Key Points
Combining CAR-T cells targeting CD19 & CD22 with a recombinant, polymer-conjugated IL15 receptor agonist (NKTR-255) was safe and feasible.
NKTR-255 was associated with increases in cytokines (IL15 and interferon-γ) and related chemokines (CXCL9, CXCL10).
Visual Abstract
Although chimeric antigen receptor (CAR) T-cell (CAR-T) therapy has revolutionized the treatment of B-cell malignancies, many patients relapse and therefore strategies to improve antitumor immunity are needed. We previously designed a novel autologous bispecific CAR targeting CD19 and CD22 (CAR19-22), which was well tolerated and associated with high response rates but relapse was common. Interleukin-15 (IL15) induces proliferation of diverse immune cells and can augment lymphocyte trafficking. Here, we report the results of a phase 1 clinical trial of the first combination of a novel recombinant polymer-conjugated IL15 receptor agonist (NKTR-255), with CAR19-22, in adults with relapsed/refractory B-cell acute lymphoblastic leukemia. Eleven patients were enrolled, 9 of whom successfully received CAR19-22 followed by NKTR-255. There were no dose-limiting toxicities, with transient fever and myelosuppression as the most common possibly related toxicities. We observed favorable efficacy with 8 of 9 patients (89%) achieving measurable residual disease–negative remission. At 12 months, progression-free survival for NKTR-255 was double that of historical controls (67% vs 38%). We performed correlative analyses to investigate the effects of IL15 receptor agonism. Cytokine profiling showed significant increases in IL15 and the chemokines CXCL9 and CXCL10. The increase in chemokines was associated with decreases in absolute lymphocyte counts and CD8+ CAR T cells in the blood and 10-fold increases in cerebrospinal fluid CAR-T cells, suggesting lymphocyte trafficking to tissue. Combining NKTR-255 with CAR19-22 was safe, feasible, and associated with high rates of durable responses. This trial was registered at www.clinicaltrials.gov as #NCT03233854.
Introduction
Chimeric antigen receptor T-cell (CAR-T) therapy targeting CD19 (CAR19) has transformed the treatment of relapsed/refractory B-cell malignancies including B-cell acute lymphoblastic leukemia (B-ALL). However, relapse after CAR19 occurs in the majority of patients and is associated with dismal outcomes.1,2 Antigen loss is a frequent cause of immune evasion, thus CAR-T constructs targeting alternate antigens may overcome this mechanism of relapse.3,4 Our group previously generated a bispecific CAR-T targeting CD19 and CD22 with a 4-1BB costimulatory domain (CAR19-22).5 In a phase 1 trial, CAR19-22 was safe, with no dose-limiting toxicities (DLTs) and led to complete remissions (CR) in 100% of patients with B-ALL (N = 17), with measurable residual disease (MRD)-negativity in 88%.6 However, relapse occurred in 10 patients, leading to a median progression-free survival (PFS) of 5.8 months (95% confidence interval [CI], 2.6 to not reached). Of 10 patients, 5 retained CD19 and CD22 expression at the time of relapse indicating mechanisms other than antigen loss may be responsible for disease progression as other studies have suggested.7 Improving the antitumor response of CAR19-22 in an antigen agnostic manner may therefore promote durable remissions.
Interleukin-15 (IL15), a proinflammatory member of the common γ-chain family of cytokines, has been proposed as a novel immunotherapy that may synergize with CAR-T therapy through multiple mechanisms.8 IL15 drives proliferation of natural killer (NK), NK-T, and CD8+ T cells, and promotes differentiation of CD8+ T effector cells into memory T cells.8-11 IL15 also promotes tissue migration of NK and CD8+ T cells.12 Enhanced homeostatic proliferation of T cells after lymphodepleting chemotherapy is mediated, in part, by IL15,9 and in patients who received axicabtagene ciloleucel, peak IL15 levels correlated with CAR expansion and response.13,14 Several preclinical models demonstrate that enhanced IL15 signaling increases the potency of adoptively transferred T cells.10,11,15,16 Together, evidence supports a role for physiologic elevations in circulating IL15 in augmenting the potency of adoptive cell therapies and that IL15 administration could enhance the efficacy of CAR-T therapy through multiple mechanisms.
Previous attempts to leverage the salutary antitumor effects of IL15 have been limited by its short half-life.12,17 To address this, NKTR-255 was developed as a novel recombinant IL15 receptor agonist attached to a polyethylene glycol moiety. NKTR-255 has been shown to prolong IL15 half-life and activate NK and CD8+ T cells durably.18 In preclinical studies, NKTR-255 improves CAR19 proliferation and persistence in murine models of lymphoma and in vitro assays of human cell lines.19 We conducted a phase 1 trial combining NKTR-255 with CAR19-22 to evaluate the safety and feasibility of this approach in relapsed/refractory B-ALL.
Methods
Trial design and oversight
This phase 1 single-center, single-arm, dose-escalation study was approved by Stanford University’s institutional review board (IRB# 41382) and registered at www.ClinicalTrials.gov as NCT03233854. Patients provided informed consent in accordance with the Declaration of Helsinki. A data safety and monitoring committee oversaw the trial conduct.
Experimental interventions
The CAR19-22 construct includes a murine anti-CD19 FMC63 single-chain variable fragment linked to a fully human anti-CD22 m971 single-chain variable fragment (ɑCD19 vH-ɑCD22 vL-linker-ɑCD22 vH-ɑCD19 vL) with the following additional domains: human CD8 hinge and transmembrane, 4-1BB costimulatory, and CD3ζ activation. Patients underwent apheresis, and CAR19-22 manufacturing was performed in a Miltenyi CliniMACS Prodigy closed-system device, as previously described.6 Patients received standard lymphodepletion (LD) with fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 on days −5, −4, and −3 (supplemental Figure 1, available on the Blood website). The CAR19-22 dose of 3 × 106 cells per kg was the recommended phase 2 dose (RP2D) identified in the phase 1 trial.6 Escalating doses of intravenous NKTR-255 were tested in a 3 × 3 design: dose level 1 = 1.5 μg/kg; dose level 2 = 3.0 μg/kg; and dose level 3 = 6.0 μg/kg. NKTR-255 was administered on day +14; patients were eligible to receive additional monthly NKTR-255 infusions for a maximum of 6 cycles; however, feasibility was determined based on ability to receive day +14 NKTR-255. Criteria for NKTR-255 discontinuation were prespecified in the protocol and are listed in supplemental Methods. We initially planned for a total of 10 patients to allow for at least 3 patients to receive NKTR-255 at each dose level, however, there was insufficient lentiviral vector to allow for additional trial enrollment and therefore only 2 patients received NKTR-255 dose level 3.
Eligibility
Adult patients (aged ≥18 years) with B-ALL with stable or progressive disease (refractory) after a single line of therapy (chemotherapy or tyrosine kinase inhibitor) or who relapsed after achieving CR were eligible for enrollment. MRD-only relapses required confirmation with a second test within 4 weeks. CD19 expression via immunohistochemistry or flow cytometry was required; CD22 expression was not. A full list of inclusion and exclusion criteria is included in the supplemental Data.
End points
The primary outcomes of this study were feasibility and safety. Feasibility was measured by the number of patients who successfully received CAR19-22 and day +14 NKTR-255. Safety of CAR19-22 was evaluated for all patients (N = 11), and safety of NKTR-255 was evaluated for those who received the combination (n = 9). Incidence and severity of adverse events (AEs) at each dose level were recorded, with the goal of identifying the RP2D, defined as the highest dose level of NKTR-255 tested and tolerated without DLT after CAR19-22.
AEs were graded using the Common Terminology Criteria for Adverse Events version 4.0.20 American Society for Transplantation and Cellular Therapy consensus grading was used to assess cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), and immune effector cell–associated hemophagocytic lymphohistiocytosis–like syndrome (IEC-HS).21,22
Secondary end points were assessed for patients who received NKTR-255 (n = 9) and included pharmacokinetics of NKTR-255 as measured by IL15 levels in blood, and efficacy as measured by PFS. Exploratory end points included cytokine profiling; CAR19-22 expansion in the blood, bone marrow, and cerebrospinal fluid (CSF); and long-term CAR19-22 persistence. MRD was assessed by multiparameter flow cytometry, polymerase chain reaction (PCR), or next-generation sequencing of the immunoglobulin receptor (Clonoseq; Adaptive Biotechnologies, Seattle, WA).
Controls
For secondary and exploratory end points, patients in this study were compared with a noncontemporaneous control cohort of patients who received CAR19-22 as part of the initial phase 1 clinical trial without the addition of NKTR-255. Control patients were enrolled between December 2017 and October 2020 and were matched primarily based on disease burden at time of screening, because leukemic burden has been shown to most closely associate with toxicity and efficacy in ALL CAR-T studies.1,23,24 Low disease burden was defined as <5% marrow involvement of ALL and absence of bulky extramedullary disease.
Correlative analyses
We aimed to collect peripheral blood samples before LD, during the first week after infusion, on day +14 before NKTR-255, and after NKTR-255 infusion of day +15, +16, +21 and +28 for patients receiving NKTR-255. We aimed to collect samples for controls before LD, on day 0, and then weekly thereafter until at least day +28. Details for laboratory assessment of correlates are provided in supplemental Methods. Briefly, cytokines were quantified in batch via multiplexed immunofluorescence (Luminex). A CD19 anti-idiotype monoclonal antibody (generous gift from MD Anderson) was used to quantify CAR+ T cells by flow cytometry including CD4+ and CD8+ subsets in an assay hereafter referred to as CARFACS in the peripheral blood, bone marrow, and CSF.6,25 DNA was extracted, and measured via quantitative PCR using previously published primers.6
Statistical analyses
Continuous variables were compared using Mann-Whitney U tests, with paired methods as needed. PFS was estimated using the Kaplan-Meier method. All statistical analyses were performed in R version 4.2.2.
Results
Patient characteristics and feasibility
Eleven patients were enrolled between February 2022 and July 2023. CAR19-22 manufacturing was successful in all patients and all 11 patients received infusion. Of 11 patients who received CAR19-22, 2 (18%) were ineligible to receive NKTR-255 at day +14 because of active grade 3 infection in 1 case, and ongoing grade 3 IEC-HS in the other. The baseline characteristics of these 2 patients were similar to those of the 9 patients who received NKTR-255 (supplemental Table 1).
Among the 9 patients who received the combination of CAR19-22 and NKTR-255, a majority had Philadelphia chromosome–negative disease (89%) and received multiple lines of prior therapy (median, 3; range, 1-5) including prior allogeneic hematopoietic stem cell transplantation (HCT) (56%), blinatumomab (56%), inotuzumab (44%), and CAR T cells (11%; Table 1). Of 9 patients, 8 had low tumor burden, and 1 patient had high burden because of the presence of bulky extramedullary disease.
Baseline characteristics
| . | NKTR-255 + CAR19-22 (n = 9) . | CAR19-22 historical controls (n = 8) . |
|---|---|---|
| Age (median, y) | 36 | 49 |
| Hispanic | 5 (56%) | 5 (62%) |
| Ph-negative | 8 (89%) | 2 (25%) |
| Prior lines of therapy (range) | 3 (1-5) | 4 (2-10) |
| CD19 expression | 9 (100%) | 8 (100%) |
| CD22 expression∗ | 7 (78%) | 7 (88%) |
| Prior blinatumomab | 5 (56%) | 5 (63%) |
| Prior inotuzumab | 4 (44%) | 4 (50%) |
| Prior CAR19† | 1 (11%) | 1 (13%) |
| Prior HCT | 5 (56%) | 6 (75%) |
| Low disease burden | 8 (89%) | 8 (100%) |
| CNS disease | 3 (33%) | 4 (50%) |
| CAR19-22 dose | ||
| 1 × 106 cells per kg | 0 | 3 (37%) |
| 3 × 106 cells per kg | 9 (100%) | 5 (63%) |
| CRS related to CAR19-22 | ||
| Grade 1 | 4 (44%) | 1 (13%) |
| Grade 2 | 0 (0%) | 3 (38%) |
| ICANS related to CAR19-22 | ||
| Grade 1 | 0 (0%) | 1 (13%) |
| NKTR-255 dose | ||
| 1.5 μg/kg | 3 (33%) | — |
| 3.0 μg/kg | 4 (44%) | — |
| 6.0 μg/kg | 2 (22%) | — |
| Number of NKTR-255 infusions (median, range) | 2 (1-5) | — |
| . | NKTR-255 + CAR19-22 (n = 9) . | CAR19-22 historical controls (n = 8) . |
|---|---|---|
| Age (median, y) | 36 | 49 |
| Hispanic | 5 (56%) | 5 (62%) |
| Ph-negative | 8 (89%) | 2 (25%) |
| Prior lines of therapy (range) | 3 (1-5) | 4 (2-10) |
| CD19 expression | 9 (100%) | 8 (100%) |
| CD22 expression∗ | 7 (78%) | 7 (88%) |
| Prior blinatumomab | 5 (56%) | 5 (63%) |
| Prior inotuzumab | 4 (44%) | 4 (50%) |
| Prior CAR19† | 1 (11%) | 1 (13%) |
| Prior HCT | 5 (56%) | 6 (75%) |
| Low disease burden | 8 (89%) | 8 (100%) |
| CNS disease | 3 (33%) | 4 (50%) |
| CAR19-22 dose | ||
| 1 × 106 cells per kg | 0 | 3 (37%) |
| 3 × 106 cells per kg | 9 (100%) | 5 (63%) |
| CRS related to CAR19-22 | ||
| Grade 1 | 4 (44%) | 1 (13%) |
| Grade 2 | 0 (0%) | 3 (38%) |
| ICANS related to CAR19-22 | ||
| Grade 1 | 0 (0%) | 1 (13%) |
| NKTR-255 dose | ||
| 1.5 μg/kg | 3 (33%) | — |
| 3.0 μg/kg | 4 (44%) | — |
| 6.0 μg/kg | 2 (22%) | — |
| Number of NKTR-255 infusions (median, range) | 2 (1-5) | — |
Ph, Philadelphia chromosome.
Two patients in the NKTR-255 cohort and 1 in the control group did not have a formal CD22 evaluation.
One patient in the NKTR-255 cohort previously received the same CAR19-22 and achieved CR and underwent allogeneic HCT before eventual relapse and was retreated with CAR19-22 + NKTR-255 after HCT. One patient in the control cohort received an investigational CAR19 product twice before CAR19-22.
The cohort of control patients previously treated with CAR19-22 was older (median age, 49 vs 36 years), less likely to have Philadelphia chromosome–negative disease (63%), and received more lines of therapy (median, 4; range, 2-10) including higher rates of prior transplant (75%; Table 1). Rates of prior CAR19, blinatumomab, and inotuzumab were similar between the 2 cohorts. All 8 patients had low tumor burden at screening. Three of the historical control patients received CAR19-22 at a lower dose than the RP2D (1.5 × 106 cells per kg vs 3.0 × 106 cells per kg), however, no dose response was observed in the prior CAR dose-finding study.6
Safety
Of 9 patients who ultimately received NKTR-255, 4 (44%) patients experienced CRS after CAR19-22 (Table 1). All cases of CRS were grade 1 and resolved before the first dose of NKTR-255. Of 8 control patients, 4 (50%) developed CRS after CAR19-22; 3 cases were grade 2, and the remaining case was grade 1. There were no cases of ICANS either before or after NKTR-255; 1 patient in the control cohort experienced ICANS (Table 1). There was 1 case of IEC-HS related to CAR19-22 in the NKTR-255 cohort that occurred in 1 of 2 patients who did not receive NKTR-255; there were no cases of IEC-HS in the control cohort.
No DLTs related to NKTR-255 were observed. Fevers after NKTR-255 were common, occurring after 55% of patients’ first infusion and 55% of subsequent infusions (Table 2). The onset of fever was typically between 4 and 12 hours after infusion and resolved within 24 hours of onset. All fevers after NKTR-255 were grade 1 or 2 in severity and managed with acetaminophen. No episode of fever required treatment with steroids or cytokine blockade (eg, tocilizumab and anakinra).
Safety of NKTR-255
| Adverse effect . | Cycle 1 (n = 9 infusions), n (%) . | Subsequent cycles (n = 12 infusions), n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Anemia | — | 4 (44) | — | — | 1 (8) | — | 1 (8) | — |
| Neutropenia | — | — | 2 (22) | 2 (22) | 2 (17) | — | 1 (8) | — |
| Thrombocytopenia | 4 (44) | — | — | 1 (11) | 1 (8) | — | — | — |
| Fevers | 4 (44) | 1 (11) | — | — | 5 (42) | — | — | — |
| Chills | 2 (22) | — | — | — | 2 (17) | — | — | — |
| Nausea | 2 (22) | — | — | — | — | — | — | — |
| Vomiting | 1 (11) | — | — | — | — | — | — | — |
| Myalgias | — | — | — | — | 2 (17) | — | — | — |
| Infusion reaction | 1 (11) | 1 (11) | — | — | — | — | — | — |
| Fatigue | — | 1 (11) | — | — | — | — | — | — |
| Headache | — | 1 (11) | — | — | — | — | — | — |
| Dizziness | 1 (11) | — | — | — | — | — | — | — |
| Sinus tachycardia | 1 (11) | — | — | — | — | — | — | — |
| Hypotension | — | — | — | — | — | — | 1 (8) | — |
| Dyspnea | — | — | — | — | — | — | 1 (8) | — |
| Hypoxia | — | — | — | — | — | — | 1 (8) | — |
| Diarrhea | — | — | — | — | — | 1 (8) | — | — |
| Adverse effect . | Cycle 1 (n = 9 infusions), n (%) . | Subsequent cycles (n = 12 infusions), n (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | |
| Anemia | — | 4 (44) | — | — | 1 (8) | — | 1 (8) | — |
| Neutropenia | — | — | 2 (22) | 2 (22) | 2 (17) | — | 1 (8) | — |
| Thrombocytopenia | 4 (44) | — | — | 1 (11) | 1 (8) | — | — | — |
| Fevers | 4 (44) | 1 (11) | — | — | 5 (42) | — | — | — |
| Chills | 2 (22) | — | — | — | 2 (17) | — | — | — |
| Nausea | 2 (22) | — | — | — | — | — | — | — |
| Vomiting | 1 (11) | — | — | — | — | — | — | — |
| Myalgias | — | — | — | — | 2 (17) | — | — | — |
| Infusion reaction | 1 (11) | 1 (11) | — | — | — | — | — | — |
| Fatigue | — | 1 (11) | — | — | — | — | — | — |
| Headache | — | 1 (11) | — | — | — | — | — | — |
| Dizziness | 1 (11) | — | — | — | — | — | — | — |
| Sinus tachycardia | 1 (11) | — | — | — | — | — | — | — |
| Hypotension | — | — | — | — | — | — | 1 (8) | — |
| Dyspnea | — | — | — | — | — | — | 1 (8) | — |
| Hypoxia | — | — | — | — | — | — | 1 (8) | — |
| Diarrhea | — | — | — | — | — | 1 (8) | — | — |
Cytopenias were also common after NKTR-255 (Table 2). Anemia (44%) and thrombocytopenia (55%) were common after the first infusion and recurred at lower rates upon subsequent NKTR-255 doses (anemia, 16%; and thrombocytopenia, 8%). Cytopenias were typically self-limited, although 1 patient had persistent grade 3 neutropenia and grade 4 thrombocytopenia after CAR19-22 plus day +14 NKTR-255 that were ongoing at time of preplanned allogeneic transplantation (supplemental Figure 2B-C). Although neutropenia occurred at all dose levels, both patients treated at the highest dose level of NKTR-255 (6.0 μg/kg) experienced grade 3 or 4 neutropenia compared with 2 cases of grade 3/4 neutropenia at lower doses (supplemental Table 2). Supportive care for neutropenia followed institutional protocols including granulocyte colony-stimulating factor use for absolute neutrophil counts of <1000 cells per μL, and mold and bacterial prophylaxis. There were no bacterial or fungal infections after treatment with NKTR-255. Cytopenias were also common among the control cohort after CAR19-22, including anemia (n = 6, 75%), neutropenia (n = 7, 88%), and thrombocytopenia (n = 5, 63%). One control patient developed prolonged grade 3/4 panctyopenia after CAR19-22 without count recovery (supplemental Figure 2D-F).
Patients received a median of 2 cycles of NKTR-255 (range, 1-5; Figure 1A). Causes for NKTR-255 discontinuation included ALL relapse (n = 3), allogeneic HCT (n = 2), infection (n = 1), inflammation (n = 1), and clinician decision (n = 2). The discontinuation related to infection occurred in a patient who developed a viral respiratory tract infection that was deemed unlikely related to NKTR-255 but took several weeks to resolve. The discontinuation because of inflammation occurred in a patient with abdominal pain and rise in C-reactive protein after starting hormone replacement therapy before the sixth dose of NKTR-255. Both the pain and rise in C-reactive protein were self-limited and deemed unrelated to NKTR-255.
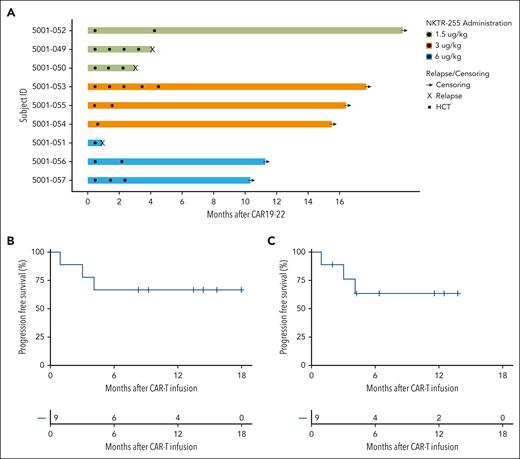
Clinical Outcomes. (A) Swimmer plot for patients who received CAR19-22 and NKTR-255; 3 patients received NKTR-255 dose-level 1 (1.5 μg/kg, green), 4 patients received dose-level 2 (3.0 μg/kg, orange), and 2 patients received dose-level 3 (6.0 μg/kg, blue). Circles represent doses of NKTR-255, squares represent allogeneic stem cell transplant, and X’s represent relapse. (B) PFS for patients who received NKTR-255 (n = 9) with events as death or relapse and without censoring for allogeneic HCT. (C) PFS considering allogeneic HCT as censoring event.
Clinical Outcomes. (A) Swimmer plot for patients who received CAR19-22 and NKTR-255; 3 patients received NKTR-255 dose-level 1 (1.5 μg/kg, green), 4 patients received dose-level 2 (3.0 μg/kg, orange), and 2 patients received dose-level 3 (6.0 μg/kg, blue). Circles represent doses of NKTR-255, squares represent allogeneic stem cell transplant, and X’s represent relapse. (B) PFS for patients who received NKTR-255 (n = 9) with events as death or relapse and without censoring for allogeneic HCT. (C) PFS considering allogeneic HCT as censoring event.
Efficacy
Of 9 patients, 8 (89%) achieved a CR with or without hematologic recovery, all without detectable MRD (Table 3). Two patients received allogeneic HCT in CR (Figure 1A). Three patients (33%) relapsed (1 with loss of CD19), all within 6 months of CAR19-22. For patients who received NKTR-255, 12-month PFS was 67%, and with 14.4 months of follow-up, the median PFS has not been reached (Figure 1B). Similar results were obtained if treating allogeneic transplantation as a censoring event (Figure 1C) and in the intention-to-treat population, which includes the 2 patients who were ineligible for NKTR-255 infusion (supplemental Figure 3). After median follow-up of 24.5 months, the median PFS of the control cohort was 3.9 months, and 12-month PFS was 38% (supplemental Figure 4).
Efficacy of CAR19-22 with NKTR-255
| . | CAR19-22 + NKTR-255 (n = 9) . | CAR19-22 control (n = 8) . |
|---|---|---|
| CR/CRi | 8 (89%) | 7 (88%) |
| MRD-negative | 8 (89%) | 6 (75%) |
| Progression/relapse | 3 (33%) | 5 (62%) |
| Consolidative HCT | 2 (23%) | 1 (13%) |
| 6-month RFS (%, 95% CI) | 67% (42-100) | 38% (15-92) |
| 12-month RFS (%, 95% CI) | 67% (42-100) | 38% (15-92) |
| . | CAR19-22 + NKTR-255 (n = 9) . | CAR19-22 control (n = 8) . |
|---|---|---|
| CR/CRi | 8 (89%) | 7 (88%) |
| MRD-negative | 8 (89%) | 6 (75%) |
| Progression/relapse | 3 (33%) | 5 (62%) |
| Consolidative HCT | 2 (23%) | 1 (13%) |
| 6-month RFS (%, 95% CI) | 67% (42-100) | 38% (15-92) |
| 12-month RFS (%, 95% CI) | 67% (42-100) | 38% (15-92) |
CRi, complete response with incomplete count recovery; RFS, relapse free survival; MRD, measurable residual disease.
IL15 pharmacokinetics and cytokine dynamics
During the first week after CAR19-22, we observed no significant difference in IL15 levels between the interventional and control patients (Figure 2A). However, after day +14 NKTR-255, we observed significant increases in IL15 compared with preinfusion levels on the same day (median mean fluorescent intensity [MFI], 15 898 vs 69; P = .039; Figure 2B). IL15 levels gradually returned to preinfusion baseline by day +21. From day +14 before NKTR-255 infusion to day +15, we observed significant increases in interferon-γ (median MFI, 35.0 vs 71.3; P = .031) and related proinflammatory cytokines, namely IL10 (median MFI, 78.0 vs 272.3; P = .031) and IL6 (median MFI, 119.0 vs 184.0; P = .031; Figure 2C). We also observed significant increases in chemokines induced by interferon-γ including CXCL9 (median MFI, 3020 vs 8508; P = .031) and CXCL10 (median MFI, 5394 vs 14 277; P = .031). Among common γ-chain cytokines, we observed significant increases in IL4 levels on day +14 after infusion (median MFI, 28.0 vs 116.8; P = .023) but not IL2 or IL7. In the subset of patients for whom serial blood samples were available after day +28 (n = 4), we continued to observe increases in IL15 after additional NKTR-255 infusions (supplemental Figure 5).
Cytokine profiling. (A) IL15 during the first month after CAR19-22 infusion. Shown are medians and interquartile ranges for MFIs of IL15 for control patients (red) and those treated with NKTR-255 (blue). NKTR-255 administration is denoted by the red dashed line. The number of observations at each time point is shown below the graph. “PLD” indicates time points before lymphodepleting chemotherapy. Control patients did not have samples at day +15 or +16 available for analysis. Cytokine levels before and after NKTR-255 administration for patients with both samples available for analysis. (B) Comparison of day +14 preinfusion with postinfusion values (n = 8); (C) comparison day +14 preinfusion with day +15 values (n = 6). P values were calculated with the paired Mann-Whitney U test. Cytokines were grouped according to common γ-chain cytokines (top), interferon-γ–related (middle), and proinflammatory cytokines (bottom).
Cytokine profiling. (A) IL15 during the first month after CAR19-22 infusion. Shown are medians and interquartile ranges for MFIs of IL15 for control patients (red) and those treated with NKTR-255 (blue). NKTR-255 administration is denoted by the red dashed line. The number of observations at each time point is shown below the graph. “PLD” indicates time points before lymphodepleting chemotherapy. Control patients did not have samples at day +15 or +16 available for analysis. Cytokine levels before and after NKTR-255 administration for patients with both samples available for analysis. (B) Comparison of day +14 preinfusion with postinfusion values (n = 8); (C) comparison day +14 preinfusion with day +15 values (n = 6). P values were calculated with the paired Mann-Whitney U test. Cytokines were grouped according to common γ-chain cytokines (top), interferon-γ–related (middle), and proinflammatory cytokines (bottom).
CAR expansion, persistence, and trafficking
All patients who received CAR19-22 and NKTR-255 had detectable CAR+ T cells in the blood during the first month after infusion. After day +14 NKTR-255 infusion, there was no change in CAR19-22 as measured by quantitative PCR (Figure 3A) or CARFACS (Figure 3B). However, on day +15 compared with day +14 before infusion, we observed a decrease in CD4+ (median, 0.65 vs 1.45 cells per μL; P = .109) and CD8+ CAR19-22 cells (median, 0.43 vs 2.38 cells per μL; P = .148; Figure 3C-D). Given the relatively larger decrease in CD8+ CAR19-22 cells in blood, the CD4:CD8 ratio rose after day +14 NKTR-255 administration (0.776 vs 1.38, P = .055; Figure 3E).
CAR19-22 expansion and persistence. Shown are CAR19-22 levels (medians with interquartile ranges) in the peripheral blood in the first month after CAR infusion as measured by (A) quantitative PCR and (B) CARFACS. NKTR-255 administration is denoted by the red dashed line. The number of observations at each time point is shown below the graph. (C) CD4 and (D) CD8 CAR subsets assessed by CARFACS. (E) CD4:CD8 CAR+ ratio during the first month after CAR19-22. (F) ALC (circles) and total CD3+ T cells by CARFACS (triangles).
CAR19-22 expansion and persistence. Shown are CAR19-22 levels (medians with interquartile ranges) in the peripheral blood in the first month after CAR infusion as measured by (A) quantitative PCR and (B) CARFACS. NKTR-255 administration is denoted by the red dashed line. The number of observations at each time point is shown below the graph. (C) CD4 and (D) CD8 CAR subsets assessed by CARFACS. (E) CD4:CD8 CAR+ ratio during the first month after CAR19-22. (F) ALC (circles) and total CD3+ T cells by CARFACS (triangles).
Next, we assessed total lymphocyte dynamics at the same time points after day +14 NKTR-255. On day +15, absolute lymphocyte counts (ALCs) decreased significantly in patients treated with NKTR-255 compared with day +14 before infusion (median, 95 vs 460 cells per μL, P = .016; Figure 3F). By day +28, we observed a significant rebound of lymphocytes compared with day +15 (median ALC, 95 vs 1160 cells per μL; P = .021). The vast majority of these rebounding lymphocytes did not express the CAR construct as assessed by flow cytometry (supplemental Figure 6). At their peak on day +21, 42% of the rebounding lymphocytes were CD3+, indicating expansion of both T cells and non–T-cell lymphocyte subsets (Figure 3F). CAR19-22 remained detectable in the peripheral blood at similar levels up to day +180 in both control patients and those treated with NKTR-255 (supplemental Figure 7).
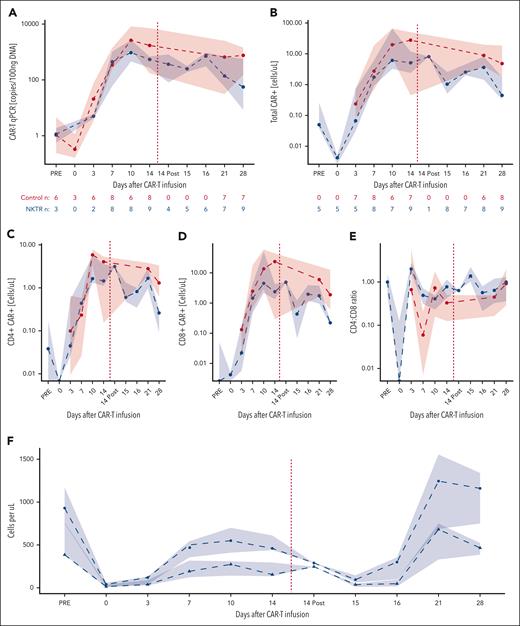
Given the role of IL15 in lymphocyte trafficking, the increases in CXCL9 and CXCL10 and decreases in ALC and CD8+ CAR19-22 in the blood, we hypothesized that NKTR-255 may be driving lymphocytes into tissues. We, therefore, profiled CAR levels in the blood and CSF in 2 patients in the NKTR-255 cohort with confirmed central nervous system (CNS) disease. ALCs and CD8+ CARs decreased in THE blood in both patients after NKTR-255 administration (Figure 4A-B). Both patients had a 10-fold increase in CAR+ cells in the CSF, with the increase in Patient 1 occurring on day +17 compared with day +28 in Patient 2 (Figure 4C), suggestive of trafficking to the CNS. In comparison, among 3 control patients with CSF samples available at day +28, we did not see elevations in CAR+ cells in the CSF: 2 patients had <1 white blood cell per μL; the third patient had 10-fold fewer CAR+ cells than the 2 patients treated with NKTR-255 (median, 0.00911 vs 0.292; Figure 4C).
CAR trafficking. CAR dynamics for patients with CNS leukemia. Shown are (A) peripheral blood ALC, (B) CD8+ CAR-Ts, and (C) CSF absolute white blood cell count (WBC; circles) and CAR19-22+ cells (triangles) for patients treated with NKTR-255 with CNS disease (n = 2). (D) WBC count (circles) and CAR+ cells in the CSF on day +28 evaluated by flow cytometry for the same patients treated with NKTR-255 (blue, n = 2) and the historical control patient with evaluable CSF (red, n = 1).
CAR trafficking. CAR dynamics for patients with CNS leukemia. Shown are (A) peripheral blood ALC, (B) CD8+ CAR-Ts, and (C) CSF absolute white blood cell count (WBC; circles) and CAR19-22+ cells (triangles) for patients treated with NKTR-255 with CNS disease (n = 2). (D) WBC count (circles) and CAR+ cells in the CSF on day +28 evaluated by flow cytometry for the same patients treated with NKTR-255 (blue, n = 2) and the historical control patient with evaluable CSF (red, n = 1).
We also assessed CAR levels via flow cytometry in the bone marrow for patients with aspirates available. There was a numerically higher percentage of CD3+ cells in patients treated with NKTR-255 (median, 42.0% vs 23.7%; P = .4; supplemental Table 4). However, the proportion of these CD3+ cells expressing the CAR construct was low in both groups and there was no evidence of enhanced migration of CAR19-22 to the bone marrow in patients treated with NKTR-255 (supplemental Table 3).
Discussion
Our trial represents, to our knowledge, the first attempt to combine a recombinant cytokine product with CAR-T therapy, and demonstrates this approach to be both feasible and safe. All patients had successful manufacturing and infusion of CAR19-22, and 88% received at least 1 dose of NKTR-255. The most common AEs in patients receiving NKTR-255 were fevers, chills, and myelosuppression, which were manageable with supportive care and were comparable with toxicities seen after CAR19-22 alone. We did not observe DLTs at any of the doses of NKTR-255 tested; however, we did note more prominent cytopenias at the highest dose of 6.0 μg/kg.
Outcomes in this population were favorable, with high rates of MRD-negative responses (88%). Our prior study of CAR19-22 showed similarly high initial response rates, but 58% of patients relapsed within 6 months of CAR infusion. With a median follow-up of 14.4 months, only 3 patients receiving combination therapy (33%) relapsed, which may suggest that administration of NKTR-255 helps prevent early disease recurrence although the nonrandomized nature of our study precludes definitive efficacy assessment. Although longer-term follow-up is needed to assess for late relapses, all relapses in the initial phase 1 trial of CAR19-22 occurred within 6 months of CAR infusion as was seen in this study’s control cohort.
Our correlative analyses suggest that NKTR-255 might influence lymphocyte trafficking to tissues. After NKTR-255 administration, we observed dramatic increases in cytokines typically secreted from activated T cells, including CXCL9 and CXCL10, that promote migration of lymphocytes into tissues in which they exert their effects on tumor cells. This hypothesis is supported by the decrease in CD8+ CAR-Ts and endogenous lymphocytes in the peripheral blood and the increase in CAR19-22 in the CSF of patients with confirmed CNS disease shortly after NKTR-255 administration. Prior studies of recombinant IL15 also support the notion that IL15 activates T cells and promotes migration of lymphocytes and NK cells to tissue.12,17 After this decrease in lymphocytes and CD8+ CAR19-22, we observed a rebound in a heterogenous group of lymphocytes including both CAR and non-CAR cells, as has been described in a nonhuman primate model of NKTR-255.19 Further studies should address the nature of these lymphocytes and how they interact with CAR-Ts to shape the immune response against leukemia.
Our study has several important limitations. The noncontemporaneous control arm received the same CAR19-22 construct and had similar disease burden, but, without randomization, differences in outcomes might reflect residual confounding. The relatively small study cohort prohibited more robust matching. Comparisons between the 2 cohorts should therefore be interpreted with caution. Furthermore, we were unable to replace participants who did not receive NKTR-255 because of limited availability of lentiviral vector for CAR19-22 manufacturing. Longer-term follow-up and larger sample sizes are needed to assess both late relapses and OS. Although we hypothesize that NKTR-255 might improve lymphocyte trafficking to tissue and activate both CAR and non-CAR mediators of antitumor immunity, we did not have enough contemporaneous tissue and blood samples to robustly test this.
In conclusion, our study demonstrates that administration of a novel CAR-T product followed by infusion of a pegylated IL15 receptor agonist is feasible. We speculate that NKTR-255 might improve lymphocyte trafficking to tissue and activate both CAR and non-CAR mediators of antitumor immunity. Larger, randomized trials are ultimately needed for accurate assessment of efficacy and toxicity. Future correlative studies to understand the effect of NKTR-255 on CAR trafficking, function, and phenotype are warranted.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grants 2P01CA049605-29A1 (CM. and D.M.) and 5P30CA124435 (C.M.), and by the Virginia and D.K. Ludwig Fund for Cancer Research. C.M is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program.
Authorship
Contribution: D.M., C.M., and L. Muffly conceived and designed the study; H.S., C.J., P.S., N.J., M.H., E.E., S.M., B.S., N.A., A.K., A.M.K., S.A., S.B., S.D., H.H., L.J., V.K., M.L., R.L., L. Mikkilineni, R.N., A.R., S.S., J.S., M.S., W.-K.W., S.F., M.J.F., D.M., C.M., and L. Muffly collected the data and wrote the manuscript; and all authors contributed to writing and editing the manuscript.
Conflict-of-interest disclosure: D.M. reports consulting for Kite Pharma-Gilead, Juno Therapeutics-Celgene, Novartis, Janssen, and Pharmacyclics; and reports research support from Kite Pharma-Gilead, Allogene, Cargo Therapeutics, Pharmacyclics, Miltenyi Biotec, and Adaptive Biotechnologies. C.M. is a founder of, holds equity in, and consults for, CARGO Therapeutics, Link Cell Therapies, and GBM NewCo; holds equity in and consults for Ensoma and Red Tree Capital; consults for Immatics; receives research funding from Tune Therapeutics and Lyell Immunopharma; receives royalties from the National Institutes of Health and Stanford University for CD22-CAR; and holds multiple patents related to CAR-T therapies. S.S. reports research funding for Magenta Therapeutics, Bristol Myers Squibb, Allogene, Janssen, and Novartis; and reports consultancy to Magenta Therapeutics, Bristol Myers Squibb, Janssen, Sanofi, Oncopeptides, Takeda, Regeneron, AbbVie, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Lori Muffly, Stanford University, Medicine, 780 Welch Rd, CJ250B, Palo Alto, CA 94304; email: lmuffly@stanford.edu.
References
Author notes
Original data are available upon request from the corresponding author, Lori Muffly (lmuffly@stanford.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal