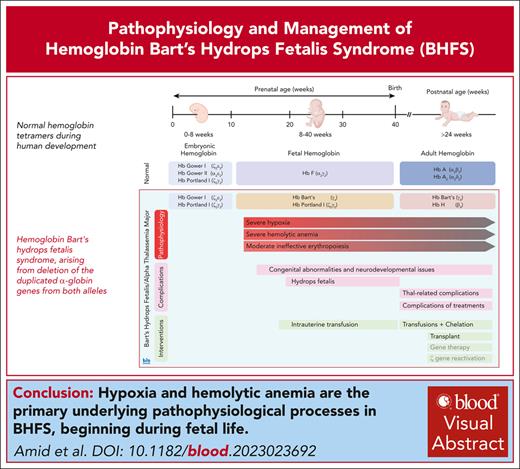
Visual Abstract
Hemoglobin Bart’s hydrops fetalis syndrome (BHFS) represents the most severe form of α-thalassemia, arising from deletion of the duplicated α-globin genes from both alleles. The absence of α-globin leads to the formation of nonfunctional hemoglobin (Hb) Bart’s (γ4) or HbH (β4) resulting in severe anemia, tissue hypoxia, and, in some cases, variable congenital or neurocognitive abnormalities. BHFS is the most common cause of hydrops fetalis in Southeast Asia; however, owing to global migration, the burden of this condition is increasing worldwide. With the availability of intensive perinatal care and intrauterine transfusions, an increasing number of patients survive with this condition. The current approach to long-term management of survivors involves regular blood transfusions and iron chelation, a task made challenging by the need for intensified transfusions to suppress the production of nonfunctional HbH–containing erythrocytes. Although our knowledge of outcomes of this condition is evolving, it seems, in comparison to individuals with transfusion-dependent β-thalassemia, those with BHFS may face an elevated risk of complications arising from chronic anemia and hypoxia, ongoing hemolysis, iron overload, and from their respective treatments. Although stem cell transplantation remains a viable option for a select few, it is not without potential side effects. Looking ahead, potential advancements in the form of genetic engineering and innovative therapeutic approaches, such as the reactivation of embryonic α-like globin gene expression, hold promise for furthering the treatment of this condition. Prevention remains a crucial aspect of care, particularly in areas with high prevalence or limited resources.
Introduction
α-thalassemia is the most common form of inherited anemia in the world.1 This is because carriers of α-thalassemia are protected from severe forms of Plasmodium falciparum malaria, providing a clear example of evolutionary selection.2,3 Consequently, α-thalassemia is common in ethnic groups originating from areas where malaria is, or was, previously endemic.4
In the 1970s and 1980s, the genetics of α-thalassemia was largely solved providing 1 of the first examples of a human genetic disease to be understood at the molecular level.5 From previous studies of families with structural variants of the α-globin proteins, it was suggested that normally individuals have 2 α-globin genes on each allele with a genotype of αα/αα. This was subsequently shown to be true when a phage-λ contig spanning the human α-globin cluster was characterized.6 This also revealed that the common allele had arisen via duplication of a ∼4–kilobase segment of DNA containing the α-globin gene. This ultimately explained how 2 common deletions of the α cluster (−α3.7 and −α4.2) had arisen via homologous recombination, again, providing a paradigm for understanding the molecular basis of other human diseases caused by variation in gene copy number.5,7
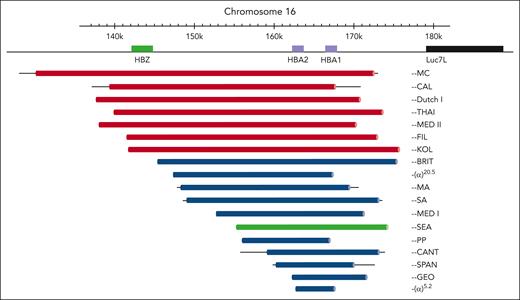
Two severe forms of α-thalassemia were known to occur in regions where it was common. The first to be recognized was hemoglobin H (HbH) disease,5 in which parents who are carriers of α-thalassemia give rise to individuals with a moderate hemolytic anemia with hypochromic and microcytic red blood cells associated with excess β-globin chains, which form the tetrameric HbH (β4). Such individuals could be easily identified by the presence of insoluble HbH inclusions in a proportion of red blood cells. The second condition found in association with α-thalassemia (which is the subject of this review) is a lethal form of fetal anemia associated with excess γ-globin chains forming another Hb called Hb Bart’s (γ4).5 By using soluble hybridization, 2 studies showed that affected fetuses had inherited no α-globin genes at all, and subsequently the most common deletion underlying these severe forms of α-thalassemia (referred to as the SEA deletion or −−SEA) was characterized8 (Figure 1).
Deletions of 2 α-genes giving rise to α0-thalassemia. Deletions that involve the ζ-globin gene (HBZ) are depicted in red. By far the most common α0-thalassemia deletion involved in Hb Bart’s hydrops fetalis (−−SEA deletion) is depicted in green. Bold lines indicate sequences known to be deleted. Thin lines indicate regions of uncertainty around the breakpoints. Reproduced with permission, with minor modifications, from Harteveld and Higgs.9
Deletions of 2 α-genes giving rise to α0-thalassemia. Deletions that involve the ζ-globin gene (HBZ) are depicted in red. By far the most common α0-thalassemia deletion involved in Hb Bart’s hydrops fetalis (−−SEA deletion) is depicted in green. Bold lines indicate sequences known to be deleted. Thin lines indicate regions of uncertainty around the breakpoints. Reproduced with permission, with minor modifications, from Harteveld and Higgs.9
Together, this work spanning ∼20 years solved the common patterns of genetic inheritance of α-thalassemia at the molecular level. Normally, individuals have 4 intact α-globin genes (αα/αα genotype). Carriers of α-thalassemia have either a single-gene deletion (α+-thal; −α/αα genotype) or a 2-gene deletion in cis (α0-thal; −−/αα genotype). Homozygotes for the α+-thal deletions (−α/−α genotype) have a mild hypochromic microcytic anemia. Individuals with HbH disease are compound heterozygotes for α0-thal and α+-thal deletions (−−/−α genotype) and infants with Bart’s hydrops fetalis syndrome (BHFS, or α-thalassemia major [ATM]) are homozygous for α0-thal deletions (−−/−− genotype). In subsequent years >200 alleles, including deletions and point mutations in the α-globin cluster, and their interactions, have been described and have illustrated many of the principles by which gene expression can be perturbed in human genetic disease; these have been summarized elsewhere.9 In addition, this knowledge has now been applied worldwide for molecular diagnosis, including prenatal screening for α-thalassemia.
In this article, we review how our knowledge of the BHFS has developed since it was first described, and discuss unique challenges and possible strategies for the management of this clinically important inherited condition. In this review, we use the terms “BHFS” to refer to this condition at the prenatal and perinatal stages and “ATM” for long-term survivors and their management.
Hb Bart’s hydrops fetalis syndrome
Shortly after Hb Bart’s was first characterized in St Bartholomew’s Hospital in London in 1958,10 the association of hydrops fetalis with a large amount of Hb Bart’s was described in an Indonesian infant who was stillborn.11 With observation of further cases, this condition became known as BHFS. Subsequently, studies over the next 2 decades offered a clearer picture of the underlying genetic basis for BHFS as well as its clinical and pathological features.
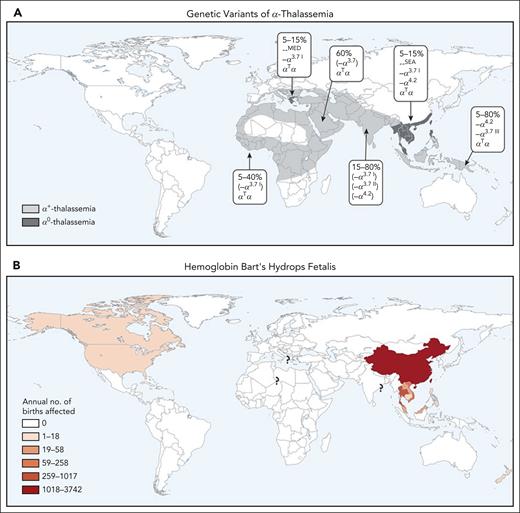
Although many different genotypes have been described, BHFS most frequently results from the inheritance of the −−SEA/−−SEA genotype, and, less frequently, from compound heterozygosity of the −−SEA with the slightly larger −−FIL deletion (−−SEA/−−FIL). The frequency of these α0-thal variants reaches up to 5% in certain regions of Southeast Asia. Consequently, it has been estimated that each year >400 pregnancies may be affected by BHFS in Thailand alone, with even more pregnancies being at risk for this condition in China (Figure 2).1,12 Although BHFS is most commonly seen in families from Southeast Asia, it has also been identified in many different population groups,13 and thousands of pregnancies are at risk each year worldwide. With increasing mobility and migration, many such families are now living in areas beyond Southeast Asia and, consequently, α-thalassemia syndromes, including BHFS, now represent a global health issue alongside other hemoglobinopathies such as β-thalassemia and sickle cell disease.1
Worldwide distribution of α-thalassemia. Predominant global distribution of α-thalassemia mutations (including α0 deletions) (A) and Hb Bart’s hydrops fetalis (B). It should be noted that, as a result of migration, these genotypes are now widely present in many other areas. Reproduced with permission, with minor modifications, from Piel and Weatherall.1
Worldwide distribution of α-thalassemia. Predominant global distribution of α-thalassemia mutations (including α0 deletions) (A) and Hb Bart’s hydrops fetalis (B). It should be noted that, as a result of migration, these genotypes are now widely present in many other areas. Reproduced with permission, with minor modifications, from Piel and Weatherall.1
From the earlier observations, it became clear that infants with BHFS die either in utero or soon after birth with features of nonimmune hydrops fetalis. Affected fetuses generally have severe anemia, gross edema, and serous effusions of peritoneal, pleural and pericardial cavities. There is marked hepatomegaly and a variable degree of splenomegaly due to extramedullary hematopoiesis. The significant expansion of the erythroid compartment and generalized deposition of hemosiderin indicate severe hemolysis, and the resulting severe anemia leads to marked cardiac hypertrophy and underdeveloped pulmonary tissue. In addition, hydrops fetalis leads to a higher incidence of maternal complications including mirror syndrome (maternal edema, hypertension, and proteinuria), labor dystocia, and postpartum hemorrhage. Curiously, many infants are observed to have other developmental abnormalities such as limb defects and abnormalities of the male genitourinary system, a finding that is uncommon in other forms of nonimmune hydrops fetalis.1,15-20
Many aspects of the underlying pathophysiology of this presentation most likely occur because, unlike the β-hemoglobinopathies, BHFS has the potential to cause anemia and tissue hypoxia from the earliest stages of fetal development, although the exact timing of this is not fully established. The α-globin genes are normally expressed in both primitive and definitive erythropoiesis.21 In primitive erythropoiesis, in an embryo with BHFS with at least 1 intact ζ-globin gene, the embryonic Hbs Gower I (ζ2ϵ2) and Portland I (ζ2γ2) are produced but, because the affected fetus does not have any α-globin genes, no Hb Gower II (α2ϵ2) is formed. It is likely that Hb Gower I and Hb Portland I are sufficient to provide adequate oxygenation during primitive erythropoiesis. However, with the start of definitive erythropoiesis, by ∼8 weeks of gestation, the embryonic genes are normally switched off.22 Because the fetus with BHFS does not have any intact α-globin genes, physiologically normal Hb F (α2γ2) is not formed and, instead, the excess γ-globin chains form Hb Bart’s (γ4). Hb Bart’s does not display any Bohr effect and has an extremely high oxygen affinity, making it unsuitable for oxygen delivery.23 This leads to a severe impairment of oxygen delivery throughout fetal life, usually leading to the development of severe hydrops fetalis in the second and third trimesters of pregnancy.
Surviving the turbulent prenatal life
Early studies presented BHFS as a universally fatal condition, although the development of infants with BHFS in utero seemed to have a variable course. Although some fetuses had severe hydrops and early intrauterine demise, remarkably, others survived to term despite Hb Bart’s having no capacity to supply oxygen to fetal tissue. The explanation for this is that fetuses with BHFS continue to express, to some extent, the embryonic ζ-globin gene leading to the persistence of Hb Portland I (ζ2γ2) beyond the period of primitive erythropoiesis (Figure 3). The small amount of Hb Portland I enables oxygen transport because it has a normal physiological oxygen dissociation curve.17,18,24 Furthermore, some fetuses were noted to have a higher proportion of Hb Portland I than others, which might explain the variable clinical course. Indeed, the first 2 reported long-term survivors with BHFS were noted to have relatively high levels of Hb Portland I at the time of delivery.25,26 These fascinating observations were best summarized by David Weatherall: “Surely any student of oxygen transport must be intrigued by a situation in which a fetus can survive until term despite the fact that it carries more than 80% of a Hb, which, as far as is known, is physiologically useless!”27
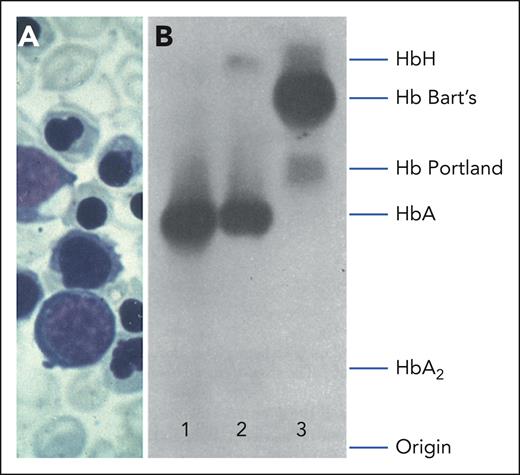
Clinical and hematological features of Hb Bart’s hydrops fetalis. (A) Peripheral blood smear of an infant showing immature red cell precursors, hypochromic microcytosis, and anisopoikilocytosis. (B) Starch gel electrophoresis of hemolysates from a normal adult (lane 1); an individual with HbH disease (lane 2), and the cord blood of an infant with Hb Bart's hydrops fetalis (lane 3). Reproduced with permission, with minor modifications from Higgs13 and Pressley et al.14
Clinical and hematological features of Hb Bart’s hydrops fetalis. (A) Peripheral blood smear of an infant showing immature red cell precursors, hypochromic microcytosis, and anisopoikilocytosis. (B) Starch gel electrophoresis of hemolysates from a normal adult (lane 1); an individual with HbH disease (lane 2), and the cord blood of an infant with Hb Bart's hydrops fetalis (lane 3). Reproduced with permission, with minor modifications from Higgs13 and Pressley et al.14
The next chapter in the care of patients with BHFS started in the mid-1990s with implementation of successful intrauterine transfusions28 and, then, bone marrow transplantation of survivors.29 With ever-improving perinatal care over the past 4 decades, there have been an increasing number of reports describing infants with BHFS who were first recognized at birth because they remarkably survived without intrauterine transfusion, and others who were identified earlier in development and were treated with intrauterine transfusion.30-34 Collectively, these observations demonstrated the benefit of early intrauterine transfusion in improving perinatal survival. Nevertheless, all surviving individuals have required lifelong blood transfusions with iron chelation, but few have received a successful bone marrow transplant freeing them of blood transfusion.31,35
The observation of improved survival of affected fetuses with the availability of intrauterine transfusions and advances in prenatal care has potentially opened a new era in the management of these patients. However, because more patients have survived beyond the neonatal period, some concerns have come to light. The first issue was the fact that many of the children suffered from neurodevelopmental delay, often quite severe, whereas others did meet normal developmental milestones.36 These observations, along with high rate of other congenital abnormalities (discussed hereafter) raised the ethical dilemma as to whether intrauterine interventions are justified.13 To better understand the long-term outcome of survivors, we initiated an international registry of survivors in 2013.31 Currently, there are 90 patients in the Bart’s Hydrops Fetalis Syndrome Registry, with ages ranging from birth to 36 years. The registry contains patients with significant neurodevelopmental delay in those who received intrauterine transfusion and those who did not, although there may have been significant variation in the timing and frequency of intrauterine transfusions, given the retrospective and international nature of the registry. Furthermore, neurodevelopmental delay remained in 3 of 10 patients who had successfully received a transplantation, indicating that neurodevelopmental delay may not always be recoverable by early intervention. The cause of unrecoverable neurodevelopmental delay in these patients may stem from the observation that affected fetuses have reduced brain weight relative to gestational age from 8 weeks onward.13 A more recent study reported improved early neurodevelopmental outcomes in those who have received ≥2 intrauterine transfusions compared with those with none or 1 intrauterine transfusion.34 Whether such promising results will be maintained as patients get older remains to be seen. The authors have personal experience with adolescents and young adult patients who had achieved early developmental milestones but have demonstrated neurodevelopmental challenges at an older age.
The second concern has been the observation that long-term survivors with BHFS have a high rate of congenital defects regardless of whether they have received intrauterine transfusions, even in fetuses without hydrops fetalis.31,32,37,38 The most common malformations are genitourinary defects and limb abnormalities. Indeed, genitourinary defects are seen in almost all affected males to a variable degree, ranging from undescended testes or mild hypospadias to severe forms of hypospadias with chordee, micropenis, and ambiguous genitalia.39-41 Limb abnormalities are also fairly common and vary in severity.31,32,42
Although not definitive, it has been suggested that both urogenital and limb malformations result from fetal hypoxia during the critical period of early development. Interestingly, the urogenital abnormalities are predominantly observed in males suggesting that the pathophysiology of these anomalies arises after the development of the testes with subsequent differentiation of Wolffian ducts, regression of Müllerian ducts, and masculinization of the urogenital sinus and external genitalia beginning at the eight to ninth weeks of gestation.43 This is of particular interest because this coincides with the initiation of hypoxia in fetuses with BHFS and suggests hypoxia at this stage may also contribute to urogenital abnormalities. Furthermore, the process of masculinization is completed by the 14th week of gestation.43 Even if diagnosed at this young stage, fetuses are too small for current interventions before the development of these anomalies.
Of note, congenital abnormalities at the more extreme end of the spectrum are likely to have their origins as early as 8 to 12 weeks of gestation, before significant fetal anemia is thought to manifest in BHFS.44 This raises the question whether other processes may contribute to the development or severity of these anomalies. One possibility is that the −−SEA deletion may have unpredictable effects on expression of other genes within and around the α-globin locus through ectopic interactions of its regulatory elements. Although these interactions have been characterized in erythroid cells,45 the role of these elements and the associated structural organization of chromatin in nonerythroid cells is unknown. The −−SEA deletion might cause unforeseen changes in expression of genes involved in organ development.46
It should be emphasized that although some of these malformations are mild and may not have a significant impact on patients, others may indeed have a significant effect on the individual’s function or quality of life. For example, most cases of hypospadias are correctable with a single operation but the more severe forms with chordee often require staged operations. The success of these operations depends on the experience of the surgeon, and for some individuals, multiple operations are required.47
These observations highlight the fact that BHFS has specific challenges that are unlike similar diseases for which intrauterine therapy is now routinely applied. Such challenges include the effects of nonfunctional Hb in addition to anemia and the potential for developmental perturbation arising directly from the deletion at an early stage of development. The full impact of these early embryonic events is not yet understood as they manifest before it is possible to detect with screening or to deliver intrauterine therapy. In some cases, some of these effects are unrecoverable, even with curative therapy.
Long-term management of survivors: more questions than answers
It is tempting to assume that once a newborn with ATM has successfully navigated the antenatal and perinatal period, their clinical care would follow a relatively straightforward course. Indeed, although the overall long-term management of ATM is based on similar principles to those of transfusion-dependent β-thalassemia (TDT-β; ie, chronic transfusion and iron chelation), there are some major differences and challenges that should be highlighted (Table 1).
Summary of the management of patients with Hb Bart’s hydrops fetalis/ATM
| Recommendations . | Comments . |
|---|---|
| Screening and prenatal diagnosis | |
| Carrier screening is recommended for couples with genetic ancestry from populations with high prevalence of α0-thal deletions. |
|
| Prenatal diagnosis should be offered to all at-risk pregnancies. |
|
| Prenatal care of affected pregnancies | |
| Couples with affected pregnancies should receive detailed and nondirective counseling regarding all aspects of prenatal and long-term outcomes, as well as available therapeutic options. |
|
| Couples with affected pregnancies should be referred to centers with expertise in prenatal care for BHFS. |
|
| IUT should be initiated as soon as technically possible. |
|
| The goal of IUT is to resolve hydrops and/or prevent development of hydrops, allowing for normal growth and development of the fetus, and preventing maternal complications. |
|
| Delivery and neonatal care | |
| Delivery should be planned at a tertiary care center. |
|
| Resuscitation and management of acute illness should be initiated immediately after birth. |
|
| Transfusions should be started as soon as possible. |
|
| Assessments and management for associated congenital anomalies and neurological evaluation should be offered. |
|
| Long-term management of patients with ATM | |
| Infants should be referred to a comprehensive thalassemia clinic for regular transfusion therapy. |
|
| Monitoring and treatment of iron overload should be initiated after 1 y of age, balancing their benefit and side effects. |
|
| Patients should be closely monitored for complications of anemia, ineffective erythropoiesis, chronic hemolysis, regular transfusion, and chelation therapy. |
|
| Management of congenital abnormalities |
|
| Management of neurocognitive compromise |
|
| Curative therapy should be considered at an early age. |
|
| Recommendations . | Comments . |
|---|---|
| Screening and prenatal diagnosis | |
| Carrier screening is recommended for couples with genetic ancestry from populations with high prevalence of α0-thal deletions. |
|
| Prenatal diagnosis should be offered to all at-risk pregnancies. |
|
| Prenatal care of affected pregnancies | |
| Couples with affected pregnancies should receive detailed and nondirective counseling regarding all aspects of prenatal and long-term outcomes, as well as available therapeutic options. |
|
| Couples with affected pregnancies should be referred to centers with expertise in prenatal care for BHFS. |
|
| IUT should be initiated as soon as technically possible. |
|
| The goal of IUT is to resolve hydrops and/or prevent development of hydrops, allowing for normal growth and development of the fetus, and preventing maternal complications. |
|
| Delivery and neonatal care | |
| Delivery should be planned at a tertiary care center. |
|
| Resuscitation and management of acute illness should be initiated immediately after birth. |
|
| Transfusions should be started as soon as possible. |
|
| Assessments and management for associated congenital anomalies and neurological evaluation should be offered. |
|
| Long-term management of patients with ATM | |
| Infants should be referred to a comprehensive thalassemia clinic for regular transfusion therapy. |
|
| Monitoring and treatment of iron overload should be initiated after 1 y of age, balancing their benefit and side effects. |
|
| Patients should be closely monitored for complications of anemia, ineffective erythropoiesis, chronic hemolysis, regular transfusion, and chelation therapy. |
|
| Management of congenital abnormalities |
|
| Management of neurocognitive compromise |
|
| Curative therapy should be considered at an early age. |
|
For more details, please refer to Amid et al.48
ECHO, echocardiography; GU, genitourinary system; IUT, intrauterine transfusions; MRI, magnetic resonance imaging; US, ultrasound.
The first challenge arises from the need to commence chronic transfusions immediately after birth, in infants that most probably have already received intrauterine transfusions.32,49 This is in contrast to even the most severe forms of TDT-β, for which patients usually do not require transfusions in the first few months of life. In addition to the significant burden of this early initiation of regular transfusions on the quality of life of the affected infants and their caregivers, this will inevitably lead to early transfusional iron overload.32,49 Here, even the most adept clinician will be challenged by the conundrum of tolerating early iron overload versus starting iron chelation in an infant, because each of these approaches can be associated with irreversible long-term side effects.50-52
After early infancy, the pathophysiology of ATM is driven by the formation of erythroid cells that are almost exclusively composed of HbH (β4), and a small fraction of Hb Bart’s (γ4). Similar to Hb Bart’s, HbH does not exhibit any Bohr effect, has a very high oxygen affinity, and does not participate in oxygen delivery.53,54 Thus, these cells, which we refer to as “HbH cells,” are essentially useless. This is in direct contrast to TDT-β, in which the circulating endogenous cells are composed of mostly HbF and small amounts of HbA2, and are functional oxygen transporters. Similarly, in HbH disease, the majority of intracellular Hb is normal adult Hb (HbA).
Another unique aspect of ATM is its prominent hemolytic phenotype in comparison with the degree of ineffective erythropoiesis. HbH, a relatively unstable Hb, predominantly precipitates in “senescent” cells, which are then trapped within the spleen microvasculature because of their rigidity. Here, erythrophagocytosis leads to considerable extravascular hemolysis and splenomegaly. Hence, the predominant mechanism of anemia in ATM is hemolysis, in contrast to ineffective erythropoiesis that is observed in β-thalassemia.13,55
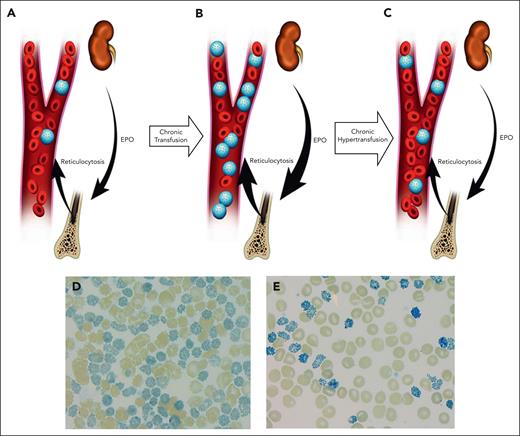
These unique pathophysiologic features have important implications for the management of individuals with ATM. It has been demonstrated that patients with ATM show features of hypoxia, severe hemolysis, and marked reticulocytosis even when regularly transfused using the protocols developed for TDT-β.55,56 Because circulating endogenous HbH cells are considered nonfunctional, the “total” Hb in patients with ATM on chronic transfusions overestimates the oxygen carrying capacity of the blood, leading to a disproportionately elevated level of erythropoietin. Over time, the erythropoietin-driven increased erythropoiesis further elevates circulating HbH levels and extravascular hemolysis, thus perpetuating the cycle (Figure 4).
Schematic representation of different transfusion strategies in ATM underlying its unique pathophysiology. In patients who received conventional transfusion (A), over time, preserved reticulocytosis will lead to an increased proportion of endogenous erythrocytes that exclusively contain nonfunctional HbH (HbH cells; shown as “blue golf ball” cells) (B). In this setting, despite a stable pretransfusion Hb, the proportion of functional Hb is decreased. The tissue hypoxia will subsequently lead to further increase of erythropoietin (EPO) and expansion of bone marrow erythron. Furthermore, the increased circulating HbH cells will lead to increased extravascular and intravascular hemolysis. (C) In contrast, a more aggressive transfusion (hypertransfusion) aimed at reduction of HbH and increase in functional Hb (non-HbH) will result in improved tissue oxygenation, reduction in EPO, reduced erythropoiesis, and diminished hemolysis. (D- E) Peripheral blood smear supravital stain of an adolescent with ATM, before (HbH, 64%) and after 4 cycles of exchange transfusion and subsequent hypertransfusion (HbH, 14%), respectively. Note the significant reduction in the HbH cells in peripheral blood smear, leading to improved functional Hb.
Schematic representation of different transfusion strategies in ATM underlying its unique pathophysiology. In patients who received conventional transfusion (A), over time, preserved reticulocytosis will lead to an increased proportion of endogenous erythrocytes that exclusively contain nonfunctional HbH (HbH cells; shown as “blue golf ball” cells) (B). In this setting, despite a stable pretransfusion Hb, the proportion of functional Hb is decreased. The tissue hypoxia will subsequently lead to further increase of erythropoietin (EPO) and expansion of bone marrow erythron. Furthermore, the increased circulating HbH cells will lead to increased extravascular and intravascular hemolysis. (C) In contrast, a more aggressive transfusion (hypertransfusion) aimed at reduction of HbH and increase in functional Hb (non-HbH) will result in improved tissue oxygenation, reduction in EPO, reduced erythropoiesis, and diminished hemolysis. (D- E) Peripheral blood smear supravital stain of an adolescent with ATM, before (HbH, 64%) and after 4 cycles of exchange transfusion and subsequent hypertransfusion (HbH, 14%), respectively. Note the significant reduction in the HbH cells in peripheral blood smear, leading to improved functional Hb.
Considering this preserved erythropoiesis and high level of nonfunctioning HbH cells in the circulation, a more aggressive transfusion regimen aimed at achieving optimal “functional” (ie, non-HbH) Hb concentration is required to improve tissue oxygenation, decrease erythropoiesis, and to diminish the significant hemolysis in these patients.55,56 Unfortunately, although this intensive transfusion strategy is effective in improving the hematological features of ATM, it is also associated with a significant increase in transfusional iron burden, which will inevitably require an escalation of iron chelation therapy.32,56 This may pose significant challenges with treatment costs, patient adherence, and, most importantly, complications of iron overload or chelator-related side effects.
Preventing both suboptimal transfusions and iron overload can be challenging in the long term. Not surprisingly, patients with ATM demonstrate a higher rate of clinical complications than their TDT-β counterparts, including a higher incidence of endocrinopathies, bone disease, growth delay, and silent ischemic infarcts of the white matter of the brain. Similarly, a higher number of patients with ATM demonstrate side effects that can be attributed to either the earlier initiation of iron chelation or a need for higher doses of chelation medications (eg, high-frequency hearing loss, poor growth, and bone disease).31,32,56 We have also observed metaphyseal dysplasia and nephrocalcinosis in our patients. Furthermore, because most patients with ATM are still in their childhood, with the oldest patient being in their mid-30s, many of the less common complications that typically arise with the advancement of age in patients with thalassemia (eg, extramedullary hematopoietic pseudotumor) have not been reported in these patients. It remains to be seen whether these complications are more common in patients with ATM if the ineffective erythropoiesis is not fully suppressed. Therefore, judicious monitoring, thorough surveillance testing, and implementing timely interventions are paramount for preventing clinical complications in these patients.
Clearly, other treatment approaches are needed to improve the long-term management of these patients. One possible approach is to use exchange transfusions, with the intent to reduce the undesired HbH cells in peripheral circulation and improve functional Hb levels, while also decreasing net transfusional iron loading (Figure 4).55,57 Although an attractive option, exchange transfusions require specialized resources and are associated with a considerably higher use of packed red cell units. Further studies are required to determine the effectiveness and possible complications of this approach.
Another intervention commonly used in patients with TDT-β or HbH disease is splenectomy. Splenectomy can improve Hb concentrations in both individuals with β-thalassemia58,59 and HbH disease.60 However, not only can splenectomy be associated with a variety of long-term complications61 but also, specifically in patients with ATM, removing the spleen would likely prolong the lifespan of nonfunctional HbH cells. This would not lead to any physiological improvement and instead would make effective transfusion even more challenging. The authors are aware of 1 patient with ATM who has undergone splenectomy, resulting in no clinical benefit and the development of a life-threatening thromboembolic complication.57 Thus, at this point, splenectomy is not recommended in patients with ATM.
Recently, there have been significant advances toward the development of novel therapeutic options in patients with either β-thalassemia or HbH disease, mainly through improving the survival of red blood cells or restoring normal erythropoietic differentiation.62,63 However, given that endogenous HbH cells lack any function in ATM, these novel options would not offer benefit for these patients.
Curative therapy for ATM: a promising option but not a definitive solution
Considering the challenges that are associated with the management of patients with ATM and the higher rate of clinical complications, curative therapy for these patients should be considered. To date, there are close to 20 patients with ATM reported in the literature who have undergone stem cell transplantation.29,32,35,64-72 Although a promising option, curative hemopoietic stem cell transplantation is not a panacea for ATM because it is restricted by the availability of both donors and resources, especially in areas where the burden of ATM is highest. Therefore, most patients currently remain transfusion dependent throughout their lives. For those who are treated, there are also transplant-related complications, including acute skin and gut graft-versus-host disease, posttransplant lymphoproliferative disorder, and hearing loss resulting from chemotherapeutic agents.73 In our registry,31 we encountered 4 patients who had unsuccessful transplants, resulting in lethality in 1 case and graft rejection in 3 cases, all of whom remain transfusion dependent.
Of note, our current approach to hemopoietic stem cell transplantation for patients with ATM is based on guidelines used for β-thalassemia. However, as discussed, the pathophysiology of ATM differs from that of β-thalassemia. This may have implications when considering the conditioning/immunosuppressive regimen, engraftment, and monitoring, and, ultimately, the transplant outcome. For example, one should consider the effect of the very high degree of erythropoiesis in individuals with ATM and the futility of endogenous HbH cells when contemplating the acceptability of mixed chimerism and the need for transfusions. For example, a patient who has achieved a total Hb concentration of 100 g/L after engraftment but has only 70% donor erythrocyte chimerism (which can be easily measured using Hb analysis or by supravital stain smear of the peripheral blood), may be falsely considered transfusion independent, with the functional (non-HbH) Hb only being 70 g/L.
The potential for genetic engineering to cure severe forms of α-thalassemia
One therapeutic approach to the treatment of ATM may be to reintroduce the α-globin genes via lentiviral delivery of an expression cassette in an analogous approach to that used to treat TDT-β74 and such protocols are currently being developed.75 This approach, if proven safe and effective, would potentially provide a curative option for patients with ATM.
The most frequent cause of ATM (−−SEA/−−SEA) leaves the ζ-globin genes intact and available for reactivation. We know that Hb Portland I (ζ2γ2) and Portland II (ζ2ß2) are functional, allosteric oxygen transporters and, at least in mice, ζ-globin can substitute for α-globin in adult life.76 Reversing ζ-globin transcriptional silencing would open up the possibility of reactivating this gene in fetuses, infants, and adults affected by ATM and severe HbH disease, in a similar way to reactivating γ-globin gene expression to ameliorate the β-hemoglobinopathies.77 The key question is, can we reactivate the ζ-globin gene in definitive erythroid cells?
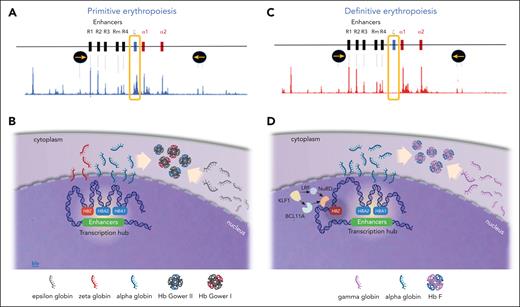
To investigate the mechanism of ζ-globin repression, we have shown that many of the chromatin marks associated with transcriptional silencing, including those associated with heterochromatin (H3K9me3), polycomb complex repression (H3K27me3 & H2AK119ub) are not present at the silenced ζ-globin genes. Furthermore, to date, we have found no role for DNA methylation in silencing the ζ-globin gene promoter.78 Of interest, this work has shown that the chromatin over the ζ-globin gene is accessible and acetylated when active in primitive erythropoiesis. This enables the ζ-globin promoter to interact with the α-globin enhancers. By contrast, in definitive erythropoiesis the ζ-globin gene is inaccessible and hypoacetylated, does not interact with the enhancers, and is therefore silent. Relevant to this is our observation that the trans-acting factors LRF and BCL11A, which recruit the nucleosome remodeling and deacetylation complex to chromatin, are thought to play a role in ζ-globin gene silencing (Figure 5).78 Expression of these factors is regulated by KLF1 and, of interest, individuals with mutations in KLF1 have been shown to have persistent expression of ζ-globin in adult life.79 The extent to which histone deacetylation plays a direct role in gene repression remains unclear,80 however, a role for deacetylases in silencing ζ-globin expression is indicated irrespective of the mechanism.
ζ- and α-globin expression in primitive and definitive erythropoiesis. (A) The mouse α-globin cluster showing the enhancers, and the embryonic (ζ) and adult (α) genes flanked by insulators (yellow arrows in black circles), and assay for transposase-accessible chromatin using sequencing (ATAC-seq) showing the pattern of chromatin accessibility during primitive erythropoiesis. The peaks on the ATAC-seq correspond to accessible chromatin. Note that the ζ-gene is accessible and expressed in primitive (embryonic) erythropoiesis as indicated in the yellow box. (B) Illustration showing the expression of α globin and ζ globin leading to production of embryonic Hbs (Gower I and II). Note the globin gene expression takes place within a non–membrane bound transcriptional hub (white area) in the nucleus. (C) The mouse α-globin cluster and ATAC-seq assay in definitive erythropoiesis. Note the absence of ζ-gene peak on the ATAC-seq (yellow box) in definitive erythropoiesis indicating the ζ-gene is silenced and may be sequestered outside of the transcriptional hub. (D) illustration showing the expression of α globin but absent ζ globin in definitive erythropoiesis. LRF and BCL11A both interact possibly via the nucleosome remodeling and deacetylation (NuRD) complex leading to ζ-globin hypoacetylation and silencing in definitive (fetal and adult) erythroid cells. Both BCL11A and LRF are positively regulated by KLF1.
ζ- and α-globin expression in primitive and definitive erythropoiesis. (A) The mouse α-globin cluster showing the enhancers, and the embryonic (ζ) and adult (α) genes flanked by insulators (yellow arrows in black circles), and assay for transposase-accessible chromatin using sequencing (ATAC-seq) showing the pattern of chromatin accessibility during primitive erythropoiesis. The peaks on the ATAC-seq correspond to accessible chromatin. Note that the ζ-gene is accessible and expressed in primitive (embryonic) erythropoiesis as indicated in the yellow box. (B) Illustration showing the expression of α globin and ζ globin leading to production of embryonic Hbs (Gower I and II). Note the globin gene expression takes place within a non–membrane bound transcriptional hub (white area) in the nucleus. (C) The mouse α-globin cluster and ATAC-seq assay in definitive erythropoiesis. Note the absence of ζ-gene peak on the ATAC-seq (yellow box) in definitive erythropoiesis indicating the ζ-gene is silenced and may be sequestered outside of the transcriptional hub. (D) illustration showing the expression of α globin but absent ζ globin in definitive erythropoiesis. LRF and BCL11A both interact possibly via the nucleosome remodeling and deacetylation (NuRD) complex leading to ζ-globin hypoacetylation and silencing in definitive (fetal and adult) erythroid cells. Both BCL11A and LRF are positively regulated by KLF1.
We also find that knock out of BCL11A, has a much more modest effect on ζ-globin than on γ-globin expression. Nevertheless, knock out of both BCL11A and LRF together causes reexpression of ζ-globin such that it accounts for ∼15% of α-like globin expression. Although an erythroid-specific enhancer of BCL11A has been identified, no such regulatory element has been found for LRF and knock out of both factors in tandem would be required to generate therapeutically useful levels of ζ-globin expression. If these proteins are found to repress ζ-globin through specific binding sites, it may be possible to edit these and produce a larger effect while conserving the wider role of both proteins in hematopoiesis. Given that the likelihood of complications arising from α-thalassemia increases when α-globin levels fall below 30% of normal, a contribution of ∼15% ζ-globin would be likely to be therapeutically useful in patients with HbH disease.78 However, it will be essential to learn how to reexpress ζ-globin to a greater extent to treat patients with BHFS/ATM and further work is required in this area.
The way forward
In the ∼35 years since the most common deletions causing α-thalassemia were characterized,8 enabling rapid diagnostic testing to be established,81 screening followed by prenatal prevention of BHFS births has become routine in areas in which risk is greatest.82 The effect of prenatal diagnosis on the prevention of severe α-thalassemia should not be overlooked. As stated above, it is estimated that in Thailand alone, without intervention, ∼400 pregnancies may be affected by BHFS each year. In China, α-thalassemia is prevalent mainly in the southern regions of the Yangtze River, involving 10 provinces with a population of ∼500 million.83 For example, in the Guangdong province, the carrier rate of the α0-thal mutation (−−SEA) is ∼4%,84 therefore, the incidence of the homozygous state would be ∼1 per 2000. Considering an annual birth rate of 1 million in this region (Guangzhou Municipal Bureau of Statistics) and assuming a low rate of consanguinity in the population, every year ∼500 pregnancies would be affected with BHFS in this province if no preventive measures were taken. Reports from local referral centers are consistent with these conclusions.85 Considering the numbers of affected individuals coupled with advances in perinatal care and availability of intrauterine transfusion, providing comprehensive care for all affected fetuses would represent a significant burden on public health systems in countries in which the α0-thal mutations are frequent.
A key and completely unanswered question in the management of ATM is to what extent the various developmental abnormalities seen in patients with ATM are established before a firm diagnosis can be made (12-16 weeks of gestation). Salvaging fetuses with irreversible developmental changes presents difficult issues for both clinicians and parents. We would advocate that parents at risk of having a pregnancy affected by BHFS should be meticulously counseled about the full implications of this diagnosis and that this counseling should include the etiology, clinical manifestations, prognosis, maternal complications, the full range of unpredictable long-term outcomes, as well as available resources. We especially wish to emphasize that this counseling should be realistic and allow parents to make an informed choice about the conditions that they are willing to accept for their child because it is likely they may require lifelong transfusion and iron chelation. Although affected fetuses can survive fetal life, current care for these individuals is complex, challenging, costly, and associated with a high rate of clinical complications.
We anticipate that, in future, it may be possible to reexpress the embryonic α-like ζ-globin gene or reintroduce the α-globin genes via genome editing. However, research in these areas is at an early stage and not yet ready for clinical application. Genome editing approaches show great promise for the treatment of other hemoglobinopathies, however, this methodology may only be possible in a limited number of patients, therefore a druggable target to reactivate ζ-globin expression would offer an alternative therapeutic approach with the potential to be low cost, effective, and widely applicable.
Acknowledgments
The authors are grateful to Thidarat Suksangpleng (Thalassaemia Center, Faculty of Medicine, Mahidol University, Bangkok, Thailand) for her help in reviewing the frequency and outcome of BHFS in Thailand.
A.A.’s research is funded in part by the Naiman-Vickars Endowment Fund and a BC Children's Hospital Research Institute Investigator Grant Award. D.R.H. is supported by the UK Medical Research Council and the Chinese Academy of Medical Sciences Oxford Institute.
Authorship
Contribution: A.A., C.B., and D.R.H. contributed to the conceptualization of the article; and all authors participated in the literature review, drafted the initial and subsequent versions of the manuscript, and approved the final version.
Conflict-of-interest disclosure: A.A. has provided consultancy services for Novo Nordisk, Octapharma, Shire, Agios, Pfizer, Chiesi, and Vertex. The remaining authors declare no competing financial interests.
Correspondence: Ali Amid, Division of Pediatric Hematology/Oncology, Department of Pediatrics, BC Children's Hospital, University of British Columbia, 4480 Oak St, Vancouver, BC V6H 3V4, Canada; email: ali.amid@cw.bc.ca; Christian Babbs, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; email: christian.babbs@imm.ox.ac.uk; and Douglas R. Higgs, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; email: doug.higgs@imm.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal