In this issue of Blood, Jiang et al1 suggest an intriguing role for red blood cells (RBCs) in regulating hemostasis through both platelet-dependent and -independent mechanisms. Using a combination of mouse and in vitro microfluidic models to control RBC and platelet counts, the authors demonstrate a hemostatic effect of RBCs via platelet activation in settings of moderate thrombocytopenia, and, more surprisingly, a platelet-independent effect via fibrin formation in conditions resembling severe pancytopenia. Mechanistically, platelet aggregation is influenced by RBC membrane deformability and adenosine 5′-diphosphate (ADP) release. The authors extend their observations to a clinically relevant state by inclusion of human samples from patients undergoing placement of left ventricular assist devices, linking RBCs with platelet hyperactivity in pathologically high-shear conditions.
RBCs are well appreciated for their role as life-sustaining deliverers of oxygen. Yet performing this essential task may pigeonhole our understanding of RBC function, which has recently expanded to recognize a contribution to hemostasis and thrombosis in physiologic and pathophysiologic states (as reviewed by Weisel and Litvinov2). Prothrombotic characteristics of RBCs are in fact multiple, ranging from the release of procoagulant substances like ADP3 or phosphatidylserine-exposed extracellular vesicles4 to promoting platelet aggregation, or even to altering clot composition and thereby impairing fibrinolysis (see figure).5 In addition, increased RBC membrane rigidity or RBC aggregation may damage the vascular endothelium with thrombotic consequences, as seen in hereditary disorders like sickle cell disease2 or resulting from acquired abnormalities associated with sepsis or COVID-19.6
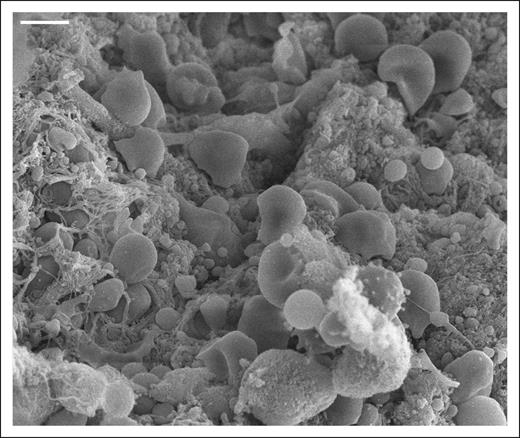
Despite being a prominent feature in blood clots, RBCs have an underappreciated role in hemostasis. Scanning electron microscopy image of an arterial thrombus. Scale bar = 4 μm.
Despite being a prominent feature in blood clots, RBCs have an underappreciated role in hemostasis. Scanning electron microscopy image of an arterial thrombus. Scale bar = 4 μm.
Perhaps the best understood hemostatic role of RBCs relates to platelet margination, which is influenced by blood viscosity, shear stress, and overall blood rheology. Others have demonstrated the influence of hematocrit on measures of platelet function using in vitro7 or even simulated models,8 and such studies support long-standing clinical observations associating anemia with prolonged bleeding, which may be improved by RBC transfusion.9 Nevertheless, mechanistic detail accounting for these observations has been lacking, as have experimental models investigating the hemostatic capabilities of RBCs under physiologic or pathophysiologic shear conditions.
Jiang et al now provide a panoply of provocative data highlighting the impact of RBCs on hemostasis in multiple ways. They first demonstrate a direct relationship between hematocrit and platelet thrombi formation on collagen (ie, modeling vascular injury) under flow conditions, a finding that holds true in the setting of normal or moderately decreased platelet counts but not in severe thrombocytopenia. They also identify RBC-induced platelet aggregation under physiologic shear conditions that appears to be independent of blood viscosity but related to RBC membrane deformability. Using a murine model of pancytopenia, the authors determine that RBC transfusion increases fibrin clot formation at a site of induced vascular injury, even in the near absence of platelet participation. Finally, after demonstrating that high-shear conditions cause RBC release of ADP and prime platelet activation in vitro, they suggest platelet activation occurs via RBC-derived ADP in patients with left ventricular assist devices. Notably, on this latter point, hemolysis is thought to contribute to platelet activation and clot formation on multiple levels.
Although the authors present compelling data, several important areas remain to be explored before the study findings can be translated to benefit patients. First, the molecular mechanisms contributing to the observed phenomenon, including RBC-induced platelet margination and deposition to subendothelial components, must be further defined. It will also be important to determine whether alterations in RBC deformability are modifiable, and, if so, the downstream impact of membrane normalization on platelet function in various settings of anemia or thrombocytopenia. Since the authors suggest hemostasis may be achieved following vascular injury in the setting of pancytopenia not by canonical platelet recruitment but by RBC-related fibrin deposition, additional investigation into the involved molecular mediators, its link to blood coagulation, and the applicability to patients with cytopenia receiving transfusion support are anticipated to be informative.
Nevertheless, the study by Jiang et al holds potential relevance for a number of patient populations, including those with cytopenia-related diseases or those undergoing manipulations involving pathologically elevated shear conditions. It is quite interesting to consider the possibility that RBC transfusion may have a greater impact on hemostasis than platelets in some patients with thrombocytopenia. This work likely represents an important preliminary step toward redefining transfusion thresholds and protocols, with RBCs moving into the spotlight for understanding hemostasis and the optimal management of bleeding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal