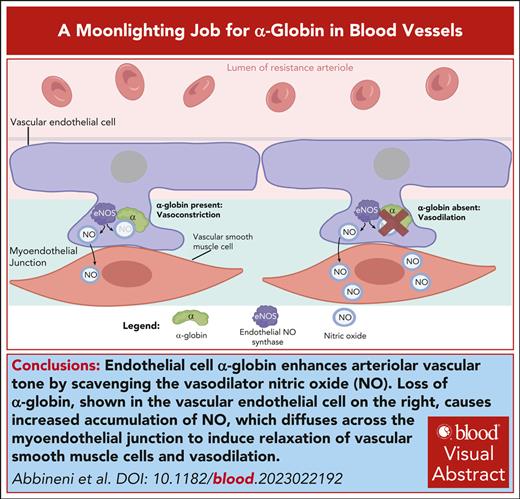
Visual Abstract
Red blood cells express high levels of hemoglobin A tetramer (α2β2) to facilitate oxygen transport. Hemoglobin subunits and related proteins are also expressed at lower levels in other tissues across the animal kingdom. Physiological functions for most nonerythroid globins likely derive from their ability to catalyze reduction–oxidation (redox) reactions via electron transfer through heme-associated iron. An interesting example is illustrated by the recent discovery that α-globin without β-globin is expressed in some arteriolar endothelial cells (ECs). α-globin binds EC nitric oxide (NO) synthase (eNOS) and degrades its enzymatic product NO, a potent vasodilator. Thus, depletion of α-globin in ECs or inhibition of its association with eNOS causes arteriolar relaxation and lowering of blood pressure in mice. Some of these findings have been replicated in isolated human blood vessels, and genetic studies are tractable in populations in which α-thalassemia alleles are prevalent. Two small studies identified associations between loss of α-globin genes in humans and NO-regulated vascular responses elicited by local hypoxia-induced blood flow or thermal stimulation. In a few larger population-based studies, no associations were detected between loss of α-globin genes and blood pressure, ischemic stroke, or pulmonary hypertension. In contrast, a significant positive association between α-globin gene copy number and kidney disease was detected in an African American cohort. Further studies are required to define comprehensively the expression of α-globin in different vascular beds and ascertain their overall impact on normal and pathological vascular physiology.
Diverse functions for hemoglobin and related proteins
Hemoglobin A (HbA; α2β2) is best known as the major red blood cell (RBC) oxygen (O2) transporter.1,2 To support this function, each subunit of the HbA heterotetramer contains a planar heme molecule with a central iron that binds O2 reversibly. Hemoglobin and related heme-containing proteins, including myoglobin, neuroglobin, and cytoglobin, are expressed in numerous nonerythroid tissues.3-6 Myoglobin facilitates intercellular O2 transport in cardiac and skeletal muscle.7 However, most other nonerythroid globins are expressed at concentrations too low to support physiological O2 transport.8-12 Indeed, primordial hemoglobin proteins likely arose in low-O2 environments as O2 sensors and/or nitric oxide (NO) scavengers rather than as O2 transporters.13,14 Hemoglobins (also referred to here as globins) bind numerous ligands in addition to O2, including NO, carbon monoxide (CO), peroxides, and nitrite (NO2−), and participate in reduction and oxidation (redox) reactions mediated by heme iron.15,16 These enzymatic functions are well established for bacterial globins and are being increasingly appreciated for mammalian hemoglobins.17,18 Although the physiological roles of mammalian cytoglobin and neuroglobin are not fully understood, they are thought to function as antioxidants and regulate NO levels through various enzymatic activities.17,18
Low levels of α- and/or β-globin proteins have been detected in multiple cell types and tissues, including macrophages, alveolar epithelial cells, retinal pigment epithelium, neurons, the endometrium, and the cervix.8 In many of these cases, globin gene expression has not been validated rigorously by multiple laboratories and the associated functions are unknown. Studies of hemoglobin expression in nonerythroid tissues are challenging. Low-level RBC and reticulocyte contamination can cause false positive results. Moreover, traces of α- and/or β-globin messenger RNA (mRNA) detected by sensitive methods may simply represent “transcriptional noise” with no associated physiological function. One exceptional case is the expression of α-globin in blood vessels. Several independent studies have detected α-globin (without β-globin) mRNA and protein in mouse and human arteriolar endothelial cells (ECs; Table 1). Extensive data from mice models indicate that EC α-globin degrades NO to enhance the contractility of vascular smooth muscle cells (VSMCs), thereby regulating blood pressure and, perhaps, regional blood flow.19-22 The implications of these findings for human health and disease are uncertain. Here, we review the body of work supporting a physiological function for EC-expressed α-globin in mice and preliminary studies hinting at potential roles in human vascular physiology.23-28
Expression of α-globin and β-globin in mouse and human blood vessels
| Blood vessel . | Detection approach . | α-globin . | β-globin . | Reference . |
|---|---|---|---|---|
| Mouse TD arteries | TEM of artery with immunostaining | Detected | Not assessed | 19 |
| Immunofluorescence analysis of sectioned artery | Detected | Not assessed in reference 1,2; not detected in reference 3 | 19,21,29 | |
| Immunofluorescence analysis of isolated ECs | Detected | Not detected | 19 | |
| Immunoblot of homogenized artery | Detected | Detected in reference 1; not in reference 2 | 19,21 | |
| RT-PCR analysis of ECs cocultured with VSMCs to recapitulate MEJs. | Detected | Not assessed | 19 | |
| RT-PCR analysis of whole artery | Detected | Not detected | 21 | |
| Mouse carotid arteries | Immunofluorescence analysis of sectioned artery | Detected | Not assessed | 19 |
| TEM of artery with immunostaining | Detected | Not detected | 19 | |
| RT-PCR analysis of whole artery | Detected | Not assessed | 19 | |
| Mouse aorta | Immunofluorescence analysis of sectioned artery | Not detected | Not detected | 29 |
| Human coronary artery | Immunoblot of ECs expanded in culture | Detected | Not detected | 19 |
| Immunoblot of ECs cocultured with VSMCs to recapitulate MEJs | Detected | Not detected | 19 | |
| RT-PCR and immunoblot of ECs overexpressing KLF2/KLF4 | Detected | Not detected (data now shown) | 30 | |
| Human mesenteric arterioles | IHC of artery section | Detected | Not detected | 21 |
| Human skeletal muscle arterioles | Immunofluorescence analysis of artery section | Detected | Not assessed | 19 |
| Human TD arteries | IHC of artery section | Detected | Detected | 19 |
| Human aorta | Immunoblot of homogenized artery | Not detected | Not detected | 31 |
| Human adipose tissue arteriole | Immunoblot of homogenized artery | Detected | Not detected | 31 |
| IHC of artery section | Detected | Not detected | 21,29 | |
| Human pulmonary artery | Immunoblot of homogenized artery | Detected | Detected | 32 |
| Blood vessel . | Detection approach . | α-globin . | β-globin . | Reference . |
|---|---|---|---|---|
| Mouse TD arteries | TEM of artery with immunostaining | Detected | Not assessed | 19 |
| Immunofluorescence analysis of sectioned artery | Detected | Not assessed in reference 1,2; not detected in reference 3 | 19,21,29 | |
| Immunofluorescence analysis of isolated ECs | Detected | Not detected | 19 | |
| Immunoblot of homogenized artery | Detected | Detected in reference 1; not in reference 2 | 19,21 | |
| RT-PCR analysis of ECs cocultured with VSMCs to recapitulate MEJs. | Detected | Not assessed | 19 | |
| RT-PCR analysis of whole artery | Detected | Not detected | 21 | |
| Mouse carotid arteries | Immunofluorescence analysis of sectioned artery | Detected | Not assessed | 19 |
| TEM of artery with immunostaining | Detected | Not detected | 19 | |
| RT-PCR analysis of whole artery | Detected | Not assessed | 19 | |
| Mouse aorta | Immunofluorescence analysis of sectioned artery | Not detected | Not detected | 29 |
| Human coronary artery | Immunoblot of ECs expanded in culture | Detected | Not detected | 19 |
| Immunoblot of ECs cocultured with VSMCs to recapitulate MEJs | Detected | Not detected | 19 | |
| RT-PCR and immunoblot of ECs overexpressing KLF2/KLF4 | Detected | Not detected (data now shown) | 30 | |
| Human mesenteric arterioles | IHC of artery section | Detected | Not detected | 21 |
| Human skeletal muscle arterioles | Immunofluorescence analysis of artery section | Detected | Not assessed | 19 |
| Human TD arteries | IHC of artery section | Detected | Detected | 19 |
| Human aorta | Immunoblot of homogenized artery | Not detected | Not detected | 31 |
| Human adipose tissue arteriole | Immunoblot of homogenized artery | Detected | Not detected | 31 |
| IHC of artery section | Detected | Not detected | 21,29 | |
| Human pulmonary artery | Immunoblot of homogenized artery | Detected | Detected | 32 |
IHC, immunohistochemistry; TD, thoracodorsal; TEM, transmission electron microscopy; RT-PCR, reverse transcription polymerase chain reaction.
α-Globin expression in vascular ECs
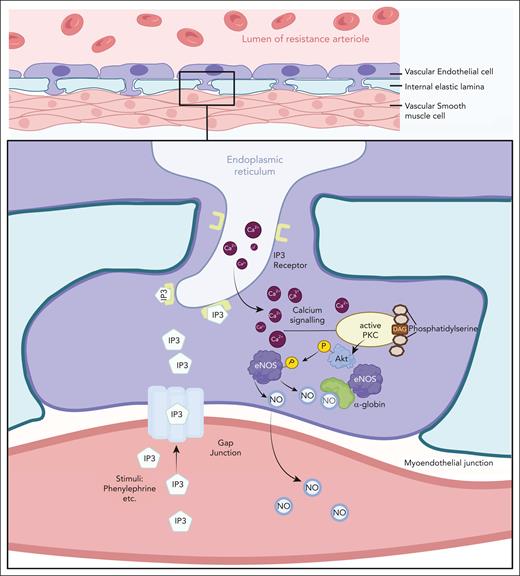
A pivotal study by Straub et al identified α-globin but not β-globin in an unbiased proteomic analysis of arteriolar myoendothelial junctions (MEJs).19 These specialized blood vessel EC structures traverse the basal lamina to contact VSMCs and mediate crosscommunication through specialized membrane channels (Figure 1).33 In response to VSMC signals, endothelial NO synthase (eNOS) generates NO, which diffuses through MEJs into VSMCs in which it activates soluble guanylyl cyclase to induce relaxation and vasodilation. Follow-up studies used immunoblotting and mRNA analysis to confirm that α-globin was expressed in isolated arteries from mouse and/or human skeletal muscle, mesentery, the heart, adipose tissue, and the lungs, particularly in resistance arterioles, which regulate blood pressure and regional blood flow19,21,29-32,34,35 (Table 1). Immunostaining showed that α-globin was enriched in arteriolar ECs and colocalized with MEJ markers. α-globin expression in blood vessels appears to be conserved in evolution, as evidenced by its detection in retinal vascular ECs of Antarctic icefish.36 Although some studies identified α-globin with β-globin protein by immunoblotting of whole mouse or human arteriole homogenates (Table 1), this approach is susceptible to false positive results from RBC contamination. In published studies to date, only α-globin has been detected in ECs by more specific methods, including immunostaining of whole arterioles and/or analysis of EC/VSMC cocultures19,32 (Table 1). A preprint reports the detection of both α-globin and β-globin in small arteries from human omental and adipose tissues using several orthogonal methods, which contrasts with a published study.29,37 Overall, it appears that some arterioles express α-globin exclusively, whereas others may express both HbA subunits. Notably, other globin proteins are also expressed in blood vessels. Cytoglobin and myoglobin are present in VSMCs of large and small arteries,29,38-41 and neuroglobin is expressed in pericytes that surround blood vessels.42
The MEJ facilitates communication between vascular ECs and smooth muscle cells to regulate vascular tone. Upon adrenergic stimulation of vascular smooth muscle, inositol triphosphate (IP3) generated by phospholipase C diffuses into ECs via gap junctions and triggers release of Ca2+ from the endoplasmic reticulum. Cytosolic Ca2+-mediated signaling leads to phosphorylation and activation of eNOS, which catalyzes the production of NO from L-arginine. NO diffuses through the MEJ into smooth muscle cells and activates guanylate cyclase, leading to an increase in cyclic guanosine monophosphate (cGMP), causing smooth muscle relaxation and vasodilation. EC α-globin can bind and degrade NO to limit its bioavailability. The figure was created using BioRender.
The MEJ facilitates communication between vascular ECs and smooth muscle cells to regulate vascular tone. Upon adrenergic stimulation of vascular smooth muscle, inositol triphosphate (IP3) generated by phospholipase C diffuses into ECs via gap junctions and triggers release of Ca2+ from the endoplasmic reticulum. Cytosolic Ca2+-mediated signaling leads to phosphorylation and activation of eNOS, which catalyzes the production of NO from L-arginine. NO diffuses through the MEJ into smooth muscle cells and activates guanylate cyclase, leading to an increase in cyclic guanosine monophosphate (cGMP), causing smooth muscle relaxation and vasodilation. EC α-globin can bind and degrade NO to limit its bioavailability. The figure was created using BioRender.
α-Globin inhibits arteriolar relaxation by degrading NO
α-globin in ECs coimmunoprecipitated with, and colocalized with, eNOS, which led investigators to test the effects of this pairing on local NO concentration.19 Hemoglobins can degrade NO via the heme iron-catalyzed reaction termed dioxygenation: [NO + O2 (Fe2+) → NO3− + (Fe3+)]. Thus, free HbA released from RBCs during intravascular hemolysis can cause vasoconstriction by degrading NO.43 Depletion of α-globin in isolated arterioles by short interfering RNAs caused increased NO diffusion across the vessel wall, decreased reactivity to the vasoconstrictor phenylephrine, and enhanced response to the vasodilator acetylcholine. These effects were abrogated by the NOS inhibitor NG-nitroarginine methyl ester (L-NAME), indicating that α-globin reduces the accumulation of eNOS-derived NO. In a follow-up study, germ line ablation of the α-globin gene Hba1 in mice resulted in dilated arteries in vivo, reduced vasoreactivity of isolated arterioles, and lowering of systemic blood pressure.21 These findings persisted after the Hba1−/− mice were reconstituted with normal hematopoietic stem cells, which corrected the α-globin deficiency in RBCs but not in ECs. Another study identified a peptide, termed HbαX, that interferes with the eNOS-α-globin interaction.22,31,34 Treatment of isolated mouse or human arterioles with HbαX caused enhanced NO signaling and reduced vasoreactivity, both of which were dependent on eNOS activity.22,31,34 Systemic administration of HbαX to mice caused lowering of blood pressure. Furthermore, EC-specific deletion of Hba1 in mice also reduced blood pressure.29 Together, these findings indicate that α-globin degrades NO in arteriolar ECs and reduces its diffusion across the MEJ, causing VSMC contraction and vasoconstriction. According to mathematical modeling, this mechanism is plausible if sufficient levels of oxygenated ferrous (Fe2+) α-globin and eNOS are present at the MEJ.44
Redox regulation of α-globin in vascular ECs
The dioxygenation reaction in ECs converts oxygenated Fe2+ α-globin to the ferric (Fe3+) form, which can no longer bind O2 and generates unstable, cytotoxic degradation products that are eliminated by protein quality control mechanisms.45 Several EC-expressed proteins can stabilize Fe3+ α-globin and recycle it to the Fe2+ form to support another round of dioxygenation. ECs express α-Hb–stabilizing protein (AHSP), a molecular chaperone that binds and stabilizes nascent free α-globin during HbA assembly in erythroid cells.46,47 Homozygous disruption of the Ahsp gene in mice accelerated the degradation of α-globin in ECs and phenocopied the vasomotor effects caused by loss of Hba1.21 Of relevance to EC biology, AHSP has an ∼100-fold greater affinity for Fe3+ α-globin than for the Fe2+ form.46-48 Moreover, binding of Fe3+ α-globin to AHSP facilitates its reduction by chemical and enzymatic reductase systems.49 Cytochrome b5 reductase 3 (CYB5R3), the major reductase for HbA in RBCs,50 forms a complex with eNOS and α-globin in ECs and reduces oxidized (Fe3+) α-globin to its Fe2+ form.19 Additionally, eNOS contains a reductase domain capable of reducing AHSP-bound Fe3+ α-globin approximately sixfold faster than did CYB5R3.21,51 In solution, purified α-globin bound eNOS or AHSP but not both together, indicating that different protein complexes containing α-globin exist in ECs.
α-Globin acts as an NO2− reductase under hypoxic conditions
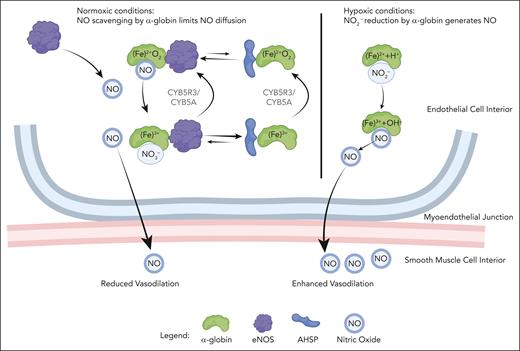
Hemoglobin-mediated degradation of NO consumes O2 and generates nitrate (NO3−) (Figure 2). Conversely, under hypoxic conditions, deoxy-HbA can act as a NO2− reductase, leading to the production of NO and consequent vasodilation [Fe2+ + NO2− + H+ → Fe3+ + NO + OH−]. After this reaction, EC-expressed CYB5R3 and/or eNOS may be capable of reducing Fe3+α-globin back to the Fe2+ form.52 Lysates from thoracodorsal arteries, which express α-globin, were found to reduce NO2− to form NO.29 In the same study, mice harboring an EC-specific deletion of Hba1 exhibited significant reductions in intracellular NO2− consumption, exercise capacity, and vasodilation after exposure to hypoxia. Of note, heme oxygenase 1 in ECs can degrade heme to generate CO, which has been shown to stimulate NO production and vasodilation.53-55 Although it is theoretically possible that α-globin can promote these effects in ECs by serving as a source of CO, levels of the protein may be too low to support this mechanism.
Molecular model for α-globin function in the MEJ. In the presence of O2, Fe2+ α-globin bound to eNOS degrades NO by dioxygenation, thereby enhancing vascular tone. eNOS and/or CYB5R3/CYB5A can reduce Fe3+ α-globin to the Fe2+ state to support another round of dioxygenation. Alternatively, under oxidizing conditions, Fe3+ α-globin may be released from eNOS and bind α-hemoglobin stabilizing protein (AHSP) to form a stable complex that cannot catalyze dioxygenation. When reducing conditions are restored, CYB5R3/CYB5A or eNOS can convert AHSP-bound Fe3+ α-globin to the Fe2+ form, which favors its transfer to eNOS for continued NO scavenging. During hypoxia, deoxy-Fe2+ α-globin can reduce NO2− to generate NO, thereby enhancing vasodilation. The figure was created using BioRender.
Molecular model for α-globin function in the MEJ. In the presence of O2, Fe2+ α-globin bound to eNOS degrades NO by dioxygenation, thereby enhancing vascular tone. eNOS and/or CYB5R3/CYB5A can reduce Fe3+ α-globin to the Fe2+ state to support another round of dioxygenation. Alternatively, under oxidizing conditions, Fe3+ α-globin may be released from eNOS and bind α-hemoglobin stabilizing protein (AHSP) to form a stable complex that cannot catalyze dioxygenation. When reducing conditions are restored, CYB5R3/CYB5A or eNOS can convert AHSP-bound Fe3+ α-globin to the Fe2+ form, which favors its transfer to eNOS for continued NO scavenging. During hypoxia, deoxy-Fe2+ α-globin can reduce NO2− to generate NO, thereby enhancing vasodilation. The figure was created using BioRender.
A model for α-globin function in arteriolar ECs
Biochemical and mouse genetic studies support a model for the regulation of NO levels by α-globin in arteriolar ECs (Figure 2). α-globin can either degrade NO by dioxygenation when O2 is present or generate NO from NO2− during hypoxia. With O2 present, Fe2+ α-globin bound to eNOS degrades NO by dioxygenation. The Fe3+ α-globin end product may be rapidly reduced by eNOS or CYB5R3 and CYB5A to generate Fe2+ α-globin, which can reengage in NO scavenging. Alternatively, Fe3+ α-globin can be released from eNOS and bind AHSP. Ferric (Fe3+) α-globin bound to AHSP is relatively stable but no longer capable of NO scavenging, which may favor vasodilation during conditions associated with oxidative stress, such as hypoxia or acidosis.46,49 Reductase-mediated conversion of AHSP-bound Fe3+ α-globin to the Fe2+ form favors its release from AHSP and transfer to eNOS for another round of dioxygenation. According to this model, NO scavenging in ECs is regulated by redox-dependent shuttling of α-globin between eNOS and AHSP. During hypoxia, deoxy Fe2+ α-globin can reduce NO2− to generate NO. In these ways α-globin, AHSP, and eNOS can sense oxidant stress and/or hypoxia and adjust NO levels to regulate arteriolar tone accordingly.
Regulation of α-globin expression in ECs
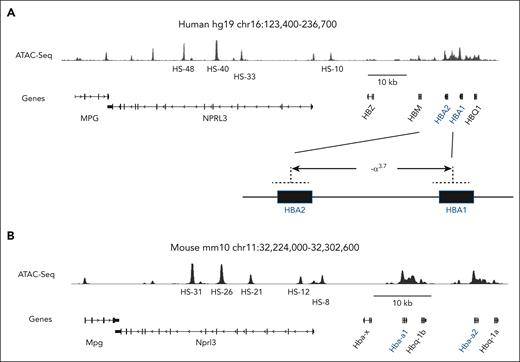
Defining how ostensibly erythroid-specific proteins, including α-globin and AHSP, are selectively expressed in arteriolar ECs represents an interesting problem in gene regulation. Adult-type human and mouse α-globin are both encoded by 2 tandem genes that arose from an ancestral gene duplication (Figure 3).56-58 High levels of expression during RBC development are driven by a multicomponent upstream enhancer with functional modules designated R1, R2, R3, Rm, and R4 in mice and multispecies conserved sequence R1-R4 in humans. In erythroid cells, the α-globin genes closest to the enhancer (mouse Hba1 or human HBA2) are expressed at twofold to fivefold higher levels than enhancer-distal Hba2 or HBA1, respectively.56-62 Whether the same is true for α-globin expression in ECs is not known.
The human and mouse extended α-globin loci. (A) Human and (B) mouse α-globin gene clusters are diagrammed. The major adult-expressed genes are indicated with blue font. Corresponding transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) peaks indicate open chromatin in adult RBC precursors. Enhancer modules associated with open chromatin (ATAC-seq signal) are indicated as multispecies conserved sequence (MCS) R1-R4 (human) or R1, R2, R3, Rm, and R4 (mouse). Human HBA2 is nearest to the enhancer and is expressed at twofold- to fivefold-higher levels than HBA1 in adult RBCs. Similarly, mouse Hba-a1 is nearest to the enhancer and is expressed at twofold- to threefold-higher levels than Hba-a2 in adult RBCs. The common α-thalassemia deletion, −α3.7 shown at the bottom of panel A generates a single α-globin fusion gene.
The human and mouse extended α-globin loci. (A) Human and (B) mouse α-globin gene clusters are diagrammed. The major adult-expressed genes are indicated with blue font. Corresponding transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) peaks indicate open chromatin in adult RBC precursors. Enhancer modules associated with open chromatin (ATAC-seq signal) are indicated as multispecies conserved sequence (MCS) R1-R4 (human) or R1, R2, R3, Rm, and R4 (mouse). Human HBA2 is nearest to the enhancer and is expressed at twofold- to fivefold-higher levels than HBA1 in adult RBCs. Similarly, mouse Hba-a1 is nearest to the enhancer and is expressed at twofold- to threefold-higher levels than Hba-a2 in adult RBCs. The common α-thalassemia deletion, −α3.7 shown at the bottom of panel A generates a single α-globin fusion gene.
α-globin expression during erythropoiesis is regulated by general and lineage-specific transcription factors including CCCTC-binding factor, GATA-binding factor 1 (GATA1), Krüppel-like factor 1 (KLF1), stem cell leukemia, E2-α, Lim-only 2, LIM domain-binding protein 1, and nuclear factor–erythroid 2, which bind DNA regulatory modules including promoters, enhancers, and boundary elements.56-58 These regulatory modules are associated with open chromatin that becomes more accessible during the course of erythroid differentiation.63 Endothelial and hematopoietic cells derive from a common mesodermal progenitor and express overlapping sets of related transcription factors.64 Thus, KLF2 and KLF4 can drive α-globin expression in ECs,30 likely by substituting for KLF1 present in erythroid cells. Similarly, EC-expressed GATA2 may substitute for GATA1 to facilitate α-globin expression, as occurs during early stages of erythropoiesis.65 Interestingly, α-globin is enriched in ECs with MEJ contacts to VSMCs and coculture with VSCMs induces α-globin expression in ECs in vitro.19,35 These findings suggest that unknown signals from VSMCs induce α-globin transcription in ECs. In erythroid cells, the α-globin and β-globin genes are regulated largely by identical sets of transcription factors, yet arteriolar ECs examined thus far appear to express only α-globin. This difference could be explained by several structural features of the extended α-globin locus that may render its resident genes more amenable to activation in nonerythroid cells.35,66-69
Diverse populations of ECs exist in different tissues and across different vascular beds.70-72 It will be interesting and informative to use single-cell RNA-sequencing approaches to identify those ECs that express α-globin and to study their chromatin structure using assay for transposase-accessible chromatin with high-throughput sequencing or related methods. This approach should produce insights into the transcription factors and DNA regulatory regions that facilitate the expression of α-globin, and other erythroid proteins including AHSP, in ECs.
Effects of EC-expressed α-globin on human vascular physiology
The physiological consequences and health implications of α-globin expression in human arterioles are unknown. A preclinical study showed that EC α-globin was upregulated in distal pulmonary arteries of individuals with idiopathic pulmonary hypertension and mice with hypoxia-induced pulmonary hypertension.32 Treatment of isolated mouse pulmonary arteries with HbαX to disrupt the α-globin–eNOS interaction caused increased NO signaling and enhanced acetylcholine-induced vasodilation. Hence, upregulation of α-globin in pulmonary vascular ECs may contribute to the pathophysiology of idiopathic pulmonary hypertension by accelerating NO degradation, and interfering with this process may produce therapeutic benefits.
Insights into the biology of EC-expressed α-globin in humans will likely be gained by studying individuals with α-thalassemia, who lack functional HBA genes.73,74 Humans and mice have 4 α-globin genes, 2 on each chromosome (Figure 3A). Loss of 1 gene, termed “silent carrier state,” has no clinical phenotype. Loss of 2 genes, termed “α-thalassemia trait,” causes microcytosis75 with minimal hemolysis. These conditions are usually well tolerated and are selected for in human evolution because they confer resistance to malaria.76,77 Loss of 3 α-globin genes usually causes “HbH disease” with accumulation of unstable, nonfunctional β-globin tetramer (HbH) and mild to moderate hemolytic anemia. Loss of all 4 α-globin genes causes Hb Barts hydrops fetalis syndrome, with accumulation of nonfunctional γ-globin tetramer (Hb Bart) and severe hemolytic anemia resulting in intrauterine death if untreated.78
Most α-thalassemia mutations are kilobase (kb)-scale deletions caused by unequal crossing over between duplicated regions of the α-globin locus, resulting in the elimination of 1 HBA gene or both in cis.75,79 A high-frequency 3.7-kb deletion (−α3.7) that converts HBA2 and HBA1 into a single HBA fusion gene is common in individuals of Southeast Asian and African descent (Figure 3A).80-82 Unequal crossovers can also produce a reciprocal product with 3 tandem α-globin loci. Although not selected for in evolution, this triplicated gene haplotype occurs infrequently, with heterozygotes and homozygotes having 5 or 6 total α-globin genes, respectively.
In principle, individuals with α-globin gene deletions in ECs should exhibit relative increases in eNOS-derived NO with consequent vasodilation and reduced blood pressure, whereas the opposite effects should occur in individuals with extra α-globin genes. Testing this hypothesis is subject to several potential confounders. First, the effects of specific HBA1 and/or HBA2 mutations on the levels of α-globin in ECs are not known. Second, loss of 3 or 4 α-globin genes causes intravascular hemolysis with RBC release of both free hemoglobin, which scavenges intravascular NO via dioxygenation, and arginase, which degrades l-arginine, the major precursor for NO biosynthesis.83 Both of these effects oppose the predicted consequences of α-globin deficiency in ECs. For example, preclinical studies indicate that disrupting the NO scavenging activity of α-globin in ECs may protect against idiopathic pulmonary hypertension.32 However, pulmonary hypertension is associated with symptomatic α-thalassemia and other hemoglobinopathies, likely due to depletion of intravascular NO by hemolysis.83-85 In this case, the deleterious effects of severe α-thalassemia on RBC stability are likely to outweigh potential benefits of α-globin deficiency in ECs, resulting in an overall reduction of vascular NO signaling and vasoconstriction. Third, different vascular beds are likely to exhibit distinct regulation by EC-expressed α-globin. Accordingly, the effects of α-globin deficiency may be manifested by tissue-specific differences in regional blood flow according to various stresses that may not occur at baseline. Fourth, blood pressure and regional blood flow are highly regulated through feedback loops and homeostatic mechanisms that may mask the effects of EC α-globin deficiency on vascular phenotypes.
Two studies have assessed the impact of α-globin gene loss on experimentally induced, NO-regulated vascular responses in humans with 1 or 2 α-thalassemia alleles, which is not usually associated with hemolysis. One study examined flow-mediated vasodilation (FMD), a noninvasive test that measures dilation of the brachial artery in response to increased blood flow following the release of transient ischemia, which is believed to be mediated by EC production of NO.86 Fifteen individuals with 1 or 2 defective α-globin genes exhibited increased FMD compared with 12 controls with 4 intact α-globin genes.23 Low FMD is associated with cardiovascular disease including myocardial infarction and stroke.87 Another study of 19 individuals with sickle cell disease (SCD) and loss of 1 or 2 α-globin genes showed increased NO-dependent, heat-induced microvascular blood flow in the skin compared with 18 individuals with SCD and 4 intact α-globin loci.24 The results of both studies are consistent with the hypothesis that α-globin deficiency in human vascular ECs causes accumulation of NO and vasodilation.
Human airway epithelial cells are reported to express α-globin and β-globin.88 A recent study showed that fractional exhalation of NO, a marker of lung inflammation, was increased in Black individuals who are homozygous for the −α3.7 deletion.89 Hence, α-globin in airway epithelial cells may function to scavenge NO, similar to what occurs in vascular ECs.
Of note, loss of 1 or 2 α-globin genes reduces the incidence and severity of some SCD complications, including cerebral vasculopathy, acute chest syndrome, leg ulcers, and kidney disease.90-94 These protective effects likely occur because α-globin deficiency in RBCs lowers the concentration of sickle hemoglobin (HbS) and inhibits its polymerization, leading to improved RBC rheology and reduced hemolysis. It is also possible that reduced α-globin expression in ECs contributes to the disease-modifying effects of α-thalassemia trait in SCD by enhancing the accumulation of eNOS-derived NO. However, proving this hypothesis will be challenging, considering the dominant effects of hemolysis on NO depletion and SCD pathophysiology.
Considering that α-thalassemia alleles are extremely common, with carrier frequencies as high as 80% to 90% in some populations,73 the effects of α-globin deficiency on NO-regulated vascular traits can be examined by gene-association studies. However, most large-scale, unbiased genome-wide association studies, including those that have examined blood pressure,95,96 interrogate single-nucleotide polymorphisms but not structural variants, which represent most α-thalassemia alleles. Common α-thalassemia deletions are not ascertained easily by DNA microarrays or by standard analysis of whole-genome sequencing data. However, several recent studies have examined associations between vascular traits and α-thalassemia alleles identified by HBA gene–specific assays (Table 2).
Association between α-globin gene copy number and NO-related vascular phenotypes
| Cohort and α-thalassemia genotype . | Phenotype . | Findings . | Reference . |
|---|---|---|---|
| 282 individuals with SCD ±1 or 2 −α3.7 HBA alleles | Pulmonary hypertension | No association | 28 |
| 623 African adolescents in Nairobi, Kenya ±1 or 2 −α3.7 alleles | Blood pressure | No association | 98 |
| 8947 AA in REGARDS cohort∗ | Ischemic stroke | No association | 25 |
| 9684 AA in REGARDS cohort | Hypertension | No association | 26 |
| 9908 AA in REGARDS cohort | Kidney disease | Prevalence of chronic and end-stage kidney disease increased with HBA gene copy number from 2 to 6 | 27 |
| 37 patients with SCA; 19 with 1 or 2 −α3.7 alleles | Microvascular blood flow to skin | No association at baseline. Increased blood flow after local heating associated with 1 or 2 −α3.7 alleles | 24 |
| 27 AA and Asian individuals: 15 with 1 or 2α-globin gene deletions | FMD | Increased FMD associated with 1 or 2 α3.7 alleles | 23 |
| Cohort and α-thalassemia genotype . | Phenotype . | Findings . | Reference . |
|---|---|---|---|
| 282 individuals with SCD ±1 or 2 −α3.7 HBA alleles | Pulmonary hypertension | No association | 28 |
| 623 African adolescents in Nairobi, Kenya ±1 or 2 −α3.7 alleles | Blood pressure | No association | 98 |
| 8947 AA in REGARDS cohort∗ | Ischemic stroke | No association | 25 |
| 9684 AA in REGARDS cohort | Hypertension | No association | 26 |
| 9908 AA in REGARDS cohort | Kidney disease | Prevalence of chronic and end-stage kidney disease increased with HBA gene copy number from 2 to 6 | 27 |
| 37 patients with SCA; 19 with 1 or 2 −α3.7 alleles | Microvascular blood flow to skin | No association at baseline. Increased blood flow after local heating associated with 1 or 2 −α3.7 alleles | 24 |
| 27 AA and Asian individuals: 15 with 1 or 2α-globin gene deletions | FMD | Increased FMD associated with 1 or 2 α3.7 alleles | 23 |
AA, African Americans; SCA, sickle cell anemia.
REGARDS (“Reasons for Geographic and Racial Differences in Stroke”)97 including 8947 to 9908 eligible AA with HBA copy number of 2 (4%), 3 (28%), 4 (67%), or ≥5 (1%).
Among 623 adolescents in Nairobi, Kenya, 55% had normal α-globin alleles, (αα/αα), 36% were heterozygous for the −3.7 kb α-globin gene deletion (−α3.7/αα), and 8% were homozygous (−α3.7/−α3.7).98 Between these groups, no differences in systolic or diastolic blood pressure were detected with 24-hour ambulatory monitoring. In agreement, analysis of a large American cohort termed “Reasons for Geographic and Racial Differences in Stroke (REGARDS)”97 showed no association between the number of α-globin gene mutations (mainly −α3.7) and the development of hypertension in 9683 Black adults (aged ≥45 years) followed-up over 13 years.26
In contrast to human studies showing no association between HBA2 deletion and blood pressure, biallelic disruption of the paralogous Hba1 gene in mice (see Figure 3) caused lowering of mean arterial blood pressure accompanied by substantial reductions in EC α-globin expression.21,29 In erythroid cells, the human and mouse α-globin genes closest to the enhancer (HBA2 and Hba1, respectively) are expressed at higher levels than the distal HBA1 or Hba2 genes (Figure 3).56-62 When the HBA2 gene is deleted, as occurs with the human −α3.7 allele, the output of the single remaining HBA fusion gene is increased by ∼1.8-fold.99 This compensatory increase does not occur with nondeletional mutations that inactivate HBA2 but leave its transcription intact, resulting in greater α-globin deficiency and more severe α-thalassemia phenotypes. Notably, both mouse models used to study the effects of α-globin deficiency on EC physiology harbored inactivating Hba1 deletions that left the associated promoter and first exon intact.29,100 These mutations are unlikely to cause compensatory increases in Hba2 expression and therefore may produce more severe α-globin deficiency in ECs than the human −α3.7 allele.101 This possibility can be tested by determining the effects of different α-thalassemia mutations on α-globin gene expression in ECs. In addition, it will be informative to perform genetic-association studies of blood pressure and other vascular phenotypes in Southeast Asian populations with high frequencies of different α-thalassemia alleles. Analyzing individuals with nondeletional HBA2 mutations may cause vascular effects more similar to those observed in mouse models with Hba1 deletions.
Two studies of African Americans in the REGARDs cohort analyzed the effects of α-globin gene dosage on stroke and kidney disease, both of which are influenced by NO levels.102-106 Analysis of 8947 individuals found no association between the −α3.7 allele and ischemic stroke.25 In contrast, a study of 9908 individuals with α-globin gene copies varying from 2 to 6 showed that a 1-copy increase was associated with 14% greater prevalence of chronic kidney disease and that the hazard of incident end-stage kidney disease was 32% higher for each additional copy of HBA present.27 This association was independent of SCD trait, another risk factor for kidney disease.107,108 The authors speculated that reductions in α-globin gene copy number in renal arteriolar ECs causes the accumulation of renoprotective NO.102-104
Conclusions and future
Identification of α-globin in MEJs within ECs of resistance arterioles and its physical and functional interactions with eNOS, AHSP, and CYB5R3/CYB5 reveal novel facets of vascular biology and hemoglobin biochemistry. Multiple studies at the molecular, cellular, and systemic levels indicate that arteriolar EC-expressed α-globin regulates NO levels and vascular contractility according to the cellular redox environment. These findings present new opportunities to understand vascular reactivity, blood pressure regulation, and regional blood flow, and perhaps manipulate them therapeutically.
Most likely, the expression of α-globin in blood vessel ECs represents an evolutionary adaptation to fine tune vascular reactivity according to local environmental influences. Hemoglobinopathy traits, including α-thalassemia, are selected for in evolution because they confer resistance to malaria.109 The associated mechanism likely originates from RBC-intrinsic effects that interfere with the malarial life cycle.76,77 However, it is interesting to consider that the pathophysiology of malaria is exacerbated by NO deficiency.110-114 Thus, loss of 1 or 2 α-globin genes in ECs may also confer resistance to malaria by enhancing NO levels in the blood vessel wall.
An important future challenge is to understand more fully the overall scope and magnitude of α-globin activities in blood vessels and their relative importance to human health. Comprehensive, quantitative analysis of EC α-globin expression in different tissues combined with future genetic-association studies of vascular health-related traits and α-thalassemia should be synergistic. For example, genetic-association studies showed that α-globin gene dosage correlates inversely with the development of renal failure in an African American cohort.27 It is now important to replicate these findings in other cohorts and to study more closely α-globin expression and function in renal blood vessels. Individuals who are missing 3 or 4 α-globin genes are likely to manifest the most profound deficiency of EC α-globin, which predicts NO accumulation, although this effect is likely to be negated by concomitant intravascular NO depletion from hemolysis. Thus, it may be informative to study blood vessel regulation and associated health risks in individuals with severe α-thalassemia who have been treated with allogeneic bone marrow transplantation, which restores normal α-globin expression to RBCs but not ECs.
Overall, studies of α-globin expression in vascular ECs highlight the concept that hemoglobins and related proteins are not simply O2 transporters. In this regard, it is important to consider that EC-expressed α-globin regulates NO levels in concert with other globin proteins expressed in blood vessels. Most current studies indicate that arteriolar ECs express α-globin but not β-globin, although a manuscript available through medRxiv shows that that β-globin is coexpressed in some human vascular ECs.37 Moreover, VSMCs express cytoglobin and myoglobin, which, like α-globin, can eliminate or generate NO via dioxygenation and NO2−-reduction reactions, respectively.29,38-41 Thus, NO homeostasis in blood vessels is regulated by the net expression of multiple globin proteins and their capacity for reduction by endogenous enzyme systems, both of which are likely to vary across different vascular beds and respond uniquely to physiological stresses.
Acknowledgments
The authors thank Lance Palmer for assistance with figure preparation and Hans Ackerman, Tom Coates, Doug Higgs, and Brant Isakson for suggestions on the manuscript.
This work was funded by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants R01HL165798, R01HL156647, and U01HL163983 (M.J.W.), NIH, National Institute of General Medical Sciences grant R00GM141268 (P.S.A.),The Assisi Foundation (M.J.W.), and the American Lebanese Syrian Associated Charities (ALSAC).
Authorship
Contribution: P.S.A., S.B., and M.J.W. wrote and approved the manuscript.
Conflict-of-interest disclosure: M.J.W. is a consultant for Novartis, Vertex Therapeutics, and bluebird bio. The remaining authors declare no competing financial interests.
Correspondence: Mitchell J. Weiss, Department of Hematology, St. Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105-3678; email: mitch.weiss@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal