation of an NF-Y binding site leads to increased HBG transcription.64 Thus, a competitive interplay between repressors and activators at the HBG promoters contributes to the HBG-to-HBB switch.
BGLT3
The region between fetal and adult globin genes contains 2 essential regulatory elements. The HBBP1 pseudogene is de facto a cis-regulatory element that is required to create an adult-specific chromatin configuration at the β-globin locus, and deleting it increases HBG transcription.48 The neighboring long noncoding RNA BGLT3 (previously known as BGL3) is expressed in conjunction with HBG.48,66 Multiple transcription start sites have been defined leading to a variety of transcripts.66,67BGLT3 has also been described as a chromatin-enriched RNA (named HIDALGO), with its transcription start sites acting as a HIDALGO RNA-dependent enhancer of HBG1.67 Ivaldi et al manipulated the genomic locus, its transcriptional activity, and the transcript itself to show that (1) the BGLT3 transcript is necessary but not sufficient for HBG transcription, (2) active transcription from the BGLT3 locus is required for HBG activation, and (3) the BGLT3 locus itself functions as an enhancer and is required for appropriate chromatin conformation and HBG expression.68
HIF1α
It has previously been proposed that hypoxia drives increased HbF production in patients with SCD or other conditions of erythroid stress, such as recovery from anemia or high altitude.69,70 Two CRISPR screens recently identified the ubiquitin ligase VHL, a key component of the hypoxia signaling pathway, as an HBG repressor.20,28 Mechanistic experiments have shown that this effect occurs through the VHL substrate hypoxia-responsive transcription factor HIF1α. HIF1α binds to the hypoxia response element within the BGLT3 region to activate HBG transcription.20 This finding provides a long-sought-after mechanistic link between hypoxia and HBG levels. Moreover, the hypoxia pathway may present a novel therapeutic target. Under normoxic conditions, HIF1α is degraded via VHL upon hydroxylation by prolyl hydroxylases. Inhibitors of these enzymes block HIF1α degradation to raise HbF levels.20 Notably, such inhibitors are already being clinically studied for anemia of chronic kidney disease, in which they increase erythropoietin production.71
BAF complex
Chromatin remodeling complexes regulate gene expression by repositioning nucleosomes to modulate chromatin accessibility and allow the binding of transcription factors. The BAF complex, a member of the SWI/SNF chromatin remodeler family, has long been known to be generally required for globin gene transcription.72,73 Guo et al recently revisited the role of this complex through studies of hemogen, a regulator of erythroid proliferation and differentiation.74 They demonstrated that hemogen facilitates recruitment of the BAF complex, including the ATPase BRG1, to the globin promoters as well as the LCR and BGLT3 regions. Hemogen depletion decreases BRG1 and BAF complex occupancy, chromatin accessibility, and transcription of the globin genes. However, hemogen function did not seem to be specific for any given globin gene but rather required for highly level expression of the developmentally dominant globin gene. Notably, recent work suggested that different subtypes of the BAF complex may convey specificity for the activation of different globin genes, with noncanonical BAF subunits showing selectivity for the HBG genes.75
Indirect repressors and corepressors
NuRD complex and ZNF410
The nucleosome remodeling and deacetylase (NuRD) complex has been implicated in HBG silencing for many years.76-78 It is an essential regulator of gene expression that serves different cell-type and context-dependent roles through paralog switching of multiple functional components including CHD-family nucleosome remodelers, histone deacetylases, MBD DNA-binding proteins, and multiple scaffolding components.79 NuRD interacts with BCL11A, contributing to its repressive function.35,80 NuRD recruitment to HBG genes has also been proposed to be aided by TR2 and TR4 orphan nuclear receptors.81 Notably, patients with a germ line missense mutation in the DNA methyltransferase DNMT1, which impairs its interaction with BCL11A, display reduced DNA methylation at the HBG genes and elevated HBG expression.82 Part of the NuRD repressive function is thought to be mediated by direct recruitment to HBG genes via the methyl-cytosine–binding NuRD component MBD2a,83 possibly explaining the HBG-activating function of the DNA methyltransferase inhibitor 5-azacytidine.84
NuRD can also function as a coactivator complex not only in the globin gene cluster85 but perhaps also at the BCL11A gene as well as the KLF1 gene, which in turn can activate BCL11A transcription, suggesting additional indirect effects on HBG silencing.77
The most parsimonious model emerging from these and other studies is that NuRD activity is brought to the HBG genes via the combined action of MBD2a and BCL11A.
New insights into the regulation of NuRD itself arose from 2 independent CRISPR-based genetic screens for HbF regulators, which identified the transcription factor ZNF410 as a strong, indirectly acting HBG repressor.26,27 A truly remarkable result from the follow-up experiments was that ZNF410 has a single functional target gene in the erythroid genome, namely CHD4, which is a core component of the NuRD complex. Specificity is conveyed by 2 dense ZNF410 motif clusters upstream of the CHD4 promoter. In spite of these many binding sites, the loss of ZNF410 reduces CHD4 expression by only ∼60%. This is important firstly because it exposes the HBG genes as exquisitely sensitive to reduced CHD4 levels in line with heterozygous loss of CHD4,77 and secondly because such reduced CHD4 levels are well tolerated by erythroid cells, and ZNF410 null mice are essentially healthy. These studies established ZNF410 as a new target for therapeutic intervention, and a vector that reduces the expression of BCL11A in combination with ZNF410 triggers higher HBG induction than either factor alone.86
In search of new ways to attenuate the NuRD complex while maintaining cell viability, a CRISPR tiling screen was carried out across functionally relevant NuRD subunits.18 Although total loss of CHD4 diminished cell fitness, the screen identified in-frame deletions at the C-terminus of CHD4 that raised HBG levels but with reduced detriment to cell fitness. Such approaches highlight the usefulness of CRISPR screening tools to define potentially druggable protein domains.
PRC
Inhibitors of the Polycomb repressive complex (PRC) components are being studied as HbF activators.87 Qin et al carried out a CRISPR screen for HbF repressors and identified the PRC component BMI1. Moreover, this study also unraveled the mechanism of PRC inhibition by showing that PRC1 and PRC2 directly repress LIN28B, IGF2BP1, and IGF2BP3 in adult erythroblasts, in turn allowing BCL11A expression. Disrupting PRC function genetically or pharmacologically increased LIN28B, IGF2BP1, and IGF2BP3 production, lowered the BCL11A expression (as described above), and increased HBG production.88 LIN28B, IGF2BP1, and IGF2BP3 thus emerged as the key intermediates between PRC and BCL11A regulation and provided a mechanistic foundation for therapeutic PRC inhibition. (PRC ⊣ [LIN28B, IGF2BP1, IGF2BP3] ⊣ BCL11A ⊣ HBG).
EHMT1/2
EHMT1 and EHMT2 (also known as G9a/GLP), responsible for the repressive H3K9me2 histone methylation mark, have been implicated as HbF silencers via unknown mechanisms.89,90 A recent screen of a protein degrader library for HbF regulators identified a molecule that triggers the degradation of WIZ, a subunit of the EHMT1 complex.91 A pharmacologically optimized version of the degrader induced HbF in a humanized mouse model and nonhuman primates. Mechanistic insights into EHMT1/2 function came from the development and study of a new chemical EHMT1/2 inhibitor.92 In particular, EHMT1/2 inhibition reduced H3K9me2 levels at the BGLT3 gene, increasing its expression along with HBG, providing one of perhaps multiple mechanisms of EHMT1/2 action.
Novel mechanisms of fetal hemoglobin regulation
The past 5 years have seen the discovery of multiple additional HbF regulators with disparate or unknown functions. For example, HEXIM1, which can stall RNA polymerase II elongation and is essential for erythropoiesis, can promote a fetal gene expression signature with downregulation of BCL11A and MYB and upregulation of HBG.93 That study provided another remarkable case in which what are thought to be components of the general transcriptional machinery may have gene selective functions, in this case, privileging HBG over HBB.
Additionally, several RNA-binding proteins have been found to increase HBG levels via BCL11A-independent posttranscriptional effects. RBM12 represses HBG through an unknown mechanism94 and PUM1 directly destabilizes and blocks the translation of HBG mRNA.95 SPOP, a substrate adapter for the CUL3 ubiquitin ligase complex, emerged from an HbF–directed CRISPR-Cas9 screen but its mechanism of action remains elusive.24
Other recent contributors to fetal hemoglobin regulation, in particular under stress conditions, have been found in FOXO3,96 SGK1, a kinase upstream of FOXO3,97 and BACH1.98 Although these new discoveries highlight the potential for new therapeutic avenues, several of them still lack a deeper mechanistic understanding required for consideration of new therapies.
Conclusions and perspective
“Progress in science depends on new techniques, new discoveries, and new ideas, probably in that order.” This quote has been attributed to the late Sydney Brenner, and based on the above considerations applies well to the progress in the field of hemoglobin regulation. It is astounding how much CRISPR technologies have accelerated advances in this mature field and shed light on new avenues for therapeutic intervention. Acceleration will likely continue in the foreseeable future, given the breathtaking progress in improving and refining these and other technologies. With more effective screening tools such as Cas12a and CRISPR-based activation systems, the entire coding genome can now be interrogated in loss-of-function and gain-of-function screens for genes relevant to a biological system of interest (eg, Traxler et al99 and Balbin-Cuesta et al100). Such improved screening tools also open up the possibility of performing combinatorial screens, for example, via pairwise gene targeting, to search for synergistic effects on processes such as HbF induction. The cooperativity of distinct pathways in HbF regulation can be exploited when devising treatments with improved therapeutic indices, especially when overly aggressive targeting of a single pathway has an unacceptable risk profile.
Historically, transcription factors have been challenging to target therapeutically because they lack the catalytic domains that are the focus of most therapies. Screening approaches address this problem by defining upstream pathways or cofactors that may be more amenable to modulation, as shown by the protein kinases, epigenetic writer enzymes, and ATPase nucleosome remodelers highlighted here. CRISPR tools are also being exploited to fine-map relevant components or protein domains of regulatory complexes (eg, Sher et al18). These types of screening experiments can now be carried out with almost a single amino acid resolution, for example, by tiling CRISPR-base editors across coding regions with relaxed PAM sequences. When combined with structural information, these approaches may pave the way for the development of small molecules that perturb functions more selectively or may serve as warheads for targeted protein degraders such as PROTACS or molecular glues,101 as discussed above for WIZ. It has also become clear over the years that the adoption of new technologies and discoveries in the field of β-hemoglobinopathies has ripple effects with an impact on other fields and diseases.
Despite great gains in knowledge in this field over the last few decades of the twentieth century, translation into new therapies has been frustratingly slow, taxing our optimism. However, with the recent burst of activities spurred by the ever-improving new technologies, renewed optimism for a better future for patients with β-hemoglobinopathies is warranted.
Acknowledgments
The authors thank Merlin Crossley and Peng Huang for their helpful comments on the manuscript.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant R01HL119479; NIH, National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK054937, R24DK106766, and K08DK128571; the American Society of Hematology Research Training Award for Fellows and Scholar Awards; the St. Jude Children’s Research Hospital Collaborative Research Consortium on Novel Gene Therapies for Sickle Cell Disease; and the DiGaetano family.
Authorship
Contribution: E.K. and G.A.B. wrote the manuscript.
Conflict-of-interest disclosure: G.A.B. received research support from Fulcrum Therapeutics. E.K. declares no competing financial interests.
Correspondence: Eugene Khandros, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104; email: khandrose@chop.edu; and Gerd A. Blobel, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Philadelphia, PA 19104; email: blobel@chop.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal