Key Points
We present updated diagnostic criteria for FHL including revised clinical criteria and guidelines on genetic and cellular diagnostic assays.
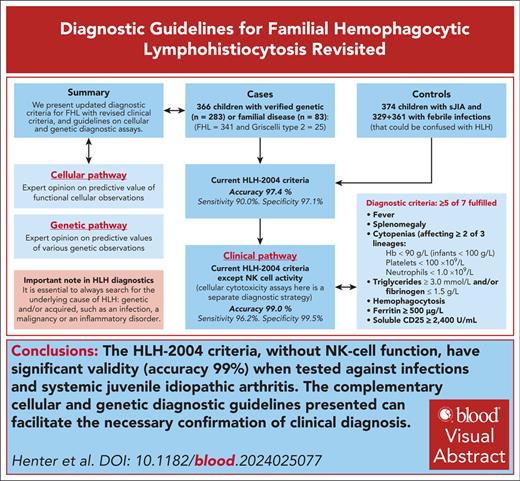
HLH-2004 criteria (without NK-cell function) have significant validity (accuracy 99%) tested against infections and systemic inflammation.
Visual Abstract
Current hemophagocytic lymphohistiocytosis 2004 (HLH-2004)–based diagnostic criteria for familial hemophagocytic lymphohistiocytosis (FHL) are based on expert opinion. Here, we performed a case-control study to test and possibly improve these criteria. We also developed 2 complementary expert opinion–based diagnostic strategies for FHL in patients with signs/symptoms suggestive of HLH, based on genetic and cellular cytotoxicity assays. The cases (N = 366) were children aged <16 years with verified familial and/or genetic FHL (n = 341) or Griscelli syndrome type 2 (n = 25); 276 from the HLH-94/HLH-2004 databases and 90 from the Italian HLH Registry. All fulfilled the HLH-94/HLH-2004 patient inclusion criteria. Controls were 374 children with systemic-onset juvenile idiopathic arthritis (sJIA) and 329 + 361 children in 2 cohorts with febrile infections that could be confused with HLH and sepsis, respectively. To provide complete data sets, multiple imputations were performed. The optimal model, based on 17 variables studied, revealed almost similar diagnostic thresholds as the existing criteria, with accuracy 99.1% (sensitivity 97.1%; specificity 99.5%); the original HLH-2004 criteria had accuracy 97.4% (sensitivity 99.0%; specificity 97.1%). Because cellular cytotoxicity assays here constitute a separate diagnostic strategy, HLH-2004 criteria without natural killer (NK)–cell function was also studied, which showed accuracy 99.0% (sensitivity, 96.2%; specificity, 99.5%). Thus, we conclude that the HLH-2004 criteria (without NK-cell function) have significant validity in their current form when tested against severe infections or sJIA. It is important to exclude underlying malignancies and atypical infections. In addition, complementary cellular and genetic diagnostic guidelines can facilitate necessary confirmation of clinical diagnosis.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL) is a severe hyperinflammatory condition with accumulation of macrophages and lymphocytes in tissues.1-3 This condition is caused by autosomal recessive variants in 1 of 4 genes FHL2-5, associated with PRF1, UNC13D, STX11, and STXBP2, all resulting in impaired lymphocyte cytotoxicity.4-9 Typical manifestations include prolonged fever, splenomegaly, cytopenias, hypertriglyceridemia, hypofibrinogenemia, and hyperferritinemia.10-12 The most frequent severe sequelae are neurological deficits associated with central nervous system (CNS) involvement.1-3,13,14
Because of the high risk for death and CNS damage if adequate treatment is delayed, early diagnosis is crucial. In clinical practice, typically the first step is to identify the hemophagocytic lymphohistiocystosis (HLH) syndrome and, secondly, confirm its underlying cause. Correspondingly, here, we present an analysis testing HLH-associated clinical and laboratory criteria and then guidelines for a genetic diagnosis, which are precise but time-consuming, and for a biological diagnosis, based on functional cellular assays.15 Such assays of lymphocyte phenotype and function can rapidly provide vital insights in patients with HLH awaiting or not subjected to genetic analysis, with genetic variants of unknown or unclear significance or altogether lacking variants in HLH-associated genes yet with suspicion of congenital HLH.
Currently, diagnosis of HLH is in most centers based on the HLH-2004 trial enrollment criteria,11 developed from the HLH-94 study criteria in which 5 of 5 criteria (fever, splenomegaly, bicytopenia [hemoglobin <90 g/L, platelets <100 × 109/L, and neutrophils <1.0 × 109/L], hypertriglyceridemia [fasting triglycerides ≥2.0 mmol/L] and/or hypofibrinogenemia [fibrinogen ≤1.5 g/L], and hemophagocytosis) were required to be included, alternatively an affected sibling (ie, familial cases).10 In the HLH-2004 study, 3 new diagnostic criteria were added; ferritin ≥500 μg/L, low/absent natural killer (NK)–cell activity, and soluble CD25 (sCD25) ≥2400 U/mL, and the level of fasting triglycerides was changed to ≥3.0 mmol/L. Altogether 5 of 8 diagnostic criteria, an affected sibling, or biallelic variants in genes associated with FHL2-5 were the criteria for enrollment (Table 1).11
Diagnostic criteria for the HLH-2004 trial, evaluated in this study
|
|
We performed a case-control study aiming to both validate the current HLH-2004 criteria, based on expert opinion, and to determine whether they improved by making up another set of diagnostic criteria or a diagnostic score. We analyzed a large cohort of children with verified molecular and/or familial diagnosis of FHL or the closely related Griscelli syndrome type 2 (GS2) in which variants in RAB27A result in impaired lymphocyte cytotoxicity.16 These data were compared with 3 cohorts of control patients, that is, children with clinical presentations similar to FHL. We aimed to find a relevant balance between a predictive model that would optimize diagnostic criteria statistically but also be reasonably easy to use clinically. For the genetic and cellular pathways, the recommendations presented are based on expert opinion.
Study population and methods
FHL cases and controls
The cases (N = 366) were children aged <16 years (according to the HLH-94 study age limit) with verified familial and/or molecular diagnosis of FHL (n = 341) or GS2 (n = 25); 82 from the HLH-94 database,10 194 from the HLH-2004 database,11 and 90 from the Italian HLH Registry; all fulfilling the HLH-94/HLH-2004 inclusion criteria. In this report, patients with GS2 are included in the term FHL. Of these 366 children, 283 had verified molecular diagnoses of FHL, and all others (n = 83) had verified familial disease (supplemental Table 1, available on the Blood website); 72 of 366 did not fulfill ≥5 diagnostic criteria.
Familial HLH is characterized by systemic inflammation, and as controls we used children with other forms of inflammation that could be confused with HLH, more specifically infections and systemic rheumatic diseases.17-19 Three such cohorts of children with available clinical and laboratory data were used: 374 “rheuma controls” with systemic juvenile idiopathic arthritis (sJIA), all without macrophage activating syndrome (MAS-HLH), and 329 separate children with infections that diagnostically could be confused with having developed MAS-HLH (in children with sJIA; “inf-controls-1”) were both obtained from a study on classification criteria for MAS-HLH complicating sJIA.20,21 The third cohort (n = 361) were children with severe sepsis (defined as presence of suspected infection, ≥2 systemic inflammatory response syndrome criteria, and ≥1 organ failure) at a pediatric intensive care unit (ICU; “inf-controls-2”); data on 368 children were received, of whom 7 were excluded because of known mutations in HLH-related genes (UNC13D = 5; PRF1 = 1; XIAP = 1).22,23 Of the inf-controls-2, 42 had an underlying malignancy but no other controls. The selection of controls is presented in detail in the supplemental Methods. Because the values from inf-controls-2 were not from the time of diagnosis but instead represent the most abnormal values from a sepsis-related ICU stay, up to 28 days in duration, this cohort was only used for analysis of sCD25, as detailed in “Statistical methods.” No control patient fulfilled ≥5 diagnostic criteria except 7 inf-controls-2; 6 had 6 criteria (2 with malignancies), and 1 had 7, but this corresponds to the most abnormal values from their sepsis-related ICU stay. Because of a high degree of missing values in the controls, it is impossible to know who would have fulfilled the diagnostic criteria had they been completely assessed.
Patient characteristics for all cases, all controls, and the respective control cohorts are presented in Table 2. The study was approved by the ethical committee in Stockholm (2016/135-31; 2018/1387-32) and the Swedish Ethical Review Authority (2020-02162).
Patient characteristics for all patients, all controls, and the respective controls cohorts
| . | Children with FHL (N = 366) . | Controls (N = 1064) . | Inf-controls-1 (n = 329) . | Inf-controls-2 (ICU) (n = 361) . | Rheuma controls (n = 374) . |
|---|---|---|---|---|---|
| Yes, N/evaluated, n/yes of total, % | |||||
| Male sex | 189/366/51.6% | 554/1064/52.1% | 166/329/50.5% | 204/361/56.5% | 184/374/49.2% |
| Fever | 331/363/90.4% | 836/1063/78.6% | 329/329/100% | 152/360/42.1% | 355/374/94.9% |
| Splenomegaly | 349/366/95.4% | 131/1059/12.3% | 20/328/6.1% | 25/361/6.9% | 86/370/23% |
| Hepatomegaly | 308/342/84.2% | 225/1060/21.1% | 32/328/9.7% | 77/361/21.3% | 116/371/31% |
| Median (mean; IQR)/evaluated, n | |||||
| Age at start of HLH-2004, y | 0.294 (1.88; 0.162-1.37)/366 | 4.48 (5.70; 1.68-9.16)/1064 | 3.33 (4.92; 1.45-7.92)/329 | 4.85 (5.92; 1.09-10.4)/361 | 5.49 (6.18; 2.58-9.56)/374 |
| Hemoglobin, g/L | 75.0 (75.8; 65.0-84.8)/342 | 105 (105; 92.0-118)/1044 | 118 (116; 108-127)/326 | 96.0 (97.9; 86.0-108)/355 | 101 (102; 90.0-113)/363 |
| Neutrophils, ×109/L | 0.60 (1.25; 0.295-1.20)/311 | 8.62 (10.2; 4.06-14.0)/910 | 6.84 (8.93; 3.73-11.8)/323 | 6.84 (8.97; 2.69-12.8)/330 | 11.6 (13.3; 7.65-17.4)/257 |
| Platelets, ×109/L | 31.0 (48.6; 17.8-56.0)/340 | 325 (348; 194-480)/1042 | 342 (361; 260-443)/327 | 149 (172; 72.3-235)/354 | 498 (510; 375-614)/361 |
| Triglycerides, mmol/L | 3.67 (4.18; 2.49-5.46)/338 | 1.40 (1.79; 1.09-1.86)/366 | 1.50 (1.75; 1.21-1.84)/122 | 1.52 (2.22; 1.01-2.99)/116 | 1.36 (1.45; 1.00-1.65)/128 |
| Fibrinogen, g/L | 1.00 (1.39; 0.690-1.80)/324 | 5.13 (5.32; 3.81-6.59)/330 | 4.01 (4.43; 2.90-5.60)/132 | 5.60 (5.91; 4.63-7.20)/198 | |
| Ferritin, μg/L | 2 590 (7 940; 1 200-7 640)/327 | 188 (1340; 76.0-624)/810 | 67.0 (156; 32.0-126)/201 | 212 (1790; 95.4-605)/361 | 462 (1660; 157-1600)/248 |
| sCD25, U/mL | 20 700 (23 000; 9 750-29 800)/113 | 2050 (2810; 1370-3080)/361 | 2050 (2810; 1370-3080)/361 | ||
| AST, U/L | 162 (317; 75.0-352)/288 | 33.0 (49.7; 24.0-44.0)/661 | 34.0 (61.7; 25.0-46.0)/311 | 32.0 (39.0; 22.0-42.0)/350 | |
| ALT, U/L | 125 (205; 56.3-266) /320 | 27.0 (87.1; 15.0-41.0)/938 | 22.0 (48.8; 14.0-36.0)/314 | 35.0 (190; 21.0-85.0)/297 | 25.0 (30.5; 13.0-35.0)/327 |
| LDH, U/L | 704 (1 070; 493-1 170)/286 | 442 (517; 314-608)/488 | 470 (529; 338-615)/236 | 427 (507; 302-598)/252 | |
| Bilirubin, μmol/L | 23.1 (58.2; 13.3-78.1)/281 | 8.55 (17.8; 5.13-15.4)/722 | 7.52 (12.8; 4.28-13.7)/215 | 10.3 (27.8; 5.13-22.2)/288 | 6.84 (9.55; 5.00-11.3)/219 |
| Creatinine, μmol/L | 27.0 (37.6; 19.1-44.2)/266 | 36.3 (50.1; 26.5-54.8)/999 | 35.4 (41.5; 25.7-52.2)/317 | 35.4 (59.0; 22.1-55.7)/361 | 44.2 (48.5; 32.7-61.9)/321 |
| Albumin, g/L | 28.0 (28.2; 24.0-32.0)/265 | 38.0 (37.4; 33.0-42.0)/521 | 39.6 (39.1; 35.4-43.0)/264 | 35.6 (35.7; 30.8-40.0)/257 | |
| . | Children with FHL (N = 366) . | Controls (N = 1064) . | Inf-controls-1 (n = 329) . | Inf-controls-2 (ICU) (n = 361) . | Rheuma controls (n = 374) . |
|---|---|---|---|---|---|
| Yes, N/evaluated, n/yes of total, % | |||||
| Male sex | 189/366/51.6% | 554/1064/52.1% | 166/329/50.5% | 204/361/56.5% | 184/374/49.2% |
| Fever | 331/363/90.4% | 836/1063/78.6% | 329/329/100% | 152/360/42.1% | 355/374/94.9% |
| Splenomegaly | 349/366/95.4% | 131/1059/12.3% | 20/328/6.1% | 25/361/6.9% | 86/370/23% |
| Hepatomegaly | 308/342/84.2% | 225/1060/21.1% | 32/328/9.7% | 77/361/21.3% | 116/371/31% |
| Median (mean; IQR)/evaluated, n | |||||
| Age at start of HLH-2004, y | 0.294 (1.88; 0.162-1.37)/366 | 4.48 (5.70; 1.68-9.16)/1064 | 3.33 (4.92; 1.45-7.92)/329 | 4.85 (5.92; 1.09-10.4)/361 | 5.49 (6.18; 2.58-9.56)/374 |
| Hemoglobin, g/L | 75.0 (75.8; 65.0-84.8)/342 | 105 (105; 92.0-118)/1044 | 118 (116; 108-127)/326 | 96.0 (97.9; 86.0-108)/355 | 101 (102; 90.0-113)/363 |
| Neutrophils, ×109/L | 0.60 (1.25; 0.295-1.20)/311 | 8.62 (10.2; 4.06-14.0)/910 | 6.84 (8.93; 3.73-11.8)/323 | 6.84 (8.97; 2.69-12.8)/330 | 11.6 (13.3; 7.65-17.4)/257 |
| Platelets, ×109/L | 31.0 (48.6; 17.8-56.0)/340 | 325 (348; 194-480)/1042 | 342 (361; 260-443)/327 | 149 (172; 72.3-235)/354 | 498 (510; 375-614)/361 |
| Triglycerides, mmol/L | 3.67 (4.18; 2.49-5.46)/338 | 1.40 (1.79; 1.09-1.86)/366 | 1.50 (1.75; 1.21-1.84)/122 | 1.52 (2.22; 1.01-2.99)/116 | 1.36 (1.45; 1.00-1.65)/128 |
| Fibrinogen, g/L | 1.00 (1.39; 0.690-1.80)/324 | 5.13 (5.32; 3.81-6.59)/330 | 4.01 (4.43; 2.90-5.60)/132 | 5.60 (5.91; 4.63-7.20)/198 | |
| Ferritin, μg/L | 2 590 (7 940; 1 200-7 640)/327 | 188 (1340; 76.0-624)/810 | 67.0 (156; 32.0-126)/201 | 212 (1790; 95.4-605)/361 | 462 (1660; 157-1600)/248 |
| sCD25, U/mL | 20 700 (23 000; 9 750-29 800)/113 | 2050 (2810; 1370-3080)/361 | 2050 (2810; 1370-3080)/361 | ||
| AST, U/L | 162 (317; 75.0-352)/288 | 33.0 (49.7; 24.0-44.0)/661 | 34.0 (61.7; 25.0-46.0)/311 | 32.0 (39.0; 22.0-42.0)/350 | |
| ALT, U/L | 125 (205; 56.3-266) /320 | 27.0 (87.1; 15.0-41.0)/938 | 22.0 (48.8; 14.0-36.0)/314 | 35.0 (190; 21.0-85.0)/297 | 25.0 (30.5; 13.0-35.0)/327 |
| LDH, U/L | 704 (1 070; 493-1 170)/286 | 442 (517; 314-608)/488 | 470 (529; 338-615)/236 | 427 (507; 302-598)/252 | |
| Bilirubin, μmol/L | 23.1 (58.2; 13.3-78.1)/281 | 8.55 (17.8; 5.13-15.4)/722 | 7.52 (12.8; 4.28-13.7)/215 | 10.3 (27.8; 5.13-22.2)/288 | 6.84 (9.55; 5.00-11.3)/219 |
| Creatinine, μmol/L | 27.0 (37.6; 19.1-44.2)/266 | 36.3 (50.1; 26.5-54.8)/999 | 35.4 (41.5; 25.7-52.2)/317 | 35.4 (59.0; 22.1-55.7)/361 | 44.2 (48.5; 32.7-61.9)/321 |
| Albumin, g/L | 28.0 (28.2; 24.0-32.0)/265 | 38.0 (37.4; 33.0-42.0)/521 | 39.6 (39.1; 35.4-43.0)/264 | 35.6 (35.7; 30.8-40.0)/257 | |
IQR, interquartile range.
The clinical diagnostic strategy
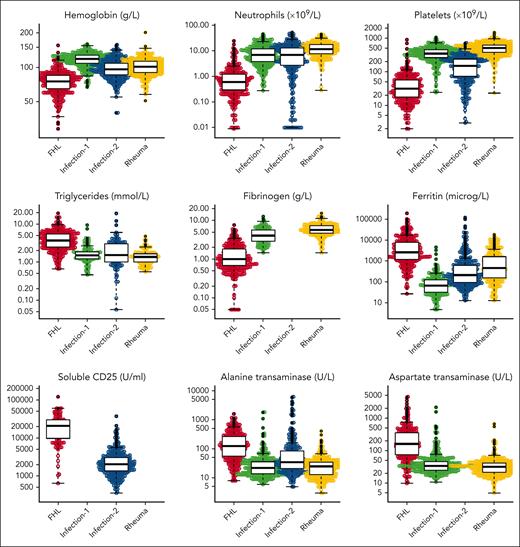
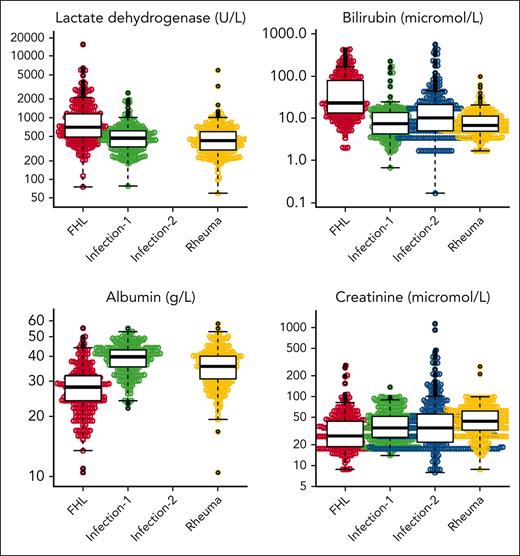
The following categorical variables were analyzed statistically: sex, fever, splenomegaly, and hepatomegaly; and the following continuous variables were analyzed: hemoglobin, absolute neutrophil count, platelet count, triglycerides, fibrinogen, ferritin, sCD25, alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), bilirubin, albumin, and creatinine (Figure 1 for distribution of original data evaluated in cases and the 3 control groups; supplemental Figure 1 for imputed data). Values for hemoglobin, neutrophils, platelets, AST, LDH, and bilirubin in newborn children, aged ≤10 days, were not included in the statistical analyses because they may be misleading. We noticed that inf-controls-1, that is, children selected so that they could be confused with MAS-HLH, were skewed in the sense that everyone had fever, and therefore, the variable fever was not included in the statistical analyses on diagnostic criteria and diagnostic score. Because the values for sCD25 were reported in U/mL in cases but in pg/mL in inf-controls-2, and because we could not find an established conversion model from picograms per milliliter to units per milliliter, we developed such a conversion model (supplemental Methods; supplemental Figure 2). To provide complete data sets for the analyses, multiple imputations were performed (see “Approach to missing data”).
Presentation of all original continuous variables evaluated in cases and controls. Beeswarm panels of all original continuous variables evaluated in cases (N = 366) and the 3 control groups: hemoglobin, absolute neutrophil count, platelet count, triglycerides, fibrinogen, ferritin, sCD25, ALT, AST, LDH, bilirubin, albumin, and creatinine. These analyzed values presented do not include any imputed values. “Infection-1” includes 329 children with systemic infections that could be confused with MAS-HLH. “Infection-2” includes 361 children with severe sepsis treated at a pediatric ICU. “Rheuma” includes 374 children with systemic juvenile idiopathic arthritis, all without MAS-HLH.
Presentation of all original continuous variables evaluated in cases and controls. Beeswarm panels of all original continuous variables evaluated in cases (N = 366) and the 3 control groups: hemoglobin, absolute neutrophil count, platelet count, triglycerides, fibrinogen, ferritin, sCD25, ALT, AST, LDH, bilirubin, albumin, and creatinine. These analyzed values presented do not include any imputed values. “Infection-1” includes 329 children with systemic infections that could be confused with MAS-HLH. “Infection-2” includes 361 children with severe sepsis treated at a pediatric ICU. “Rheuma” includes 374 children with systemic juvenile idiopathic arthritis, all without MAS-HLH.
Before initiating the statistical analyses, it was decided to statistically evaluate 3 different methods in the clinical strategy; (1) a number of diagnostic criteria fulfilled (ie, such as “5/8 criteria”); (2) a numerical score using cutoffs for continuous laboratory values; and (3) a numerical score using a continuous scale for laboratory values. The 3 methods would be compared both with regard to diagnostic preciseness and how easy they would be to use clinically and compared with current HLH-2004 criteria. All models are built on training data (70%) and evaluated on test data (30%).
The diagnostic genetic and cellular pathways
For these pathways, the criteria are based on the author’s expert opinion, in turn based on accumulated experience developed in 6 countries with long-term specific interest in HLH: Canada, China, Germany, Italy, Sweden, and the United States.
Statistical methods
The statistical methods for estimation of numerical scores using cutoffs for continuous laboratory values and for estimation of numerical scores using a continuous scale for laboratory values are presented in supplemental Methods.
Approach to missing data
To address the problem of missing values, multiple imputation was applied, using 20 imputed data sets for the case and each control group separately. In each of the imputed data sets, the missing values were replaced by imputed values sampled from the distribution of the observed data of all variables.24 The imputed data sets were then combined for the case and control groups, except for the inf-controls-2 group because these observations were only used to impute values of sCD25 to the inf-controls-1 and rheuma controls. All analyses conducted were performed on each of the 20 imputed data sets, and the results were combined using Rubin rules.25
Number of diagnostic criteria fulfilled
Of cases and controls, 70% were randomly assigned to a training set and the remaining 30% to a test set. To compare cases and controls, Fisher exact test for binary variables and Wilcoxon rank-sum test for continuous variables were used. All chosen variables except age were significantly different between the 2 groups and used in a multivariable logistic regression model, in which the binary variables were coded as absent or present, and the continuous variables were dichotomized. The cutoff for each continuous variable was determined based on a receiver operating characteristic curve analysis, in which sensitivity and specificity were maximized. All calculations above were conducted on each of the 20 imputed data sets, and the results were summarized using Rubin rules.
The binary variables from the multivariable logistic regression model that were significantly associated with the outcome at significance level P value <.20, as suggested in Hosmer et al,26 were used to calculate a sum of score for each patient in both cases and control groups, such that 1 fulfilled criterion resulted in 1 point. This sum was then used in a univariable logistic regression model to calculate the probability of FHL by converting the probability according to (for explanations on equation components, see supplemental Methods). The results are presented as probability of FHL for each sum of score with corresponding receiver operating area under the curve (AUC), accuracy (), sensitivity (), specificity (), positive predictive value (PPV; ), and negative predictive value (NPV; ).
Assessment of current HLH-2004 criteria
To test the current HLH-2004 criteria with these control cohorts, the same procedure as described in "Number of diagnostic criteria fulfilled" was applied, with the difference that the cutoff values for the continuous variables were from the current HLH-2004 criteria with some modifications. In these analyses, we used the criterion bicytopenia, as stated in the HLH-2004 criteria, and not individual cytopenias. Because data were missing regarding hemophagocytosis and NK-cell activity for cases and controls, we arbitrarily sampled 50% of the controls to have these variables to be compared with 72% and 85%, respectively, of the cases with verified FHL, as in the HLH-2004 study. In addition, accuracy values are presented for alternative frequencies of both of these variables in the controls, ranging from 0% to 100% (supplemental Table 2). For the percentage of fever in cases and controls, we used the same percentage (92%) as for fever among children with verified FHL in the HLH-2004 study. For an assumption of the prevalence of verified FHL/GS2 in the study population, we used the same percentage as in the Italian HLH Registry as of 24 January 2023, that is, 16% (99/601); for explanations, see "Proportion of cases vs controls" in the supplemental Methods.
Results
Three diagnostic strategies
We outline 3 different strategies for a diagnosis of FHL, based on (1) clinical symptoms and routine laboratory findings, (2) genetic analyses, and (3) cellular analyses of lymphocytes.
The clinical diagnostic strategy
Number of diagnostic criteria fulfilled
Comparing all 366 cases with 703 controls (inf-controls-1 and rheuma controls) for the 17 variables, except fever and sCD25 for reasons detailed in “Methods,” 7 variables were significant with P values <.20 (as suggested in Hosmer et al26): splenomegaly (P = .01), neutrophils ≤2.6 × 109/L (P = .01), platelets ≤136 × 109/L (P = .03), triglycerides ≥2.08 mmol/L (P = .08), fibrinogen ≤2.6 g/L (P = .01), ferritin ≥621 μg/L (P = .06), and bilirubin ≥17 μmol/L (P = .08). The number of missing data for each variable and diagnostic group is found in supplemental Table 3.
Assuming a prevalence of FHL at 16% in our study population (as in the Italian HLH Registry), using these 7 significant variables led to an accuracy of 97.7%. Adding fever and sCD25 (≥5369 U/mL) led to an accuracy of 99.1% (Table 3).
Summary of statistical results for various options of the clinical diagnostic pathway and various combinations of variables
| Option of clinical pathway . | Variables included . | No. of criteria∗ . | AUC† . | Accuracy . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|---|---|---|---|
| No. of diagnostic criteria fulfilled | Exact values based on current optimization‡ | 4/7 | 0.9976 | 97.7% | 98.1% | 97.6% | 89.1% | 99.6% |
| Exact values as above + fever + sCD25§ | 6/9 | 0.9991 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| Rounded values (ferritin ≥500 μg/L)|| | 5/9 | 0.9961 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| Rounded values (ferritin ≥1000 μg/L)|| | 5/9 | 0.9967 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| HLH-2004 criteria | Current criteria¶ | 5/8 | 0.9920 | 97.4% | 99.0% | 97.1% | 87.3% | 99.8% |
| Current criteria except NK-cell activity¶ | 5/7 | 0.9924 | 99.0% | 96.2% | 99.5% | 97.6% | 99.2% | |
| Ferritin ≥500 μg/L, sCD25 ≥2400 U/mL | 4/7 | 0.9924 | 93.8% | 99.0% | 92.8% | 73.3% | 99.8% | |
| Ferritin ≥1000 μg/L, sCD25 ≥2400 U/mL | 5/7 | 0.9932 | 99.4% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥1000 μg/L, sCD25 ≥2400 U/mL | 4/7 | 0.9932 | 94.6% | 99.0% | 93.8% | 76.0% | 99.8% | |
| Ferritin ≥500 μg/L, sCD25 ≥5000 U/mL | 5/7 | 0.9951 | 96.2% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥500 μg/L, sCD25 ≥5000 U/mL | 4/7 | 0.9951 | 98.2% | 99.0% | 98.1% | 91.2% | 99.8% | |
| Ferritin ≥1000 μg/L, sCD25 ≥5000 U/mL | 5/7 | 0.9954 | 99.4% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥1000 μg/L, sCD25 ≥5000 U/mL | 4/7 | 0.9954 | 99.1% | 99.0% | 99.0% | 95.4% | 99.8% | |
| Numerical score using cutoffs for continuous laboratory values | At score 115, FHL probability was 56.2%. Variables: splenomegaly, neutrophils, platelets, triglycerides, fibrinogen, ferritin, and bilirubin | 0.9980 | 98.5% | 98.1% | 98.6% | 93.2% | 99.6% | |
| Numerical score using a continuous scale for laboratory values | Variables: splenomegaly, hepatomegaly, hemoglobin, neutrophils, platelets, triglycerides, fibrinogen, ferritin, ALT, LDH, and bilirubin | 0.9976 | NA | NA | NA | NA | NA |
| Option of clinical pathway . | Variables included . | No. of criteria∗ . | AUC† . | Accuracy . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|---|---|---|---|
| No. of diagnostic criteria fulfilled | Exact values based on current optimization‡ | 4/7 | 0.9976 | 97.7% | 98.1% | 97.6% | 89.1% | 99.6% |
| Exact values as above + fever + sCD25§ | 6/9 | 0.9991 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| Rounded values (ferritin ≥500 μg/L)|| | 5/9 | 0.9961 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| Rounded values (ferritin ≥1000 μg/L)|| | 5/9 | 0.9967 | 99.1% | 97.1% | 99.5% | 97.6% | 99.4% | |
| HLH-2004 criteria | Current criteria¶ | 5/8 | 0.9920 | 97.4% | 99.0% | 97.1% | 87.3% | 99.8% |
| Current criteria except NK-cell activity¶ | 5/7 | 0.9924 | 99.0% | 96.2% | 99.5% | 97.6% | 99.2% | |
| Ferritin ≥500 μg/L, sCD25 ≥2400 U/mL | 4/7 | 0.9924 | 93.8% | 99.0% | 92.8% | 73.3% | 99.8% | |
| Ferritin ≥1000 μg/L, sCD25 ≥2400 U/mL | 5/7 | 0.9932 | 99.4% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥1000 μg/L, sCD25 ≥2400 U/mL | 4/7 | 0.9932 | 94.6% | 99.0% | 93.8% | 76.0% | 99.8% | |
| Ferritin ≥500 μg/L, sCD25 ≥5000 U/mL | 5/7 | 0.9951 | 96.2% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥500 μg/L, sCD25 ≥5000 U/mL | 4/7 | 0.9951 | 98.2% | 99.0% | 98.1% | 91.2% | 99.8% | |
| Ferritin ≥1000 μg/L, sCD25 ≥5000 U/mL | 5/7 | 0.9954 | 99.4% | 96.2% | 100% | 100% | 99.2% | |
| Ferritin ≥1000 μg/L, sCD25 ≥5000 U/mL | 4/7 | 0.9954 | 99.1% | 99.0% | 99.0% | 95.4% | 99.8% | |
| Numerical score using cutoffs for continuous laboratory values | At score 115, FHL probability was 56.2%. Variables: splenomegaly, neutrophils, platelets, triglycerides, fibrinogen, ferritin, and bilirubin | 0.9980 | 98.5% | 98.1% | 98.6% | 93.2% | 99.6% | |
| Numerical score using a continuous scale for laboratory values | Variables: splenomegaly, hepatomegaly, hemoglobin, neutrophils, platelets, triglycerides, fibrinogen, ferritin, ALT, LDH, and bilirubin | 0.9976 | NA | NA | NA | NA | NA |
A prevalence of FHL of 16% in the study population was assumed, that is, similar as in the Italian HLH Registry.
ALT, alanine transaminase; NA, not applicable.
The number of criteria presented here are the numbers that maximizes the sensitivity and specificity.
AUC is calculated based on all criteria and not just 5 of 7 criteria, or similar.
Splenomegaly, neutrophils ≤2.7 × 109/L, platelets ≤136 × 109/L, triglycerides ≥2.08 mmol/L, fibrinogen ≤2.6 g/L, ferritin ≥621 μg/L, and bilirubin ≥17 μmol/L.
As above, as well as fever and sCD25 ≥5369 U/mL.
Fever, splenomegaly, neutrophils ≤1.0 × 109/L, platelets ≤100 × 109/L, triglycerides ≥2.0 mmol/L, fibrinogen ≤2.5 g/L, ferritin ≥500 μg/L or 1000 μg/L, sCD25 ≥5000 U/mL, and bilirubin ≥20 μmol/L.
Ferritin, ≥500 μg/L and sCD25, ≥2400 U/mL.
To facilitate clinical use, we then evaluated more even cutoff values based on the author’s expert opinion but still using the same 9 variables; fever, splenomegaly, neutrophils ≤1.0 × 109/L, platelets ≤100 × 109/L, triglycerides ≥2.0 mmol/L, fibrinogen ≤2.5 g/L, ferritin ≥500 μg/L, sCD25 ≥5000 U/mL, and bilirubin ≥20 μmol/L. Here, accuracy was 99.1%, AUC 0.9961, sensitivity 97.1%, specificity 99.5%, PPV 97.6%, and NPV 99.4% (Table 3).
Assessment of the current HLH-2004 diagnostic criteria
We then analyzed the current HLH-2004 criteria (Table 1), still assuming a 16% prevalence of FHL, which revealed an accuracy of 97.4%. Because cellular assays are here considered a separate diagnostic pathway, we then estimated a model using the current HLH-2004 criteria except NK-cell function, which revealed an accuracy of 99.0%, AUC 0.9924, sensitivity 96.2%, specificity 99.5%, PPV 97.6%, and NPV 99.2% (Table 3).
Because it has been suggested that the cutoffs of ferritin at 500 μg/L and sCD25 at 2400 U/mL are too low,27-29 we also evaluated a ferritin cutoff of 1000 μg/L, by which the accuracy increased to 99.4%. By instead increasing the sCD25 cutoff to 5000 U/mL, accuracy decreased to 98.2%, and with ferritin 1000 μg/L and sCD25 5000 U/mL, the accuracy was 99.1% (Table 3).
Thus, overall, the HLH-2004 criteria demonstrated high accuracy, sensitivity, and specificity for FHL in this cohort.
Numerical score using cutoffs for continuous laboratory values
The 7 variables that were significant had an AUC of 0.9980, and at score 115, the probability for FHL was >50%, specifically 56.2%, with an accuracy of 98.5%, sensitivity 98.1%, specificity 98.6%, PPV of 93.2%, and NPV of 99.6%.
The weighted score for each independent variable, and the underlying calculations, are presented in supplemental Table 4. The total score ranged from 0 to 200. The cases had a mean score of 176 (median, 181; interquartile range, 165-200). For the controls, the corresponding values were 24, 16, and 4 to 33. The probability of FHL for various scores are presented in supplemental Table 5.
Numerical score using a continuous scale for laboratory values
In the first logistic regression model, using 14 variables with both binary and continuous outcomes, significant P values (P < .20) were obtained for the following variables: splenomegaly (P < .01), hepatomegaly (P = .01), hemoglobin (P < .01), triglycerides (P < .01), fibrinogen (P < .01), neutrophils (P < .01), platelets (P < .01), ferritin (P = .07), ALT (P = .04), LDH (P = .19), and bilirubin (P = .05; supplemental Table 6).
These significant variables were then included in a final model (supplemental Table 6). This resulted in a model with AUC 0.9976. Inserting the patient values in the equation provides the probability that the patient has FHL.
The genetic diagnostic pathway
After consensus discussions, we deem that a diagnosis of FHL, potentially necessitating curative treatments, can be based on the presence of biallelic loss-of-function variants, including large deletions, nonsense variants, or previously well-described HLH-associated rearrangements, noncoding or missense variants (Table 4). Such variants should be annotated as "pathogenic" in the ClinVar database, a freely available archive for interpretations of clinical significance of constitutional and somatic human variants of any size, type, or genomic location.30 The Human Gene Mutation Database constitutes a comprehensive collection of published germ line mutations in genes that are thought to underlie or closely associated with human inherited disease.
Alternative pathways to diagnose FHL: the genetic and the cellular pathways
| Pathway . | Predictive value . | Recommended diagnostic follow-up . |
|---|---|---|
| The genetic pathway: genetic observation | ||
| Biallelic combination of large deletions, nonsense variants, and other previously well-described disease-causing∗ genetic aberrations in FHL-associated genes | Sufficient for FHL diagnosis | Not necessary but functional validation can be supportive |
| Biallelic combination of large deletions, nonsense mutations, or previously described genetic aberrations with at least 1 rare missense or noncoding genetic aberration in an FHL-associated gene | Suspicion of FHL | Other diagnostic validation necessary, functional validation recommended |
| Genetic variant of unknown significance identified in an FHL-associated gene | Undetermined | Other diagnostic validation necessary, functional validation recommended |
| No genetic variants identified in FHL-associated genes | Undetermined | Other diagnostic validation necessary, functional validation can be supportive |
| The cellular pathway: functional cellular observation | ||
| Absent perforin expression or defective cytotoxic lymphocyte exocytosis† | Strong suspicion of FHL | Genetic analysis necessary |
| Low perforin expression or impaired cytotoxic lymphocyte exocytosis† | Suspicion of FHL | Genetic analysis necessary and repeated functional analyses recommended |
| Normal perforin expression and cytotoxic lymphocyte exocytosis† | FHL less likely | Genetic analyses suggested if FHL is clinically suspected |
| Pathway . | Predictive value . | Recommended diagnostic follow-up . |
|---|---|---|
| The genetic pathway: genetic observation | ||
| Biallelic combination of large deletions, nonsense variants, and other previously well-described disease-causing∗ genetic aberrations in FHL-associated genes | Sufficient for FHL diagnosis | Not necessary but functional validation can be supportive |
| Biallelic combination of large deletions, nonsense mutations, or previously described genetic aberrations with at least 1 rare missense or noncoding genetic aberration in an FHL-associated gene | Suspicion of FHL | Other diagnostic validation necessary, functional validation recommended |
| Genetic variant of unknown significance identified in an FHL-associated gene | Undetermined | Other diagnostic validation necessary, functional validation recommended |
| No genetic variants identified in FHL-associated genes | Undetermined | Other diagnostic validation necessary, functional validation can be supportive |
| The cellular pathway: functional cellular observation | ||
| Absent perforin expression or defective cytotoxic lymphocyte exocytosis† | Strong suspicion of FHL | Genetic analysis necessary |
| Low perforin expression or impaired cytotoxic lymphocyte exocytosis† | Suspicion of FHL | Genetic analysis necessary and repeated functional analyses recommended |
| Normal perforin expression and cytotoxic lymphocyte exocytosis† | FHL less likely | Genetic analyses suggested if FHL is clinically suspected |
With documented cases of Mendelian inheritance.
The definitions of defective, low, impaired, and normal are specific for each laboratory and cannot be generalized.
In patients carrying rare coding variants in HLH-associated genes that are novel, deemed "likely pathogenic," of "unknown significance," or "conflicting interpretation," assays of lymphocyte cytotoxic function are strongly recommended. In addition, the Genome Aggregation Database is currently the largest publicly available collection of population variation from harmonized sequencing data.31 Providing useful insights, the Genome Aggregation Database browser enables rapid and intuitive variant analysis,32 including the prediction of pathogenicity using Combined Annotation Dependent Depletion tool scores.33 In individuals for whom biallelic possible disease-causing variants are not identified but who suffer repeated episodes of HLH, cellular diagnostic pathways assessing cytotoxic lymphocyte function are strongly recommended to rule out pathogenic noncoding aberrations.
The cellular diagnostic pathway
Cellular assays of patients with FHL originally identified defective lymphocyte cytotoxicity using assays of radioactivity measuring release of 51Cr from labeled K562-cells, a cell line devoid of major histocompatibility complex class I expression and universally susceptible to NK-cell–mediated lysis, after incubation with leukocytes isolated from peripheral blood.11 Due to radioactive safety restrictions and inherent variability, such cytotoxicity assays have largely been replaced with flow cytometric assay quantifying intracellular perforin expression in NK cells as well as NK-cell exocytic responses (typically measuring induced surface CD107a expression after incubation with target K562 cells).15,34,35 Combined exocytosis, that is, degranulation, assays quantifying both NK-cell and cytotoxic CD8+ T-cell exocytotic responses provide superior sensitivity and specificity.36 Absent perforin expression or defective exocytosis by patient NK cells is indicative of familial HLH (Table 4). A suggestion on how the functional cellular assays should be best performed is provided in the supplemental Methods.
The genetic and cellular pathways also need signs and/or symptoms of HLH
Genetic or cellular data do not diagnose FHL per se; in isolation, they only indicate a risk for FHL. They should only be considered diagnostic in patients with signs and/or symptoms of HLH. The suggested signs and symptoms are the diagnostic criteria as defined in Table 5 (fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis, elevated ferritin, and elevated sCD25), as well as isolated CNS inflammation and/or isolated hepatitis/liver failure. Anyone of these could be sufficient diagnostic support in the alternative diagnostic pathways, with sufficient genetic or cellular findings.
Revised diagnostic guidelines for the diagnosis of FHL
|
|
It is always of vital importance to search for the underlying cause(s) for the HLH syndrome, which may be either genetic, such as FHL, and/or acquired, such as an infection, a malignancy, or an autoimmune or autoinflammatory disorder.
Discussion
The current criteria most widely used for diagnosis of FHL were developed as enrollment criteria for the HLH-2004 treatment protocol 20 years ago and based on expert opinion.9 Here, we have evaluated these clinical criteria relative to other inflammatory disorders. In addition, we present 2 additional strategies to make FHL diagnosis in patients with signs/symptoms suggestive of HLH: a genetic pathway and a cellular pathway.
In contrast to the situation when the HLH-2004 diagnostic criteria were developed, the current analyses of clinical criteria could be based on a large cohort of patients with genetically verified FHL/GS2. This is an enormous advantage compared with many other medical conditions.
When assessing the current HLH-2004 criteria, which was performed late in our analysis, we were surprised by their positive performance, that is, an accuracy of 97.4%. Possible reasons for this remarkably high performance can be a homogenous group of cases (all have verified FHL), and the control groups appear to be quite distinct from the cases (Figure 1). However, the possibility of a confirmation bias cannot be excluded, that is, the FHL cases were defined on fulfilling HLH-2004 criteria, which thus formed the basis for genetic analyses.
Using the current HLH-2004 criteria except NK-cell cytotoxicity, the accuracy was 99.0%. By modifying the ferritin level from 500 to 1000 μg/L or the sCD25 level from 2400 to 5000 U/mL, the accuracy was 99.4% and 98.2%, respectively (Table 3). However, the authors deem that there is no meaningful difference between the various alternatives and, therefore, not sufficient reasons to change the cutoff levels of ferritin and sCD25, also having in mind the value of easy comparison with studies over the last 20 years that used the current cutoff levels. Moreover, by using lower cutoff levels, more patients will fulfill the criteria, and with reduced costs of genetic sequencing, it is valuable that more patients are sequenced. The suggested revised clinical criteria for FHL are presented in Table 5.
When this study started, we considered 3 different methods for the clinical pathway; a number of diagnostic criteria fulfilled, a numerical score using cutoffs for continuous laboratory values resembling the HScore,37 and a numerical score using a continuous scale for laboratory values. In our corresponding “FHL-score,” at the best cutoff of 100 points, accuracy was 98.1%; that is, lower than the accuracy of 99.0% reached at 5 of 7 fulfilled HLH-2004 criteria, that is, except NK-cell activity, in this study (Table 3). We, therefore, do not suggest this somewhat more complicated model, nor a model with a numerical score using a continuous scale for laboratory values because this would need a computer or a website to estimate the probability for FHL.
The cellular diagnostic pathway appears to provide quite reliable prediction on the likelihood that the patient has FHL, and moreover, results can be provided already within a day in some laboratories (Table 4). The sensitivity of cytotoxic lymphocyte exocytosis assays can be further augmented using different triggers of NK-cell activation or also examining cytotoxic CD8+ T-cell responses.38 In patients with recurrent HLH but in whom genetic analysis fail to provide a molecular diagnosis, cytotoxicity assays should be repeated when used as a basis for instigating curative treatments associated with significant side effects. In patients with novel genetic associations, further cellular assays can be useful to determine pathophysiological processes.39 Notably, to avoid potential pitfalls, cytotoxicity assays should be performed by experienced laboratories. A drawback is that there are few well-experienced laboratories, and another drawback is that reliable results require fresh cells; that is, ideally the sample is delivered to the laboratory within ∼24 hours after sampling.
Notably, aberrations in a large number of other genes than FHL2-5 and RAB27A have been associated with HLH, including mutations in LYST (Chediak-Higashi syndrome), SAP (XLP-1), XIAP (XLP-2), and NLRC4.40 HLH can also be a less common manifestation of other genetic diseases including several immunodeficiencies, autoinflammatory diseases, and lysinuric protein intolerance. However, high-throughput sequencing technologies are now established in clinical laboratories for the rapid genome-wide investigation and diagnosis of patients suffering life-threatening constitutional genetic diseases.
Hypomorphic variants in HLH-associated genes and multiple variants in distinct genes represent a challenge to the interpretation of a molecular diagnosis, supporting the value of the cellular pathway. An example of a hypomorphic variant frequently encountered in late-onset cases of HLH is PRF1 c.272C>T (p.Ala91Val), which in a biallelic setting confers an ∼50% reduction in perforin expression and lymphocyte cytotoxic function yet has an allelic frequency of 5% in some populations.41 Furthermore, experimental evidence from animal models of HLH indicate that an increasing polygenic burden of loss-of-function variants in HLH-associated genes correlates with augmented predisposition to HLH.42 Clinical evidence supports a potential digenic or polygenic mode of inheritance of familial HLH in which single loss-of-function mutations in 2 different degranulation pathway genes cooperate to impair cellular cytotoxicity.43
When to use each diagnostic pathway depend on the clinical situation. The clinical pathway can rapidly help to decide on whether to initiate HLH-directed therapy. The cellular pathway may at a later stage give additional prediction on the likelihood of FHL. Importantly, although the clinical and cellular pathways both independently may be strongly indicative of FHL and be the basis for initiating pre-hematopoietic stem cell transplantation (HSCT) treatments, we deem that the FHL diagnosis should be confirmed through genetic analysis, such as for decisions on performing HSCT. Moreover, the genetic and cellular pathways can both diagnose patients before disease presentation, enabling rapid HSCT that reduces risk of sequelae. As a diagnostic complement, the MAS/HLH score can be used to discriminate between primary HLH and MAS-HLH.44
Our study has several weaknesses, one is that our analyses are based on the control groups used, and strictly speaking, our data are relevant only for the control groups used. Thus, because malignancies and atypical infections were not included as controls, such diseases have to be excluded in each patient. Another weakness is that there were several missing values in the data sets, and as a result, multiple imputations were applied. The extent of imputation could overestimate the PPV and NPV of the clinical FHL criteria, and some variables deemed not to be statistically different between controls and FHL may have been underestimated, but multiple imputations are still considered reasonable with limited bias.45 Because there were no controls with known secondary HLH, and because the syndrome HLH may have various possible causes,46 it is always of vital importance to search for the underlying reason(s) for the HLH, either genetic, as in FHL, or acquired, such as infections, malignancies, or inflammatory disorders, as in secondary HLH. This includes sepsis-associated HLH, so that appropriate therapy is provided. Notably, the pattern of inflammatory cytokines can help to diagnose HLH early, to discriminate HLH from non-HLH as well as the type of HLH; an approach that is encouraged.47-51 Moreover, as always in medicine, making a clinical diagnosis should be based on the entire evaluation of the patient and cannot be entirely based on predefined clinical criteria.
To conclude, we present 3 separate pathways to the diagnosis of FHL, a clinical pathway, a cellular pathway, based on cytotoxicity, and a genetic pathway. As clinical criteria, we suggest to keep the HLH-2004–based criteria, except NK-cell activity. We hope that these 3 pathways will be helpful in making the diagnosis of FHL in the future.
Acknowledgments
The authors thank Maurizio Aricò for his valuable contributions to management of familial hemophagocytic lymphohistiocytosis and involvement in the early phase of this study.
The work was supported by grants from the Swedish Childhood Cancer Fund (grant KP2021-0006), the Swedish Cancer Society (Dnr: 23 3162 Pj), the Cancer and Allergy Foundation of Sweden (reference number 10118), and the Region Stockholm (ALF-project; FoUI-960717). K.F.K. has received funding from the National Institute of General Medical Sciences, National Institutes of Health (K23GM148827).
Authorship
Contribution: J.-I.H., A.H., and M.B.J. planned the study, with J.-I.H. serving as the principal investigator; J.-I.H., E.B., A.H., M.L.C., and E.S. provided data summaries on cases; F.M., K.F.K., R.Q.C., A.R., and S.W.C. provided data summaries on controls; J.E., I.H.M., and M.B. performed statistical analyses; Y.T.B. and E.S. developed the format for the genetic and functional pathways; J.-I.H., A.H., J.E., A.R.K., K.L., A.N., Y.-M.T., M.B.J., E.S., and Y.T.B. analyzed the data; J.-I.H. drafted the manuscript, assisted by J.E. and Y.T.B.; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.-I.H., E.S., A.R.K., and K.L. serve as consultants and/or on advisory boards for Sobi. M.B.J. serves as consultant and/or on advisory board for, and has received research support from, Sobi. R.Q.C. serves as a consultant for Sobi, Pfizer, AbbVie, Curbside Consultations, Vindico Medical Education, and Lilly; serves on an advisory board for Sobi; and serves as an unpaid consultant for Apollo Therapeutics. A.H. serves as a consultant for Sobi and a speaker for Novartis. A.N. is on the advisory board for Jazz Pharmaceuticals. S.W.C. has received consulting fees from Apollo and Simcha Therapeutics and speaker fees from Sobi and Practice Point Communications. The remaining authors declare no competing financial interests.
Correspondence: Jan-Inge Henter, Childhood Cancer Research Unit, Karolinska Institutet, Tomtebodavägen 18A, SE-171 77 Stockholm, Sweden; email: jan-inge.henter@ki.se.
References
Author notes
Data cannot be shared because this was not included in the ethical applications.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal