Key Points
RBCs promote fibrin formation and hemostasis in mice in the condition of severe pancytopenia.
Altering RBC membrane deformability interferes with the ability of RBCs to support platelet aggregation in vitro.
Visual Abstract
Red blood cells (RBCs) have been hypothesized to support hemostasis by facilitating platelet margination and releasing platelet-activating factors such as adenosine 5′-diphosphate (ADP). Significant knowledge gaps remain regarding how RBCs influence platelet function, especially in (patho)physiologically relevant hemodynamic conditions. Here, we present results showing how RBCs affect platelet function and hemostasis in conditions of anemia, thrombocytopenia, and pancytopenia and how the biochemical and biophysical properties of RBCs regulate platelet function at the blood and vessel wall interface and in the fluid phase under flow conditions. We found that RBCs promoted platelet deposition to collagen under flow conditions in moderate (50 × 103/μL) but not severe (10 × 103/μL) thrombocytopenia in vitro. Reduction in hematocrit by 45% increased bleeding in mice with hemolytic anemia. In contrast, bleeding diathesis was observed in mice with a 90% but not with a 60% reduction in platelet counts. RBC transfusion improved hemostasis by enhancing fibrin clot formation at the site of vascular injury in mice with severe pancytopenia induced by total body irradiation. Altering membrane deformability changed the ability of RBCs to promote shear-induced platelet aggregation. RBC-derived ADP contributed to platelet activation and aggregation in vitro under pathologically high shear stresses, as observed in patients supported by left ventricular assist devices. These findings demonstrate that RBCs support platelet function and hemostasis through multiple mechanisms, both at the blood and vessel wall interface and in the fluidic phase of circulation.
Introduction
Growing evidence suggests that red blood cells (RBCs) may play an important role in supporting hemostasis. In clinical observation, lower RBC counts are associated with prolonged bleeding times,1-3 which improve with RBC transfusion2,4 and correction of iron deficiency.5 However, these clinical observations provide limited mechanistic insights. Furthermore, prior studies have largely focused on the prothrombotic characteristics of RBCs, such as the release of adenosine 5′-diphosphate (ADP),6,7 direct RBC-platelet interaction,8,9 interaction with fibrinogen,10 and increased procoagulant activity from phosphatidylserine-exposed RBCs and derivative extracellular vesicles (EVs),11,12 with limited information on hemostasis.
The influence of RBCs on hemostasis is often attributed to their hemorheologic role in facilitating platelet margination by pushing platelets toward the vessel wall. Using an in vitro perfusion model, Turitto and Baumgartner demonstrated that the presence of RBCs increases platelet deposition and platelet-surface reactivity along denuded endothelium by 57-fold,13 and the degree of platelet adhesion increases with rising hematocrit.14 In anemic conditions with hematocrits <40%, smaller subendothelial platelet hemostatic plugs are observed.15,16 Other experimental studies and theoretical simulations have subsequently modeled the kinetics of hematocrit-dependent platelet margination and predicted that platelet-endothelial contact is dramatically reduced when hematocrit is <20%.16-20 When low hematocrit is combined with low platelet counts (∼50 × 103/μL), platelet thrombus formation is near absent.15 Platelet margination is also modulated by shear stress generated by flowing viscous blood, although the relative impact of shear on platelet margination and adhesion to the vessel wall is complex and has been variably reported depending on experimental flow rates and RBC counts.14,16,21-23
These in vitro observations are supported by limited information from in vivo models. RBC transfusions have been shown to improve bleeding times in animal models of anemia8,24 and of combined anemia and thrombocytopenia down to a platelet count of 80 × 103/μL.25 Significant knowledge gaps remain in differentiating the effects of RBCs on hemostasis at varying platelet counts, particularly in conditions of severe thrombocytopenia. Here, we present results of a study designed to address key knowledge gaps by determining the contribution of RBCs on platelet aggregation under physiological and pathological shear stress and by elucidating the underlying biophysical and biochemical mechanisms of interactions among RBCs, platelets, and coagulation.
Methods
Mouse models
We used adult C57BL/6J mice (50% male, aged 10-20 weeks, and weighing 20-30 g; Jackson Laboratory, Bar Harbor, ME) to evaluate the hemostatic effects of RBCs under disease-related conditions. First, we decreased hematocrit by inducing hemolytic anemia using phenylhydrazine (PHZ; 60 mg/kg, intraperitoneal injection; Sigma-Aldrich, Saint Louis, MO). Second, we induced acute immune thrombocytopenia (ITP) by IV injection of a monoclonal (2 μg/g body weight; Emfret Analytics, Wurzburg, Germany) or polyclonal antiglycoprotein 1bα (GP1bα) antibody (25 μg; MyBiosource, San Diego, CA). Control mice received phosphate-buffered saline (PBS). Tail bleeding was assessed 24 hours after PHZ and 3 hours after anti-GP1bα antibody treatment (supplemental Methods, available on the Blood website).26,27 Third, mice received 6.5 Gy total body irradiation to simulate therapy–induced hypoproliferative pancytopenia (supplemental Methods). The kinetics of platelet recruitment and fibrin clot formation28,29 at the site of laser–induced vascular puncture to the cremaster arteriole were assessed in mice that received RBC transfusion and control mice 10 days after irradiation. All animal experiments were conducted in accordance with the Declaration of Helsinki and approved by the institutional animal care and use committee of the Bloodworks Research Institute.
Platelet adhesion and aggregation under flow conditions
Human blood was collected in 3.2% sodium citrate from healthy donors under a protocol approved by the Bloodworks Institutional Review Board. Platelet-rich plasma (PRP) was obtained by centrifuging whole blood (WB) at 120g for 20 minutes at room temperature (RT). After isolating the PRP and discarding the buffy coat, washed RBCs were obtained by resuspending the remaining sample in PBS and centrifuged 3 times at 1500g at RT for 9 minutes each. Platelet-poor plasma was isolated by centrifuging PRP at 2000g at RT for 5 minutes to collect the supernatant. Blood counts of the homologous components were measured (ABX Micros 60; Horiba Medical, Montpellier, France) and reconstituted to the desired hematocrit and platelet count. Reconstituted blood was perfused over immobilized collagen at an arterial wall shear stress of 2 Pa in a microfluidic chamber. The areas covered by platelet aggregates were quantified (supplemental Methods).30,31
To model RBC-platelet interaction in the fluid phase of circulation, we exposed PRP and WB to constant shear rates of 500 to 10 000 per second or to constant shear stresses of 1 to 26 Pa for 5 minutes at 37°C using a cone-and-plate viscometer (HAAKE RheoStress 1; ThermoFisher Scientific, Waltham, MA; supplemental Methods).32 Shear–induced platelet aggregation (SIPA) was defined as the percentage reduction of single platelets compared with homologous unsheared samples. For a subset of experiments, cold-stored RBCs and RBCs treated with glutaraldehyde (Sigma-Aldrich) or methyl-β-cyclodextrin (MβCD; Sigma-Aldrich), all of which altered membrane deformability, were assessed for their effect on SIPA (supplemental Methods). RBC deformability under flow conditions was also quantitatively measured as previously described (supplemental Methods).33
To measure the additive effects of high shear stress and platelet agonists, PRP was exposed to 10 Pa of constant shear stress on the cone-and-plate viscometer for 5 minutes at 37°C (unsheared samples as control). ADP- or collagen–induced platelet aggregation was then measured using optical aggregometry (supplemental Methods). ADP released from RBCs under shear stress was assessed using fluorescence spectroscopy (supplemental Methods). To further evaluate the role of ADP, RBCs were exposed to 25 Pa of constant shear stress with the following treatment regimens: (1) shear exposure alone to induce ADP release; (2) washing with PBS to remove ADP released from sheared RBCs; and (3) shear exposure in the presence of apyrase (1 U/mL, 10 minutes at 37°C; Sigma-Aldrich) followed by PBS washing. The shear-exposed RBCs were then reconstituted with homologous PRP and exposed to 8 Pa of shear stress to quantify SIPA.
To evaluate the impact of shear stress on RBC lysis, which could release ADP to activate platelets, we examined samples from patients with end-stage heart failure before and after implantation of a Heartmate II left ventricular assist device (LVAD). The patients were recruited from the Center for Advanced Heart Failure, The University of Texas Houston Health Science Center under a protocol approved by the The University of Texas Institutional Review Board (supplemental Methods).
Flow cytometry
Statistical analysis
Data from human participants are presented as box plots with medians and first (25%) and third (75%) quartiles to show heterogeneity among participants. Mouse data are presented as bar graphs because they are relatively homogenous due to their clonal nature. Continuous variables are expressed as mean ± standard deviation and categorical variables as percentages. Multiple group comparisons were made using 1-way or 2-way analysis of variance, as indicated, and individual pairwise comparisons were assessed with Tukey honestly significant difference tests if the data passed the normality test or with Kruskal-Wallis analysis of variance on ranks test if they failed. To compare data between 2 groups, Student t test (paired and unpaired) or the Mann-Whitney rank sum test was used depending on the results of normality tests. Data analyses were conducted in R (version 4.1.0) or SigmaPlot V11 (Systat Software Inc, San Jose, CA).
Results
RBCs increased platelet thrombus formation under flow conditions
To mimic platelet-subendothelial interaction during hemostasis, human blood was reconstituted at varying hematocrit (20%, 30%, and 40%)-to-platelet (10 × 103/μL, 50 × 103/μL, 100 × 103/μL, and 300 × 103/μL) ratios and perfused through a microfluidic chamber at an arterial wall shear stress of 2 Pa. The chamber was coated with collagen, which is the major component of the subendothelial matrix. Surface area coverage by platelet thrombi was significantly greater at a hematocrit of 30% than at 20% for platelet counts of 50 × 103/μL, 100 × 103/μL, and 300 × 103/μL (Figure 1A-C). However, minimal platelet thrombus was formed at a platelet concentration of 10 × 103/μL among hematocrits of 20%, 30%, and 40% (Figure 1D).
RBCs increased platelet thrombus formation on collagen under flow conditions. Human blood reconstituted to varying hematocrits (HCTs) and platelet counts was perfused over a collagen-coated surface using a flow perfusion chamber at a shear stress of 2 Pa. The proportion of surface area covered by platelet thrombus was calculated and is expressed as mean thrombus surface percentage ± standard deviation. (A-C) Representative images of platelet thrombus formed on immobilized collagen after 5-minute perfusion (scale bar, 50 μm) and collective data from multiple independent experiments with different platelet counts and HCTs (n = 49-50 per experimental condition; Student t test). (D) Representative images of thrombus surface coverage (scale bar, 50 μm) after 10-minute perfusion of blood reconstituted to a platelet concentration of 10 × 103/μL and varying HCT (n = 50-59 per experimental condition; 1-way analysis of variance [ANOVA] and Tukey honestly standard deviation).
RBCs increased platelet thrombus formation on collagen under flow conditions. Human blood reconstituted to varying hematocrits (HCTs) and platelet counts was perfused over a collagen-coated surface using a flow perfusion chamber at a shear stress of 2 Pa. The proportion of surface area covered by platelet thrombus was calculated and is expressed as mean thrombus surface percentage ± standard deviation. (A-C) Representative images of platelet thrombus formed on immobilized collagen after 5-minute perfusion (scale bar, 50 μm) and collective data from multiple independent experiments with different platelet counts and HCTs (n = 49-50 per experimental condition; Student t test). (D) Representative images of thrombus surface coverage (scale bar, 50 μm) after 10-minute perfusion of blood reconstituted to a platelet concentration of 10 × 103/μL and varying HCT (n = 50-59 per experimental condition; 1-way analysis of variance [ANOVA] and Tukey honestly standard deviation).
Effects of RBCs on hemostasis in mouse models
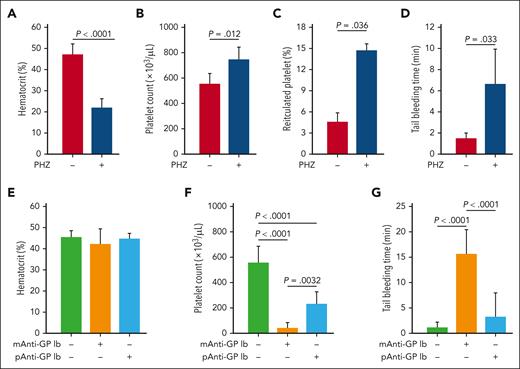
The data in Figure 1 suggest that RBCs increased platelet adhesion to and aggregation on the collagen matrix under flow conditions in vitro. We then used 3 mouse models to characterize the impact of RBCs on hemostasis in vivo by changing hematocrit and platelet counts individually and together. First, we induced hemolytic anemia with PHZ, which selectively destroys mature RBCs through oxidative stress.36,37 PHZ reduced RBC counts by 47.6% (supplemental Figure 1) and hematocrits by 45.5% (Figure 2A) relative to vehicle control. Platelet counts (Figure 2B) and percent of reticulated platelets increased in these mice (Figure 2C), consistent with a PHZ–induced reactive thrombocytosis. Tail bleeding times were prolonged in the PHZ-treated mice relative to control mice (Figure 2D).
Influence of RBCs and platelet concentration on tail bleeding time. For the hemolytic anemia model, mice were evaluated 24 hours after being treated with PHZ (n = 13) or an equal volume of buffer control (n = 15) for hematocrit (A), platelet count (B), reticulated platelet percentage (C), and tail bleeding time (D) (t test). For the ITP model, mice were evaluated for hematocrit (E), platelet count (F), and tail bleeding time (G) 3 hours after IV injection of a monoclonal (n = 6) or polyclonal (n = 10) anti-GP1bα antibody (1-way ANOVA).
Influence of RBCs and platelet concentration on tail bleeding time. For the hemolytic anemia model, mice were evaluated 24 hours after being treated with PHZ (n = 13) or an equal volume of buffer control (n = 15) for hematocrit (A), platelet count (B), reticulated platelet percentage (C), and tail bleeding time (D) (t test). For the ITP model, mice were evaluated for hematocrit (E), platelet count (F), and tail bleeding time (G) 3 hours after IV injection of a monoclonal (n = 6) or polyclonal (n = 10) anti-GP1bα antibody (1-way ANOVA).
Second, mice were infused with a monoclonal anti-GP1bα antibody, which increases antibody–mediated platelet clearance.38 The treatment did not change hematocrit (Figure 2E) but reduced platelet counts by 90% (Figure 2F) and prolonged tail bleeding times (Figure 2G). In contrast, treatment with a polyclonal anti-GP1bα antibody reduced platelet counts by 60% (Figure 2F) but did not cause statistically significant prolongation of bleeding times over controls (Figure 2G).
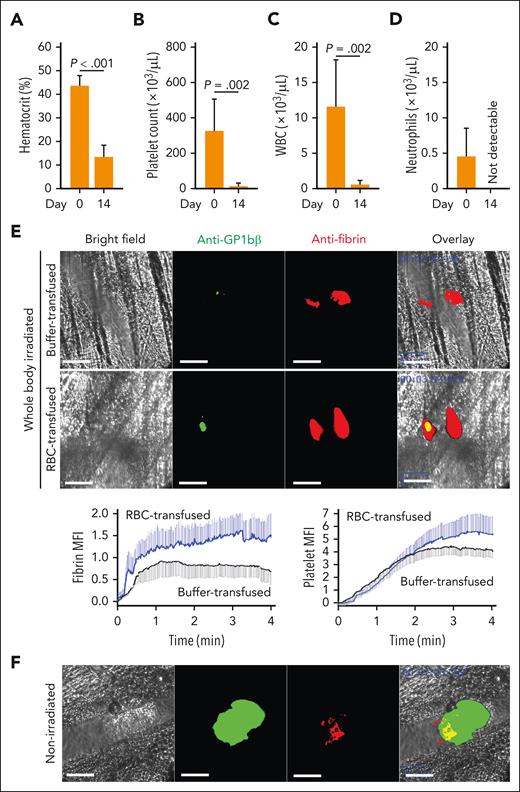
These models encapsulated hemolysis–induced anemia and acute ITP but also generated confounding conditions, such as increasing cell-free hemoglobin and oxidative stress in PHZ-treated mice and increasing hyperactive, immature platelets in both groups. To address these concerns and to model concurrent anemia and thrombocytopenia, we treated mice with a sublethal total body irradiation39 to mimic therapy-induced pancytopenia. The mice developed severe pancytopenia 10 to 14 days after irradiation (Figure 3A-D; supplemental Figure 2). At day 10, we examined local hemostasis in response to penetrating laser–induced vascular injury. There was no visible injury to the vessel wall under intravital microscopy at the site where laser injury was induced (supplemental Figure 3). Irradiated, severely pancytopenic mice formed small platelet-poor hemostatic clots that did not lead to vessel occlusion at the injury site (Figure 3E; supplemental Figure 4). RBC transfusion significantly enhanced hemostatic clot formation in irradiated mice by moderately increasing the platelet plug and, more notably, augmenting fibrin formation at the site of injury, resulting in a fibrin-rich clot that was threefold to fivefold larger than in irradiated mice receiving buffer infusion (Figure 3E). By contrast, nonirradiated mice formed a large platelet-rich hemostatic plug that partially overlapped with a small fibrin clot at the base of the platelet plug, resulting in partial vessel occlusion at the site of injury (Figure 3F). As a result, the platelet-to-fibrin ratio of irradiated mice was reversed compared with that of nonirradiated mice. These results demonstrate that RBCs promoted bleeding arrest in 3 mouse models with different platelet-to-RBC ratios, and RBC transfusion enhanced local coagulation to form fibrin-rich clots even in conditions of severe pancytopenia.
Hemostasis at the site of laser-induced injury. Mice subjected to 6.5 Gy total body irradiation had significantly reduced hematocrit (A), platelet count (B), white blood cell (WBC) count (C), and neutrophil count (D) (n = 6, paired t test). (E) Top panels: Representative images of platelet aggregation identified by Dylight 488 anti-GPIbβ antibody (green) and fibrin clot formation detected by Alexa Fluor 647 anti-fibrin antibody (red) in response to a penetrating vascular injury induced by pulse laser in cremaster arterioles of irradiated mice with or without RBC transfusion (bar = 50 μm); bottom panels: time-dependent changes in the formation of platelet and fibrin clots quantified using mean fluorescence intensity (MFI) of antibody-bound fibrin (left) and platelets (right) from 11 independent experiments (6 transfused with RBCs and 5 with cell-free plasma; 2-way ANOVA, P < .001) (bottom). (F) Representative images of platelet and fibrin hemostatic plugs at the site of an identical laser injury to nonirradiated C57BL/6J mice (bar = 50 μm).
Hemostasis at the site of laser-induced injury. Mice subjected to 6.5 Gy total body irradiation had significantly reduced hematocrit (A), platelet count (B), white blood cell (WBC) count (C), and neutrophil count (D) (n = 6, paired t test). (E) Top panels: Representative images of platelet aggregation identified by Dylight 488 anti-GPIbβ antibody (green) and fibrin clot formation detected by Alexa Fluor 647 anti-fibrin antibody (red) in response to a penetrating vascular injury induced by pulse laser in cremaster arterioles of irradiated mice with or without RBC transfusion (bar = 50 μm); bottom panels: time-dependent changes in the formation of platelet and fibrin clots quantified using mean fluorescence intensity (MFI) of antibody-bound fibrin (left) and platelets (right) from 11 independent experiments (6 transfused with RBCs and 5 with cell-free plasma; 2-way ANOVA, P < .001) (bottom). (F) Representative images of platelet and fibrin hemostatic plugs at the site of an identical laser injury to nonirradiated C57BL/6J mice (bar = 50 μm).
Effects of RBCs on SIPA
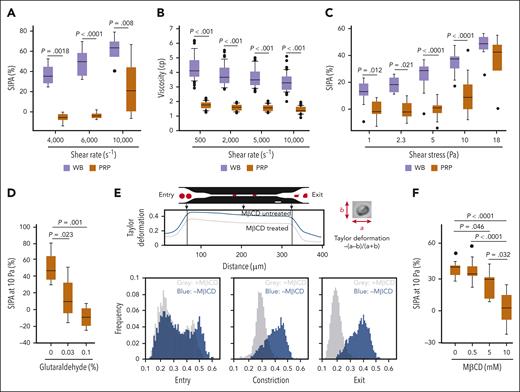
The results in Figures 1 to 3 demonstrate that RBCs improved hemostasis at the site of vascular injury (ie, blood vessel wall interface) but do not show whether RBCs also promote SIPA resulting from platelet-platelet interactions in the fluid phase of circulation. Therefore, we used a cone-and-plate viscometer, which generates a uniform shear stress, as opposed to a microfluidic channel, in which the shear stress varies spatially from a maximum value at the vessel wall to nearly 0 at the center of the channel. At shear rates of 4000, 6000, and 10 000 per second, SIPA was greater in the presence of RBCs than in their absence (Figure 4A). However, WB is more viscous than PRP (supplemental Figure 5). Although the viscosity of WB decreases with escalating shear rates (also known as shear-thinning behavior), at the rates we tested, WB remained more viscous than PRP both when tested at individual shear rates (Figure 4B) and with continuously increased shear rates (supplemental Figure 6). Therefore, at comparable shear rates, the platelets in WB experience greater shear stress than in PRP, which may account for the enhanced SIPA. To address this, we evaluated SIPA under controlled shear stress. SIPA was again significantly enhanced in WB relative to PRP at shear stresses ranging from 1 to 10 Pa (Figure 4C) but was similarly induced in WB and PRP at 18 Pa. After we exposed WB to a shear stress of 5.39 Pa, imaging flow cytometry detected 5.65% of platelets to be RBC bound (supplemental Figure 7). These results suggest that RBCs promoted SIPA in solution independent of blood viscosity.
Facilitation of SIPA and its regulation by membrane cholesterol. (A) SIPA observed after WB and PRP were exposed to different shear rates for 5 minutes at 37°C (n = 6 per condition; 2-way ANOVA). (B) Viscosities of WB and PRP under different shear rates (n = 88; 46% male; Mann-Whitney rank sum test). (C) SIPA in WB and PRP exposed different shear stresses for 5 minutes at 37°C (n = 5-26 per condition; 2-way ANOVA). (D) SIPA of platelets reconstituted with fresh, partially fixed (0.03% glutaraldehyde at 5 minutes), and completely fixed (0.1% glutaraldehyde at 1 hour) RBCs (n = 4 per condition; 1-way ANOVA, P = .0016). (E) MβCD (10 mM) treated and untreated RBCs were drawn through microfluidic channels under negative pressure (2 psi; bar = 20 μm) and their movements were recorded at 2000 fps. RBC deformability was quantified by Taylor deformation (top panel: deformation distributions at the entrance, middle, and exit of the constriction, representative of 5 independent runs). (F) Shear-induced aggregation of platelets reconstituted with MβCD-treated RBCs (n = 6-8 per condition; 1-way ANOVA, P < .0001). fps, frames per second.
Facilitation of SIPA and its regulation by membrane cholesterol. (A) SIPA observed after WB and PRP were exposed to different shear rates for 5 minutes at 37°C (n = 6 per condition; 2-way ANOVA). (B) Viscosities of WB and PRP under different shear rates (n = 88; 46% male; Mann-Whitney rank sum test). (C) SIPA in WB and PRP exposed different shear stresses for 5 minutes at 37°C (n = 5-26 per condition; 2-way ANOVA). (D) SIPA of platelets reconstituted with fresh, partially fixed (0.03% glutaraldehyde at 5 minutes), and completely fixed (0.1% glutaraldehyde at 1 hour) RBCs (n = 4 per condition; 1-way ANOVA, P = .0016). (E) MβCD (10 mM) treated and untreated RBCs were drawn through microfluidic channels under negative pressure (2 psi; bar = 20 μm) and their movements were recorded at 2000 fps. RBC deformability was quantified by Taylor deformation (top panel: deformation distributions at the entrance, middle, and exit of the constriction, representative of 5 independent runs). (F) Shear-induced aggregation of platelets reconstituted with MβCD-treated RBCs (n = 6-8 per condition; 1-way ANOVA, P < .0001). fps, frames per second.
Membrane deformability and its impact on SIPA
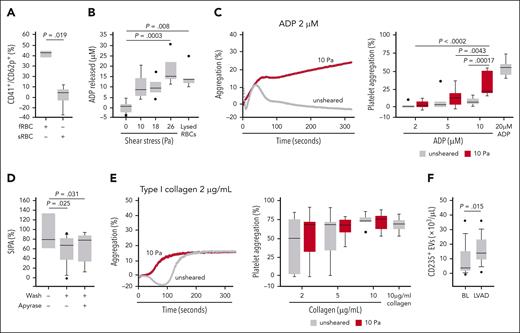
Having established that RBCs enhance platelet aggregation in solution, we examined the effects of modulating RBC membrane deformability on platelet aggregation. This is important because RBCs are highly deformable, a unique property that affects the local distribution of RBCs, platelets, and other cells in circulation.17,40 We have previously shown that glutaraldehyde-treated RBCs are less deformable when squeezed through a confinement that is smaller than the diameter of the cell.33 At 4000 per second, SIPA was progressively reduced in reconstituted blood containing fresh, partially fixed (5 minutes with 0.03% glutaraldehyde), and completely fixed RBCs (60 minutes with 0.1% glutaraldehyde; Figure 4D). However, the fixation could leave a residual amount of glutaraldehyde in the membrane, render RBCs biochemically inert,41 and change cellular osmolality in a concentration-dependent manner, distorting the cell’s shape (supplemental Figure 8).33 To address these pitfalls, we first modulated the deformability of RBCs with MβCD, which depletes membrane cholesterol without fixing cells or affecting cellular osmality.42,43 MβCD at 10 mM depleted >90% of RBC membrane cholesterol (supplemental Figure 9). To characterize the impact of MβCD on RBC deformability, we perfused RBCs through a microfluidic channel under negative pressure (supplemental Video 1) and estimated the Taylor deformation (Figure 4E). Before entering the channels, untreated and MβCD-treated RBCs showed similar deformability indices. Once in confinement, the MβCD-treated RBCs did not change shape as much as the untreated RBCs, as indicated by the more circular shape of the former and the more elongated shape of the latter, suggesting that membrane rigidity was increased by MβCD treatment. SIPA induced by 10 Pa of shear stress was reduced in the presence of MβCD-treated RBCs in a dose-dependent manner (Figure 4F). We then tested cold-stored RBCs, which are known to be less deformable.44,45 At a shear stress of 10 Pa, cold-stored RBCs induced less platelet activation compared with fresh cells (Figure 5A) and released more EVs (supplemental Figure 10), consistent with increased rupture rates of the more rigid RBCs. These results demonstrate that RBCs promote SIPA in a viscosity-independent manner, and this effect is regulated by RBC membrane deformability.
Synergistic activities of shear stress and RBC-derived ADP. (A) Levels of CD62p+ platelets in response to 10 Pa shear stress exposure after reconstitution with fRBCs or sRBCs; n = 3 per condition; t test). (B) ADP released from washed RBCs subjected to increasing levels of shear stress (n = 5-7 per condition; 1-way ANOVA). The white bar indicates the amount of ADP from lysed RBCs at 40% hematocrit (n = 5). (C) ADP-induced aggregation of resting platelets and those primed with shear stress (10 Pa for 5 minutes at 37°C; n = 7-8 per condition; 2-way ANOVA); a representative aggregation curve in response to 2 μM ADP (left). (D) SIPA in response to 8 Pa when PRP was reconstituted with unwashed, washed, and apyrase-treated RBCs exposed to high shear stress (n = 8-12 per condition; 1-way ANOVA). (E) Collagen-induced aggregation of resting platelets and those primed with shear stress (10 Pa for 5 minutes at 37°C; n = 7-8 per condition; 2-way ANOVA) with representative curve in response to 2 μg/mL collagen on the left. (F) Plasma levels of RBC-derived EVs (CD235+ EVs) of patients at baseline and after LVAD implantation (n = 12; paired t test). BL, baseline; fRBC, fresh RBC; sRBC, cold-stored RBC.
Synergistic activities of shear stress and RBC-derived ADP. (A) Levels of CD62p+ platelets in response to 10 Pa shear stress exposure after reconstitution with fRBCs or sRBCs; n = 3 per condition; t test). (B) ADP released from washed RBCs subjected to increasing levels of shear stress (n = 5-7 per condition; 1-way ANOVA). The white bar indicates the amount of ADP from lysed RBCs at 40% hematocrit (n = 5). (C) ADP-induced aggregation of resting platelets and those primed with shear stress (10 Pa for 5 minutes at 37°C; n = 7-8 per condition; 2-way ANOVA); a representative aggregation curve in response to 2 μM ADP (left). (D) SIPA in response to 8 Pa when PRP was reconstituted with unwashed, washed, and apyrase-treated RBCs exposed to high shear stress (n = 8-12 per condition; 1-way ANOVA). (E) Collagen-induced aggregation of resting platelets and those primed with shear stress (10 Pa for 5 minutes at 37°C; n = 7-8 per condition; 2-way ANOVA) with representative curve in response to 2 μg/mL collagen on the left. (F) Plasma levels of RBC-derived EVs (CD235+ EVs) of patients at baseline and after LVAD implantation (n = 12; paired t test). BL, baseline; fRBC, fresh RBC; sRBC, cold-stored RBC.
Shear-induced release of ADP from RBCs and its priming effect on platelet activation
Figure 4 raised the question of whether increased SIPA in the presence of RBCs can be partly attributed to the shear-induced release of ADP, which is primarily derived from RBCs in blood and is a potent platelet activator.7,15,16,46 We found that RBCs did not release a detectable amount of ADP at rest, but released 9.9 ± 6.3 μM and 9.8 ± 4.6 μM of ADP when exposed to 10 and 18 Pa for 5 minutes, respectively (Figure 5B). The amount of ADP released from RBCs exposed to 26 Pa was similar to the amount detected when the same number of RBCs were lysed, suggesting maximal ADP release. Consistent with this observation, a greater amount of cell-free hemoglobin was detected in the supernatant of washed RBCs exposed to 25 Pa of pathological high shear stress but not at an arterial shear stress of 5 Pa (supplemental Figure 11). Anionic phospholipids detected on RBCs by annexin V increased by 16.3 ± 9.2% from baseline levels after 10 Pa of shear exposure (P < .05). Notably, human RBCs contain ∼276 binding sites for annexin V,47 far fewer than other cells such as endothelial cells.48 Moreover, exposing platelets to a shear stress of 10 Pa primed them for aggregation induced by a subthreshold concentration of ADP at 2 μM (Figure 5C). When RBCs were exposed to 25 Pa of shear stress and then washed before being reconstituted with PRP, they were no longer effective in promoting SIPA (Figure 5D). Treatment of high shear-exposed RBCs with apyrase, which catalyzes the hydrolysis of adenosine triphosphate and ADP, also did not further reduce SIPA. In contrast, platelets pre-exposed to shear stress had a similar aggregation profile with collagen (Figure 5E).
The finding that high shear stress triggered ADP release from RBCs and primed platelets for aggregation induced by the subthreshold concentration of ADP led us to examine the rate of RBC rupture in LVAD patients, who offer a rare in vivo opportunity to study RBCs and platelets under circulatory shear stress ≥18 Pa.49 These patients often develop significant platelet activation, hemolysis, and gastrointestinal bleeding.50-53 We examined plasma samples from 12 patients collected before and after Heartmate II LVAD implantation (Table 1). Levels of RBC-derived EVs, which served as a surrogate marker for RBC rupture and hemolysis, were significantly elevated after LVAD implantation (Figure 5F). There were also higher levels of CD62p-expressing platelets and von Willebrand Factor-bound platelet-derived EVs (supplemental Figure 12). We did not quantify ADP in these plasma samples because of its short half-life in blood.54,55 These findings demonstrate that pathological high shear stress induces RBC rupture and releases ADP, which contributes to the platelet activation found in patients with LVAD.
Demographic and clinical characteristics of patients with LVAD
| Characteristic . | N = 12 . |
|---|---|
| Age, y | 65.6 ± 11.6 |
| Female sex, N (%) | 5 (41.7) |
| LVAD as destination therapy, N (%) | 10 (83.3) |
| Laboratory findings | |
| Hemoglobin, g/dL | 11.1 ± 2.5 |
| Platelet count, × 103/μL | 238.2 ± 106.9 |
| INR | 2.1 ± 0.7 |
| Creatinine, mg/dL | 1.3 ± 0.1 |
| LDH, U/L | 319.6 ± 40.7 |
| LVAD parameters | |
| RPM | 8631 ± 792 |
| PI | 5.11 ± 1.3 |
| PP, watts | 5.8 ± 1.21 |
| Characteristic . | N = 12 . |
|---|---|
| Age, y | 65.6 ± 11.6 |
| Female sex, N (%) | 5 (41.7) |
| LVAD as destination therapy, N (%) | 10 (83.3) |
| Laboratory findings | |
| Hemoglobin, g/dL | 11.1 ± 2.5 |
| Platelet count, × 103/μL | 238.2 ± 106.9 |
| INR | 2.1 ± 0.7 |
| Creatinine, mg/dL | 1.3 ± 0.1 |
| LDH, U/L | 319.6 ± 40.7 |
| LVAD parameters | |
| RPM | 8631 ± 792 |
| PI | 5.11 ± 1.3 |
| PP, watts | 5.8 ± 1.21 |
Continuous values are expressed as mean ± standard deviation.
INR, International Normalized Ratio; LDH, lactate dehydrogenase; PI, pulsatility index; PP, pump power; RPM, revolutions per minute.
Discussion
RBCs have been implicated in hemostasis based on clinical observations that hematocrit is inversely correlated with bleeding,1,2,4,5,24,56 but the underlying mechanism remains poorly defined. In this study, we investigated how RBCs regulated platelet reactivity at varying ratios of the 2 cell types to mimic specific pathologies and under altered hemodynamic conditions to examine platelet-RBC interactions at the blood vessel wall and in the fluid phase. We made several novel observations.
First, we showed in mouse models that a 45% reduction in hematocrit led to bleeding diathesis, whereas a significantly prolonged tail bleeding time was observed at 90% but not at 60% reduction in platelet counts (Figure 2), suggesting that at comparable levels, reducing the RBC count may have a larger effect size on hemostasis than reducing the platelet count. The effect of RBCs was not detected in vitro at severely low platelet counts of 10 × 103/μL (Figure 1D). To our knowledge, no previous studies have examined the effects of hematocrit on hemostasis in severe thrombocytopenia. There has been interest in the potential of raising hematocrit to mitigate bleeding because a secondary analysis of the Platelet Dose (PLADO) trial showed that a hematocrit of ≥25% was correlated with lower risk of World Health Organization grade 2A and 3 bleeding in patients with severe pancytopenia related to treatment for hematological malignancies,56 especially because other strategies such as antifibrinolytic therapy have been found ineffective in this population.57 Our findings suggest that although increased RBC concentration may facilitate platelet margination in vitro,17 the scarcity of circulating platelets in conditions of severe thrombocytopenia blunts the effect of hematocrit.
Second, mice subjected to sublethal total body irradiation developed severe pancytopenia (Figure 3A-D). They formed very small platelet plugs in response to penetrating vascular injury compared with nonirradiated mice (Figure 3E-F), consistent with results from in vitro experiments (Figure 1D). Transfusion of packed RBCs increased platelet and fibrin clot formation at the injury site. More important, fibrin clots were observed in nontransfused, irradiated mice despite minimal platelet plug formation and these increased in size with RBC transfusion without changing platelet counts, coagulation factors, or plasma volumes (Figure 3D). This observation was unexpected because fibrin clots are thought to form from proteolytically crosslinking fibrin on activated platelets at the site of vascular injury and are thus typically colocalized with a large platelet plug.58,59 Our findings demonstrate that a substantial intraluminal fibrin clot had formed without a significant number of aggregated platelets at the site of vascular injury, suggesting that RBCs can promote the formation of fibrin clots, even in the condition of severe thrombocytopenia. The cause of this unique phenotype requires further investigation but could be attributed to direct RBC-fibrin interactions based on observations that, (1) RBCs located within the fibrin meshwork can mediate clot formation and stability via a factor XIII-mediated mechanism;10,60 (2) RBCs can directly enhance fibrin deposition, because they express receptors for fibrinogen and ∼2% of fibrinogen circulates as RBC bound;61 and (3) increasing the occupancy of RBCs in plasma clots confers resistance to fibrinolysis.62,63
Third, RBCs also enhanced platelet aggregation in solution when exposed to pathologically high shear stress, independent of fluid viscosity (Figure 4). Because the hemorheologic contribution of RBCs to promoting platelet margination is no longer relevant in these viscometer experiments, we examined other mechanisms by which RBCs could promote platelet reactivity, including ADP, which is a platelet agonist that is either directly released from RBCs64 or rapidly converted from adenosine triphosphate released from RBCs.65-67 In previous studies,46,68,69 high shear stress, ranging from 5 to >150 Pa, was found to induce ADP release from RBCs that contribute up to 65% of the total ADP in the sheared blood samples.64 We show that RBCs release ADP only when exposed to pathologically high shear stress, reaching the maximal release at 26 Pa (Figure 5). These levels of high shear stress are not found in physiological circulation but can be found in LVAD-driven blood flow.70 Therefore, patients on LVAD support provide an ideal model to study the interaction between high shear stress, RBC rupture, and ADP release. Consistent with in vitro observations, we detected significantly elevated levels of RBC-derived membrane EVs in the plasma samples of patients with LVAD, indicative of systemic hemolysis.52,53 These patients also had high levels of platelet activation and microvesiculation (supplemental Figure 12), which was likely caused by the combined actions of high shear stress and ADP released from RBCs, as indicated by our in vitro results (Figure 5).
Finally, increasing membrane rigidity by glutaraldehyde fixation, cholesterol depletion, or cold storage abrogated the effects of RBCs on promoting SIPA (Figures 4 and 5). Although published reports have been inconsistent regarding the effect of cholesterol on RBC membranes,71-73 we found that cholesterol depletion with MβCD reduced RBC membrane deformability, thus affecting the ability of RBCs to regulate platelet reactivity and hemostasis. These results also corroborate previous observations that platelet aggregation is reduced with stored RBCs.74 We hypothesize that altering RBC membrane deformability impairs platelet collision frequency. Consistent with this notion, RBCs in sickle cell disease and iron deficiency have been observed to have increased rigidity and frequently marginate to the periphery, disrupting platelet-platelet75 and platelet-endothelial interactions.76,77
This study has several limitations. First, we studied the impact of RBCs on platelets at the blood vessel wall interface but did not directly examine the endothelium, which influences hemostasis under flow conditions through multiple mechanisms that intersect with RBCs. Blood vessel geometry helps dictate the distribution of RBCs in the microvasculature.78,79 RBC adhesion to the endothelium contributes to the pathophysiology of diseases such as sickle cell disease,80,81 diabetic microangiopathy,82 and retinal vascular occlusion.83 This RBC-endothelial interaction is modulated by shear stress.84,85 Second, the LVAD cohort were collected from a single center, which may limit generalizability. We also could not assess for correlations between levels of hemolysis and clinical outcomes such as bleeding with this small sample size. Further, we could not examine the mechanistic links between RBC rupture under high shear stress, ADP release, and its contribution to platelet activation in vivo. Future studies should evaluate for the clinical implications of RBC rupture rates in high shear conditions and to systematically investigate the link between shear-induced RBC rupture and platelet activation.
In summary, we provide data that support the following: (1) RBCs promote hemostasis at varying platelet-to-RBC ratios, encompassing models related to hemolytic anemia, ITP, and hypoproliferative pancytopenia; (2) RBCs differentially contribute to platelet reactivity in fluid phase in a shear stress–dependent fashion and release ADP under high shear stress; and (3) altering membrane deformability interferes with RBCs’ support of hemostasis. Together, they show that RBCs promote hemostasis through multiple mechanisms, which variably contribute depending on blood cell concentrations and levels of shear stress. Further work is warranted to examine how RBCs support hemostasis through interactions with fibrin and whether RBCs can mitigate bleeding in patients at high risk, such as those with LVADs and those undergoing therapy for hematologic malignancy.
Acknowledgments
This study is supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants T32 HL007093 (D.J.) and R01 HL152200 (J.-f.D.) and the NIH, National Institute of General Medical Sciences grant R01 GM140983 (J.-f.D and R.K.). D.J. is also supported by the National Bleeding Disorders Foundation-Takeda Clinical Fellowship Program. The anti-fibrin antibody was kindly provided by James H. Morrissey of the University of Michigan.
The visual abstract was created with BioRender.com.
Authorship
Contribution: D.J. formulated hypotheses, performed experiments, analyzed data, and wrote the manuscript; K.L.H., L.M., H.H., N.R., Z.A.C.S., and S.K.R. performed experiments and analyzed data; A.N. provided LVAD samples and wrote the manuscript; T.B.G. formulated hypotheses and wrote the manuscript; A.K.R. analyzed data and wrote the manuscript; R.A. performed experiments, analyzed data, and wrote the manuscript; R.K. wrote the manuscript; and J.-f.D. formulated hypotheses, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: T.B.G. claims no relevant conflicts of interest but reports being a member of the medical advisory board for Platelet Disorder Support Association; receiving consultation fees from Sanofi Corporation, Sobi Corporation, Alpine Immune Science: Bioproducts Laboratory, Cellphire Corporation, Novartis, and Amgen; is a member of the data safety monitoring board of Palisade Bio and Novartis; and receives honoraria from Amgen Inc and Sobi Corporation. The remaining authors declare no competing financial interests.
Correspondence: Jing-fei Dong, Bloodworks Research Institute, Medicine, 1551 Eastlake Ave E, Seattle, WA 98102; email: jfdong@bloodworksnw.org; and Debbie Jiang, Division of Hematology, Massachusetts General Hospital, 40 Blossom St, Boston, MA, 02114; email: dcjiang@mgh.harvard.edu.
References
Author notes
D.J. and K.L.H. contributed equally to this study.
Original data reported in this manuscript have not been deposited in any public database, but they are available upon reasonable requests from the corresponding authors, Debbie Jiang (dcjiang@mgh.harvard.edu) and Jing-fei Dong (jfdong@bloodworksnw.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![RBCs increased platelet thrombus formation on collagen under flow conditions. Human blood reconstituted to varying hematocrits (HCTs) and platelet counts was perfused over a collagen-coated surface using a flow perfusion chamber at a shear stress of 2 Pa. The proportion of surface area covered by platelet thrombus was calculated and is expressed as mean thrombus surface percentage ± standard deviation. (A-C) Representative images of platelet thrombus formed on immobilized collagen after 5-minute perfusion (scale bar, 50 μm) and collective data from multiple independent experiments with different platelet counts and HCTs (n = 49-50 per experimental condition; Student t test). (D) Representative images of thrombus surface coverage (scale bar, 50 μm) after 10-minute perfusion of blood reconstituted to a platelet concentration of 10 × 103/μL and varying HCT (n = 50-59 per experimental condition; 1-way analysis of variance [ANOVA] and Tukey honestly standard deviation).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/14/10.1182_blood.2024023887/2/m_blood_bld-2024-023887-gr1.jpeg?Expires=1769092271&Signature=uO-C47XMmFvjsz4Onf4rEb6X5wb2nmpC7Lizv4Z0HVlcyE9Yntzg-ZsZmPAOUPvWhLqPzPgBPrycv8GMcLfnIYfqjp1dit4ddWS4NIy2fFnDJz1-0vxdDdUw3Tu1ZiaRpU2yeyGDTQznfmkt2ubttdm96OpI-YHWgT261r~tjw~1PaFUQuEacFQYgQ2U1RMHJh6785Ql7y2XRhQ3l8fr5rYq~syUqOVZR-l15aII8YNqWtSjy~cBOZVeGUxbXKl7eYPQjq9tT~fsF8mEGRdwGlkzX4PEXeP9qK2MCSp4qhweK02gERGltStTgLYM1lDAmwgskk2F0x5VrTJf4CXEiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal