Therapeutic advances have transformed the management of chronic lymphocytic leukemia (CLL) in recent years, but many questions remain regarding the development and regulation of this B-cell tumor. In this issue of Blood, Roessner and colleagues characterize expression of the T-box expressed in T cells (T-bet) transcription factor, typically associated with the T-cell lineage, in CLL tumors.1 Moreover, they demonstrate this to be associated with suppression of cellular proliferation and improvement in clinical outcome. The work provides a range of new insights into this fascinating, and still challenging, disorder.
Transcription factors are proteins that regulate gene expression and determine cell fate. Although “master regulator” transcription factors are sometimes observed, forkhead box P3 as a determinant of the T-regulatory cell lineage being a good example, transcription factors usually work in teams to choreograph transcription of genes that define cellular phenotype. The T-bet transcription factor, encoded from the Tbx21 gene, is a canonical regulator of the T-cell lineage and expressed in T helper cell 1 and cytotoxic T cells.2
So why is it expressed in a B-cell tumor?
In fact, it is now clear that T-bet is expressed within the physiological B-cell lineage, hiding in plain sight within a small and enigmatic population sometimes referred to as age-associated B cells (ABCs). These cells, which gradually increase in frequency until middle age, play a valuable role in control of infection but also accumulate in patients with autoimmune conditions, where they are linked with the secretion of autoreactive antibodies.3,4 Interestingly, a patient with inherited T-bet gene deficiency showed a specific deficiency in the development of CD11chiCD21lo B cells, a phenotype similar to ABC populations.5 Prior expression of T-bet expression in B-cell malignancies has also been shown.6,7
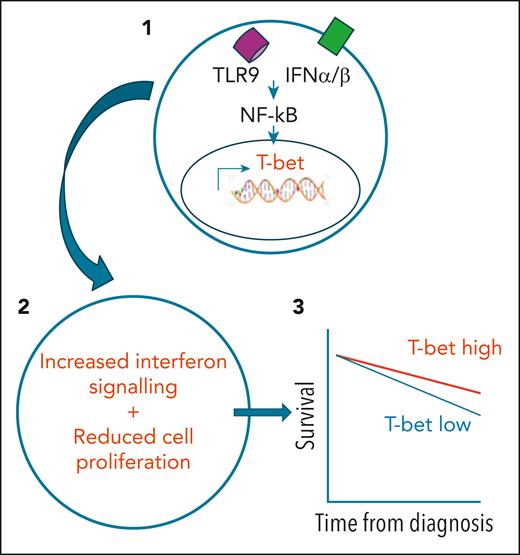
To study T-bet expression and regulation, Roessner et al accessed a range of normal and malignant human B cells and took advantage of the murine TCL-1 transgenic tumor in which the Tbx21 gene had been deleted. T-bet expression in primary CLL tumors was substantially increased compared with normal B cells and, although levels were not related to IGHV mutation status, they were positively and negatively associated with trisomy 12 or ATM mutation, respectively. The team next identified that T-bet expression was induced by stimulation with type 1 interferon or toll-like receptor 9 ligands, irrespective of IgM engagement. This was mediated through NF-κB activation and, as such, could be suppressed by Bruton tyrosine kinase inhibitors. Inflammatory mediators thus emerge as primary determinants for enhancing T-bet expression in CLL (see figure).
(1) Stimulation of CLL tumor cells by type 1 interferon (IFN) or toll-like receptor (TLR) ligands can drive NF-κB–dependent expression of the T-bet transcription factor. (2) This leads to enhanced interferon signaling and reduced tumor cell proliferation. (3) Tumors that have higher levels of T-bet at diagnosis are associated with longer patient survival.
(1) Stimulation of CLL tumor cells by type 1 interferon (IFN) or toll-like receptor (TLR) ligands can drive NF-κB–dependent expression of the T-bet transcription factor. (2) This leads to enhanced interferon signaling and reduced tumor cell proliferation. (3) Tumors that have higher levels of T-bet at diagnosis are associated with longer patient survival.
The functional consequence of T-bet expression next came under scrutiny, and overall the news was positive. Transcriptional and epigenetic analysis shows that the transcription factor behaves as a tumor suppressor, acting as a net silencer of gene transcription, and working to reduce cell proliferation. Indeed, expression was seen to be decreased in high-grade tumors, such as Richters transformation or diffuse large B-cell lymphoma. Interestingly, this effect was at least partially mediated through enhancement of the type 1 interferon signaling pathway within tumor cells.
The team was also able to assess the importance of T-bet expression in prediction of clinical outcome. Using samples from a cohort of 375 patients, it became clear that high-level expression was associated with increased longevity. Importantly, this was independent of IGHV mutation status or ZAP-70 expression. This reveals the potential for future incorporation of this transcription factor in clinical decision-making.
The work points to a range of areas requiring further investigation. It is interesting to speculate whether T-bet uncovers the ABC lineage as the cell of origin of CLL. This is unresolved. A considerable transcriptional overlap was seen between these populations, but there were also striking differences, and as such the expression of T-bet may in fact be leveraged during malignant transformation. Furthermore, it will be important to further define the role of T-bet during CLL development, and here it was noteworthy that expression did not differ between the monoclonal B cell lymphocytosis and CLL stage, potentially hinting at differential determinants of progression. The association with type 1 interferon signaling is also of interest. Type 1 interferon has been used in the management of CLL and, despite modest success, was most effective in early-stage disease, potentially mirroring cells with high T-bet expression.8 Finally, it remains unclear if approaches can be developed to enhance T-bet expression in CLL tumors as an approach for improving therapy.
The complex biology of the CLL tumor never fails to mesmerize. This comprehensive study uncovers the unexpected and novel impact of T-bet expression and holds considerable implications for fundamental understanding and clinical management.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal