Key Points
Overall survival and most safety outcomes with axi-cel were consistent across race/ethnicity in relapsed/refractory large B-cell lymphoma.
Real-world outcomes with CAR T-cell therapy are different in non-Hispanic Black patients, possibly related to barriers to access.
Visual Abstract
Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Despite extensive data supporting its use, outcomes stratified by race and ethnicity groups are limited. Here, we report clinical outcomes with axi-cel in patients with R/R LBCL by race and ethnicity in both real-world and clinical trial settings. In the real-world setting, 1290 patients who received axi-cel between 2017 and 2020 were identified from the Center for International Blood and Marrow Transplant Research database; 106 and 169 patients were included from the ZUMA-1 and ZUMA-7 trials, respectively. Overall survival was consistent across race/ethnicity groups. However, non-Hispanic (NH) Black patients had lower overall response rate (OR, 0.37; 95% CI, 0.22-0.63) and lower complete response rate (OR, 0.57; 95% CI, 0.33-0.97) than NH White patients. NH Black patients also had a shorter progression-free survival vs NH White (HR, 1.41; 95% CI, 1.04-1.90) and NH Asian patients (HR, 1.67; 95% CI, 1.08-2.59). NH Asian patients had a longer duration of response than NH White (HR, 0.56; 95% CI, 0.33-0.94) and Hispanic patients (HR, 0.54; 95% CI, 0.30-0.97). There was no difference in cytokine release syndrome by race/ethnicity; however, higher rates of any-grade immune effector cell–associated neurotoxicity syndrome were observed in NH White patients than in other patients. These results provide important context when treating patients with R/R LBCL with CAR T-cell therapy across different racial and ethnic groups. ZUMA-1 and ZUMA-7 (ClinicalTrials.gov identifiers: #NCT02348216 and #NCT03391466, respectively) are registered on ClinicalTrials.gov.
Introduction
People from racial and ethnic minority groups across the United States experience worse survival outcomes after a cancer diagnosis compared with non-Hispanic White patients.1,2 Among patients with B-cell malignancies, use of, and response rates to, first-line chemoimmunotherapy (CIT) are similar across all races and ethnicities.3 However, therapeutic approaches for relapsed or refractory (R/R) disease may be limited for people from racial and ethnic minority groups because of socioeconomic and geographic disparities.3-5 Large B-cell lymphoma (LBCL) is a common and aggressive B-cell malignancy for which 20% to 50% of patients develop R/R LBCL.6
Axicabtagene ciloleucel (axi-cel) is a CD19-directed autologous chimeric antigen receptor (CAR) T-cell therapy with a CD28 costimulatory domain that provides rapid and strong expansion and reprograms T cells to trigger target-specific cytotoxicity of cancer cells. Axi-cel is approved for the treatment of adult patients with R/R LBCL after receipt of ≥2 lines of therapy based on favorable results in the pivotal phase 1/2 ZUMA-1 study in refractory LBCL.7,8 Its use was subsequently expanded for second-line treatment of primary R/R LBCL within ≤12 months from frontline therapy after demonstration of statistically significant improvements in event-free survival and overall survival (OS) in the randomized phase 3 ZUMA-7 trial of axi-cel vs standard of care.7,9
Because of strict eligibility criteria in clinical trials, efficacy outcomes may not always reflect real-world medical practice, in which patients may exhibit more heterogenous outcomes because of biological differences, comorbidities, and racial differences.8,10 Recently, a noninterventional postauthorization safety study (PASS) was initiated using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry as a prospective, long-term, noninterventional cohort study of real-world axi-cel use in LBCL and found that efficacy outcomes were consistent with the results of the ZUMA-1 clinical trial.8
Despite extensive data supporting the efficacy and safety of axi-cel in LBCL, sparse data are available on outcomes by race and ethnicity in clinical trials and real-world studies published to date.7,9,11-14 Here, we examine outcomes with axi-cel in R/R LBCL by race and ethnicity in both clinical trials and real-world settings.
Methods
Patients and study design
The CIBMTR is a collaborative working group of >500 treatment centers worldwide managed by the Medical College of Wisconsin and the National Marrow Donor Program (NMDP). Detailed information on patient, treatment and disease characteristics, as well as demographics were longitudinally reported by participating centers. Integrity and quality of data were monitored at different levels, including onsite audits, and automated and manual checks for discrepancies. Patients signed informed consent forms to share data with the CIBMTR for research studies, and use of these data for research was overseen by the NMDP central institutional review board. A PASS was conducted using the CIBMTR data infrastructure to prospectively capture long-term outcomes of axi-cel in the real-world, with the enrollment of 1497 patients from 79 centers completed in August 2020. Data collected for the PASS up until 4 May 2022 were used for the real-world assessment.
In the real-world study population, patients received postapproval axi-cel per institutional practice or per protocol as part of the clinical trials. No treatments, therapy protocols, or procedures were mandated. Participating sites were responsible for the completion of data collection at predetermined time points that aligned with routine medical care. Patients who received axi-cel in a noncommercial setting, with prior history of nontransplant cellular therapy, and of unknown/other race (who were non-Hispanic and not Asian, Black, or White) or ethnicity were excluded.
Additionally, a post hoc assessment of axi-cel in R/R LBCL by race and ethnicity in eligible patients enrolled in ZUMA-1 (clinicaltrials.gov identifier: NCT02348216; phases 1 and 2, cohorts 1 and 2) and ZUMA-7 (clinicaltrials.gov identifier: NCT03391466; phase 3) was conducted.7,15 Data from patients treated with axi-cel enrolled in ZUMA-1 (April 2015-January 2017) and ZUMA-7 (January 2018-October 2019) were included in the clinical trial analysis.7,15 R/R disease in ZUMA-7 was defined as patients who were refractory to first-line CIT or experienced a relapse ≤12 months after first-line CIT.7 In ZUMA-1, refractory disease was defined as progressive or stable disease as the best response to the most recent therapy, or relapse ≤12 months after autologous stem cell transplantation.15 Eligibility criteria and data collection schedules of the ZUMA-1 and ZUMA-7 trials were previously described.7,15
Race (Asian, Black, White, or other) and ethnicity (Hispanic/Latino, or non-Hispanic/Latino) were self-reported. Patients treated outside of the United States were excluded.
Treatment and end points
In the real-world assessment, efficacy end points assessed in this analysis by race and ethnicity included overall response rate (ORR), complete response (CR) rate (per Lugano classification16 and institution), duration of response (DOR), progression-free survival (PFS), and OS. Safety outcomes assessed were cytokine release syndrome (CRS) graded per Lee et al17 and immune effector cell–associated neurotoxicity syndrome (ICANS; per American Society for Transplantation and Cellular Therapy [ASTCT] consensus grading)17 at follow-up 100 days after infusion, and prolonged cytopenias (defined as failure to resolve within the first 30 days after infusion) at 30 days after infusion. In the clinical trial analysis, ORR and CR were assessed centrally per Lugano classification.16 CRS (per Lee et al17) and neurologic events (graded per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03) were also analyzed.
Statistical methods
Baseline characteristics, efficacy, and safety outcomes were assessed in all eligible patients for the real-world assessment and in patients treated with axi-cel in ZUMA-1 phases 1 and 2 (cohorts 1 and 2) and ZUMA-7 for the clinical trial analysis.
For both real-world and clinical trial analyses, race (White, Black, and Asian) and ethnicity (Hispanic vs non-Hispanic) were combined as a single variable with 4 categories: non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic. Dichotomous outcomes were described using percentages with 95% exact confidence intervals (CIs). For the real-world assessment, DOR, PFS, and OS were summarized via the Kaplan-Meier estimator. Time to CRS/ICANS resolution was summarized using cumulative incidence function with death without resolution as a competing risk. In the real-world analysis, multivariate logistic and Cox regression models were used to assess the associations between race and ethnicity with efficacy and safety end points of interest while adjusting for other potential risk factors (age, sex, Eastern Cooperative Oncology Group performance status, comorbidities, and disease and treatment characteristics; please see supplemental Methods, available on the Blood website, for the complete list of covariates). The proportionality assumption for the Cox model was tested for the main race/ethnicity variable using an interaction term with the logarithm of the event time. A stepwise variable selection process was used to determine the final list of covariates for both the logistic and Cox regression models, using a P value cutoff of .2 for variables to enter the model and .05 for variables to stay in the model. Sensitivity analyses with race and ethnicity kept as separate variables were also performed. All analyses were conducted using SAS 9.4 M6.
Patients signed informed consent forms to share data with the CIBMTR for research studies, and use of these data for research was overseen by the NMDP central institutional review board.
Results
Disposition and baseline characteristics
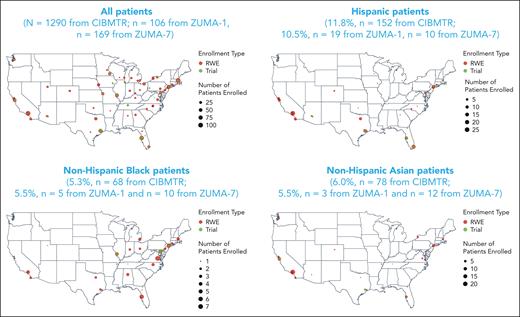
A total of 1497 patients with R/R LBCL were enrolled in the real-world cohort between October 2017 and August 2020 (supplemental Figure 1); 207 patients were excluded from the analysis because of histology of non-LBCL cancer (n = 24), prior non–hematopoietic cell transplantation (HCT) cellular therapy (n = 29), missing data on comorbidities (n = 43), missing data on time from leukapheresis to infusion (n = 2), no follow-up for efficacy or safety (n = 5), and other or missing race/ethnicity (n = 104; because of missing ethnicity [n = 73], missing race [n = 20], other race including Native Hawaiian/Pacific Islander [n = 4], American Indian/Alaska native [n = 2], and >1 race [n = 5]). In total, 1290 patients were included in the real-world analysis. Non-Hispanic Black patients were primarily treated in centers along the east coast of the United States, and those of Hispanic ethnicity were primarily treated on either coast or the southern regions of the United States (Figure 1). Most patients were non-Hispanic White (77%, n = 992); 12% were Hispanic (n = 152), and 5% and 6% were non-Hispanic Black (n = 68) and non-Hispanic Asian (n = 78), respectively. Within the Hispanic ethnicity group, 69% of patients were White, 1% were Black, <1% were Asian, and 29% were of other or unknown races (unknown, n = 28; not reported, n = 12; American Indian or Alaska native, n = 2; >1 race, n = 2).
Patient geographic distribution in the real world and clinical trials. The race and ethnicity distributions of patients enrolled in the real-world (red circles) and clinical trial (green circles) settings within authorized treatment centers at the city level are shown. The size of the circle is commensurate with the number of patients enrolled in that setting. RWE, real-world evidence.
Patient geographic distribution in the real world and clinical trials. The race and ethnicity distributions of patients enrolled in the real-world (red circles) and clinical trial (green circles) settings within authorized treatment centers at the city level are shown. The size of the circle is commensurate with the number of patients enrolled in that setting. RWE, real-world evidence.
Baseline characteristics by race and ethnicity in the real world are reported in Table 1 and consistent across non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic patient groups with a few exceptions. The median age across all groups was 62.2 years (range, 19.6-90.8 years). Of those included for analysis, both non-Hispanic Black patients (median age, 55.5 years) and Hispanic patients (median age, 58.3 years) were less likely to be aged ≥65 years than non-Hispanic White patients (median age, 63.0 years). The baseline prevalence of moderate to severe pulmonary disease was higher in non-Hispanic Black patients (41%) than non-Hispanic White patients (29%), although the prevalence of prior malignancies was lower (6% vs 17%, respectively). Hispanic patients had a lower prevalence of moderate to severe pulmonary disease (20%) and prior malignancy (7%) than non-Hispanic White patients. Furthermore, 75% of non-Hispanic Black, 60% of non-Hispanic Asian, and 58% of non-Hispanic White and Hispanic patients received axi-cel infusion ≥12 months after diagnosis.
Baseline characteristics by race and ethnicity in the real world
| Key variable of interest, n (%) unless specified . | Hispanic (n = 152) . | Non-Hispanic Asian (n = 78) . | Non-Hispanic Black (n = 68) . | Non-Hispanic White (n = 992) . |
|---|---|---|---|---|
| Age, median (range), y | 58.3 (23.9-80.6) | 61.8 (21.5-82.1) | 55.5 (21.5-84.4) | 63.0 (19.6-90.8) |
| Age ≥65 | 37 (24) | 27 (35) | 16 (24) | 411 (41) |
| Male sex | 98 (64) | 41 (53) | 42 (62) | 663 (67) |
| ECOG PS ≥2 before infusion | 4 (3) | 9 (12) | 1 (1) | 44 (4) |
| Elevated LDH at initial diagnosis | 38 (25) | 22 (28) | 17 (25) | 303 (31) |
| HCT-CI score before infusion | ||||
| 0 | 67 (44) | 29 (37) | 16 (24) | 300 (30) |
| 1 | 24 (16) | 17 (22) | 11 (16) | 178 (18) |
| 2 | 32 (21) | 9 (12) | 10 (15) | 115 (12) |
| ≥3 | 29 (19) | 23 (29) | 31 (46) | 399 (40) |
| Disease histology at diagnosis | ||||
| DLBCL | 119 (78) | 61 (78) | 61 (90) | 803 (81) |
| PMBCL | 4 (3) | 2 (3) | 2 (3) | 30 (3) |
| HGBL | 29 (19) | 15 (19) | 5 (7) | 159 (16) |
| With MYC and BCL2 and/or BCL6 rearrangements | 26 (17) | 13 (17) | 4 (6) | 147 (15) |
| NOS | 3 (2) | 2 (3) | 1 (1) | 12 (1) |
| Key comorbidities∗ | ||||
| Pulmonary, moderate to severe | 30 (20) | 14 (18) | 28 (41) | 289 (29) |
| Prior cancer | 10 (7) | 8 (10) | 4 (6) | 166 (17) |
| Obesity (BMI >35 kg/m2) | 15 (10) | 1 (1) | 9 (13) | 90 (9) |
| Renal, moderate to severe, or prior renal implant | 2 (1) | 1 (1) | 1 (1) | 28 (3) |
| Histologic transformation | 37 (24) | 18 (23) | 17 (25) | 300 (30) |
| Disease sensitivity before infusion | ||||
| Sensitive | 44 (29) | 19 (24) | 16 (24) | 213 (21) |
| Resistant | 93 (61) | 53 (68) | 46 (68) | 662 (67) |
| Unknown | 15 (10) | 6 (8) | 6 (9) | 117 (12) |
| No. of lines of prior therapies, median (range) | 3 (2-8) | 3 (1-12) | 3 (1-8) | 3 (1-18) |
| 1-2 | 40 (26) | 19 (24) | 17 (25) | 275 (28) |
| ≥3 | 102 (67) | 54 (69) | 49 (72) | 691 (70) |
| Unknown | 10 (7) | 5 (6) | 2 (3) | 26 (3) |
| Prior HCT (any type) | 32 (21) | 22 (28) | 17 (25) | 305 (31) |
| Prior ASCT | 31 (20) | 21 (27) | 17 (25) | 290 (29) |
| Bridging therapy (any type)† | 31 (20) | 12 (15) | 11 (16) | 224 (23) |
| Year of axi-cel infusion | ||||
| 2018 or before | 46 (30) | 20 (26) | 21 (31) | 284 (29) |
| 2019 | 66 (43) | 36 (46) | 29 (43) | 466 (47) |
| 2020 | 40 (26) | 22 (28) | 18 (26) | 242 (24) |
| ≥12 mo from diagnosis to infusion | 88 (58) | 47 (60) | 51 (75) | 572 (58) |
| ≥28 d from leukapheresis to lymphodepleting chemotherapy | 75 (49) | 37 (47) | 42 (62) | 488 (49) |
| Estimated trial eligibility for ZUMA-1‡ | ||||
| Eligible | 80 (53) | 39 (50) | 23 (34) | 426 (43) |
| Ineligible | 72 (47) | 39 (50) | 45 (66) | 566 (57) |
| Key variable of interest, n (%) unless specified . | Hispanic (n = 152) . | Non-Hispanic Asian (n = 78) . | Non-Hispanic Black (n = 68) . | Non-Hispanic White (n = 992) . |
|---|---|---|---|---|
| Age, median (range), y | 58.3 (23.9-80.6) | 61.8 (21.5-82.1) | 55.5 (21.5-84.4) | 63.0 (19.6-90.8) |
| Age ≥65 | 37 (24) | 27 (35) | 16 (24) | 411 (41) |
| Male sex | 98 (64) | 41 (53) | 42 (62) | 663 (67) |
| ECOG PS ≥2 before infusion | 4 (3) | 9 (12) | 1 (1) | 44 (4) |
| Elevated LDH at initial diagnosis | 38 (25) | 22 (28) | 17 (25) | 303 (31) |
| HCT-CI score before infusion | ||||
| 0 | 67 (44) | 29 (37) | 16 (24) | 300 (30) |
| 1 | 24 (16) | 17 (22) | 11 (16) | 178 (18) |
| 2 | 32 (21) | 9 (12) | 10 (15) | 115 (12) |
| ≥3 | 29 (19) | 23 (29) | 31 (46) | 399 (40) |
| Disease histology at diagnosis | ||||
| DLBCL | 119 (78) | 61 (78) | 61 (90) | 803 (81) |
| PMBCL | 4 (3) | 2 (3) | 2 (3) | 30 (3) |
| HGBL | 29 (19) | 15 (19) | 5 (7) | 159 (16) |
| With MYC and BCL2 and/or BCL6 rearrangements | 26 (17) | 13 (17) | 4 (6) | 147 (15) |
| NOS | 3 (2) | 2 (3) | 1 (1) | 12 (1) |
| Key comorbidities∗ | ||||
| Pulmonary, moderate to severe | 30 (20) | 14 (18) | 28 (41) | 289 (29) |
| Prior cancer | 10 (7) | 8 (10) | 4 (6) | 166 (17) |
| Obesity (BMI >35 kg/m2) | 15 (10) | 1 (1) | 9 (13) | 90 (9) |
| Renal, moderate to severe, or prior renal implant | 2 (1) | 1 (1) | 1 (1) | 28 (3) |
| Histologic transformation | 37 (24) | 18 (23) | 17 (25) | 300 (30) |
| Disease sensitivity before infusion | ||||
| Sensitive | 44 (29) | 19 (24) | 16 (24) | 213 (21) |
| Resistant | 93 (61) | 53 (68) | 46 (68) | 662 (67) |
| Unknown | 15 (10) | 6 (8) | 6 (9) | 117 (12) |
| No. of lines of prior therapies, median (range) | 3 (2-8) | 3 (1-12) | 3 (1-8) | 3 (1-18) |
| 1-2 | 40 (26) | 19 (24) | 17 (25) | 275 (28) |
| ≥3 | 102 (67) | 54 (69) | 49 (72) | 691 (70) |
| Unknown | 10 (7) | 5 (6) | 2 (3) | 26 (3) |
| Prior HCT (any type) | 32 (21) | 22 (28) | 17 (25) | 305 (31) |
| Prior ASCT | 31 (20) | 21 (27) | 17 (25) | 290 (29) |
| Bridging therapy (any type)† | 31 (20) | 12 (15) | 11 (16) | 224 (23) |
| Year of axi-cel infusion | ||||
| 2018 or before | 46 (30) | 20 (26) | 21 (31) | 284 (29) |
| 2019 | 66 (43) | 36 (46) | 29 (43) | 466 (47) |
| 2020 | 40 (26) | 22 (28) | 18 (26) | 242 (24) |
| ≥12 mo from diagnosis to infusion | 88 (58) | 47 (60) | 51 (75) | 572 (58) |
| ≥28 d from leukapheresis to lymphodepleting chemotherapy | 75 (49) | 37 (47) | 42 (62) | 488 (49) |
| Estimated trial eligibility for ZUMA-1‡ | ||||
| Eligible | 80 (53) | 39 (50) | 23 (34) | 426 (43) |
| Ineligible | 72 (47) | 39 (50) | 45 (66) | 566 (57) |
ASCT, autologous stem cell transplantation; BMI, body mass index; DLBCL, diffuse LBCL; ECOG PS, Eastern Cooperative Oncology Group performance status; HCT-CI, HCT-specific comorbidity index; HGBL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase; no., number; NOS, not otherwise specified; PMBCL, primary mediastinal LBCL.
Comorbidities were assessed per Sorror et al.18
The incidence of bridging therapy was derived from the number of patients who initiated a prior therapy after leukapheresis and before conditioning chemotherapy.
The rates of ZUMA-1 trial eligibility used adapted eligibility criteria and were estimated based on available registry data.
A total of 106 patients were included from ZUMA-1 (data cutoff date: 11 August 2018) and 169 patients from ZUMA-7 (data cutoff date: 18 March 2021). The median age of patients included from ZUMA-1 and ZUMA-7 was 58 years (range, 23-76 years) and 59 years (range, 21-80 years), respectively. Within the Hispanic group of ZUMA-1, 68% of patients were of White race and 32% were reported as other or unknown. In ZUMA-7, within the Hispanic group, 60% of patients were White, 10% were Black, and 30% were of other or unknown races. More baseline details on patients included from clinical trials with axi-cel can be found in supplemental Table 1.
Efficacy
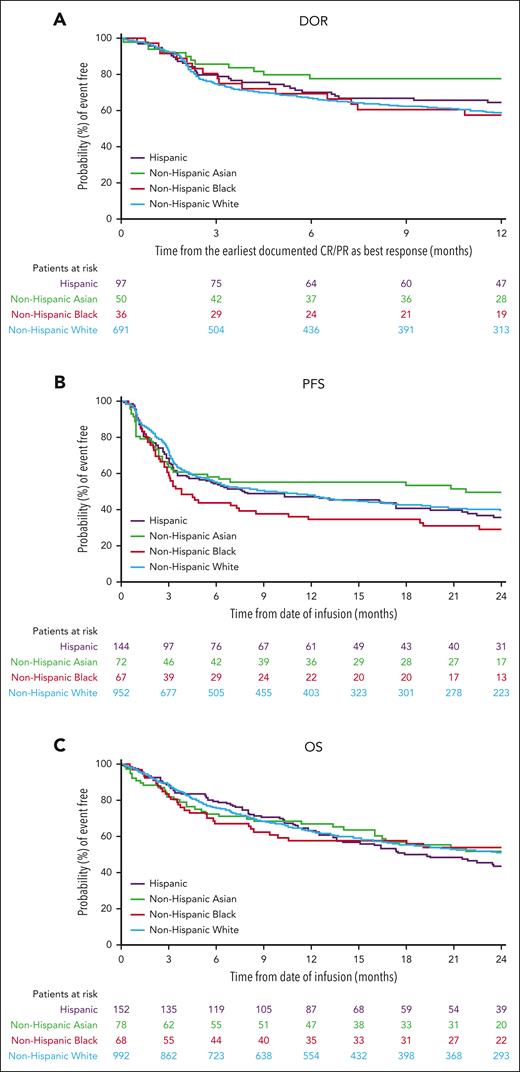
The median follow-up for patients receiving axi-cel within the real-world cohort was 24.3 months (95% CI, 24.2-24.5). ORR was 75% (CR rate, 59%), 54% (CR rate, 46%), 71% (CR rate, 58%), and 73% (CR rate, 57%) in non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic patients, respectively (Figure 2A). The median DOR in non-Hispanic White patients was 23.6 months (95% CI, 17.8-31.3); in non-Hispanic Black patients, 22.6 months (95% CI, 6.5-not estimable [NE]); in non-Hispanic Asian patients, not reached (95% CI, 25.3-NE); and in Hispanic patients, 21.3 months (95% CI, 16.4-NE; Figure 3A). The 12-month event-free probability of DOR was higher among non-Hispanic Asian patients (78%) than non-Hispanic Black (58%), non-Hispanic White (59%), and Hispanic patients (64%). Median PFS was 9.3 months (95% CI, 6.5-12.7) for non-Hispanic White patients and 3.8 months (95% CI, 2.9-8.5), 21.7 months (95% CI, 3.3-NE), and 7.8 months (95% CI, 4.1-17.4) for non-Hispanic Black, non-Hispanic Asian, and Hispanic patients, respectively (Figure 3B). Median OS was 25.9 months (95% CI, 20.7-31.4) for non-Hispanic White patients, 28.0 months (95% CI, 8.3-NE) for non-Hispanic Black patients, 24.7 months (95% CI, 16.0-NE) for non-Hispanic Asian patients, and 19.3 months (95% CI, 13.6-29.3) for Hispanic patients (Figure 3C). The 24-month PFS rate was generally consistent among all patients, except among non-Hispanic Black patients, and the cumulative incidence of relapse was higher among non-Hispanic Black patients (Figure 4A; supplemental Table 2). The primary cause of death was lymphoma related in 71% of non-Hispanic White, 73% of non-Hispanic Black, 60% of non-Hispanic Asian, and 71% of Hispanic patients. Nonadjusted odds ratio (OR) and hazard ratio (HR) are reported in supplemental Table 7.
Response by race and ethnicity in the real world and clinical trials. ORR and CR rates among patients in (A) the real world, (B) ZUMA-1, and (C) ZUMA-7. Per central assessment.
Response by race and ethnicity in the real world and clinical trials. ORR and CR rates among patients in (A) the real world, (B) ZUMA-1, and (C) ZUMA-7. Per central assessment.
Duration of response and survival outcomes by race and ethnicity in the real world. (A) DOR, (B) PFS, and (C) OS. PR, partial response.
Duration of response and survival outcomes by race and ethnicity in the real world. (A) DOR, (B) PFS, and (C) OS. PR, partial response.
Outcomes from the real world with multivariable adjustment. (A) Adjusted ORs for ORR, and CR rate; and HRs for DOR, PFS, and OS; and (B) adjusted OR for CRS, ICANS, prolonged neutropenia, and prolonged thrombocytopenia. aAdditional covariates associated with efficacy outcomes were adjusted (data not shown). bOR was used for the analysis of ORR and CR, and HR was used for the analysis of DOR, PFS, and OS. cVariables with multivariate P < .05. NHA, non-Hispanic Asian; NHB, non-Hispanic Black; NHW, non-Hispanic White.
Outcomes from the real world with multivariable adjustment. (A) Adjusted ORs for ORR, and CR rate; and HRs for DOR, PFS, and OS; and (B) adjusted OR for CRS, ICANS, prolonged neutropenia, and prolonged thrombocytopenia. aAdditional covariates associated with efficacy outcomes were adjusted (data not shown). bOR was used for the analysis of ORR and CR, and HR was used for the analysis of DOR, PFS, and OS. cVariables with multivariate P < .05. NHA, non-Hispanic Asian; NHB, non-Hispanic Black; NHW, non-Hispanic White.
After multivariate adjustment (Figure 4A), non-Hispanic Black patients had statistically lower ORR than non-Hispanic White (OR, 0.37; 95% CI, 0.22-0.63), non-Hispanic Asian (OR, 0.45; 95% CI, 0.22-0.94), and Hispanic patients (OR, 0.45; 95% CI, 0.24-0.84); a lower CR rate than non-Hispanic White patients (OR, 0.57; 95% CI, 0.33-0.97); and a shorter PFS than non-Hispanic White (HR, 1.41; 95% CI 1.04-1.90) and non-Hispanic Asian patients (HR, 1.67; 95% CI, 1.08-2.59). Non-Hispanic Asian patients had a longer DOR than non-Hispanic White (HR, 0.56; 95% CI, 0.33-0.94) and Hispanic patients (HR, 0.54; 95% CI, 0.30-0.97). There was a trend toward longer DOR in non-Hispanic Asian patients when compared with non-Hispanic Black patients (HR, 0.51; 95% CI, 0.26-1.00). No statistical differences were found in OS across all race and ethnicity, or in any efficacy outcome between Hispanic and non-Hispanic White patients. In a sensitivity analysis with race and ethnicity separated as 2 variables, efficacy results were consistent with the main analysis of the real-world assessment (supplemental Table 3).
In ZUMA-1 (n = 99), CR rates were reported as the following among non-Hispanic White (n = 74; ORR, 73%; CR rate, 55%), non-Hispanic Black (n = 4; ORR, 100%; CR rate, 100%), non-Hispanic Asian (n = 3; ORR, 67%; CR rate, 67%), and Hispanic patients (n = 18, ORR, 78%; CR rate, 44%; Figure 2B). Among patients who received axi-cel in ZUMA-7 (n = 169), ORR remained largely consistent across all race and ethnicity groups (range, 80%-83%; Figure 2C); with the following CR rates among non-Hispanic Black (CR rate, 70%), non-Hispanic White (CR rate, 66%), non-Hispanic Asian (CR rate, 58%), and Hispanic patients (CR rate, 50%). In a sensitivity analysis with race and ethnicity separated as 2 variables, efficacy results were consistent with the main analysis of clinical trials (supplemental Table 4).
Safety
As shown in Table 2, CRS rates were generally similar in all patients who received axi-cel in the real-world assessment; 83% in non-Hispanic White (grade ≥3, 9%), 82% in non-Hispanic Black (grade ≥3, 6%), 90% in non-Hispanic Asian (grade ≥3, 10%), and 81% in Hispanic patients (grade ≥3, 5%). Between race and ethnicity groups, the median time to onset of CRS after infusion was 4 days (range, 1.0-73.0 days), and most cases resolved within 3 weeks of onset regardless of race or ethnicity. Rate of ICANS of all grade and grade ≥3 in patients were reported as the following: non-Hispanic White, 59% (grade ≥3, 29%); non-Hispanic Black, 40% (grade ≥3, 19%); non-Hispanic Asian, 45% (grade ≥3, 21%); and Hispanic, 43% (grade ≥3, 16%; Table 2). The median time to onset of ICANS was 7 days (range, 1.0-36.0 days), and median time to resolution was 8 days (range, 1.0-115.0 days) after onset. For the treatment of CRS or ICANS, 59% of patients received tocilizumab and 48% received corticosteroids (Table 2). Prolonged neutropenia and prolonged thrombocytopenia (among patients who survived day 30 after infusion) was generally similar across all patients (Table 2). Nonadjusted OR/HRs are reported in supplemental Table 7.
Safety outcomes by race and ethnicity in the real world
| n (%) . | Hispanic (n = 152) . | Non-Hispanic Asian (n = 78) . | Non-Hispanic Black (n = 68) . | Non-Hispanic White (n = 992) . |
|---|---|---|---|---|
| Any-grade CRS∗ | 123 (81) | 70 (90) | 56 (82) | 824 (83) |
| Grade ≥3 CRS | 7 (5) | 8 (10) | 4 (6) | 89 (9) |
| Any-grade ICANS | 65 (43) | 35 (45) | 27 (40) | 584 (59) |
| Grade ≥3 ICANS | 24 (16) | 16 (21) | 13 (19) | 285 (29) |
| Management of CRS and ICANS | ||||
| Tocilizumab | 94 (62) | 54 (69) | 38 (56) | 571 (58) |
| Corticosteroids | 55 (36) | 42 (54) | 19 (28) | 509 (51) |
| Prolonged cytopenia† | 37 (25) (n = 148) | 25 (36) (n = 70) | 13 (20) (n = 66) | 234 (24) (n = 957) |
| Neutropenia | 11 (7) | 5 (7) | 3 (5) | 67 (7) |
| Thrombocytopenia | 35 (24) | 23 (33) | 11 (17) | 215 (22) |
| n (%) . | Hispanic (n = 152) . | Non-Hispanic Asian (n = 78) . | Non-Hispanic Black (n = 68) . | Non-Hispanic White (n = 992) . |
|---|---|---|---|---|
| Any-grade CRS∗ | 123 (81) | 70 (90) | 56 (82) | 824 (83) |
| Grade ≥3 CRS | 7 (5) | 8 (10) | 4 (6) | 89 (9) |
| Any-grade ICANS | 65 (43) | 35 (45) | 27 (40) | 584 (59) |
| Grade ≥3 ICANS | 24 (16) | 16 (21) | 13 (19) | 285 (29) |
| Management of CRS and ICANS | ||||
| Tocilizumab | 94 (62) | 54 (69) | 38 (56) | 571 (58) |
| Corticosteroids | 55 (36) | 42 (54) | 19 (28) | 509 (51) |
| Prolonged cytopenia† | 37 (25) (n = 148) | 25 (36) (n = 70) | 13 (20) (n = 66) | 234 (24) (n = 957) |
| Neutropenia | 11 (7) | 5 (7) | 3 (5) | 67 (7) |
| Thrombocytopenia | 35 (24) | 23 (33) | 11 (17) | 215 (22) |
ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome; ICANS, immune effector cell–associated neurotoxicity syndrome.
Reported on the 100-day follow-up case-report form.
Defined as failure to resolve within the first 30 days after infusion, measured among patients who survived day 30 after infusion.
Safety analyses with multivariate adjustment are briefly described in Figure 4B and included in the supplement (supplemental Tables 5 and 6). No differences were observed for CRS (any-grade or grade ≥3) or prolonged neutropenia by race or ethnicity. Non-Hispanic Asian, non-Hispanic Black, and Hispanic patients had a lower risk of any-grade ICANS vs non-Hispanic White patients (non-Hispanic Asian, OR 0.56 [95% CI, 0.35-0.90]; non-Hispanic Black, OR 0.54 [95% CI, 0.32-0.91]; and Hispanic, OR 0.58 [95% CI, 0.40-0.82]). Hispanic patients also had lower risk of grade ≥3 ICANS (OR 0.45 [95% CI, 0.28-0.71]) vs non-Hispanic White patients. Non-Hispanic Asian patients had a higher incidence of thrombocytopenia than both non-Hispanic White (OR, 1.77; 95% CI, 1.02-3.09) and non-Hispanic Black patients (OR, 2.5; 95% CI, 1.07-5.83). Safety outcomes in the sensitivity analysis were also consistent with the main analysis (supplemental Table 5).
In ZUMA-1 (n = 106), CRS rates were 92% (grade ≥3, 13%) in non-Hispanic White, 100% (grade ≥3, 40%) in non-Hispanic Black, 67% (no grade ≥3) in non-Hispanic Asian, and 95% (no grade ≥3) in Hispanic patients. Neurologic events developed in 72% (grade ≥3, 34%) of non-Hispanic White, 80% (grade ≥3, 60%) of non-Hispanic Black, 33% (no grade ≥3) of non-Hispanic Asian, and 47% (grade ≥3, 21%) of Hispanic patients (Table 3).
Safety outcomes by race and ethnicity in clinical trials
| n (%) . | Hispanic . | Non-Hispanic Asian . | Non-Hispanic Black . | Non-Hispanic White . |
|---|---|---|---|---|
| ZUMA-1 | n = 19 | n = 3 | n = 5 | n = 79 |
| Any-grade CRS | 18 (95) | 2 (67) | 5 (100) | 73 (92) |
| Grade ≥3 CRS | 0 | 0 | 2 (40) | 10 (13) |
| Any-grade neurologic events | 9 (47) | 1 (33) | 4 (80) | 57 (72) |
| Grade ≥3 neurologic events | 4 (21) | 0 | 3 (60) | 27 (34) |
| ZUMA-7 | n = 8 | n = 11 | n = 8 | n = 132 |
| Any-grade CRS | 8 (100) | 11 (100) | 7 (88) | 121 (92) |
| Grade ≥3 CRS | 1 (13) | 1 (9) | 0 | 7 (5) |
| Any-grade neurologic events | 8 (100) | 5 (45) | 4 (50) | 78 (59) |
| Grade ≥3 neurologic events | 2 (25) | 2 (18) | 2 (25) | 28 (21) |
| n (%) . | Hispanic . | Non-Hispanic Asian . | Non-Hispanic Black . | Non-Hispanic White . |
|---|---|---|---|---|
| ZUMA-1 | n = 19 | n = 3 | n = 5 | n = 79 |
| Any-grade CRS | 18 (95) | 2 (67) | 5 (100) | 73 (92) |
| Grade ≥3 CRS | 0 | 0 | 2 (40) | 10 (13) |
| Any-grade neurologic events | 9 (47) | 1 (33) | 4 (80) | 57 (72) |
| Grade ≥3 neurologic events | 4 (21) | 0 | 3 (60) | 27 (34) |
| ZUMA-7 | n = 8 | n = 11 | n = 8 | n = 132 |
| Any-grade CRS | 8 (100) | 11 (100) | 7 (88) | 121 (92) |
| Grade ≥3 CRS | 1 (13) | 1 (9) | 0 | 7 (5) |
| Any-grade neurologic events | 8 (100) | 5 (45) | 4 (50) | 78 (59) |
| Grade ≥3 neurologic events | 2 (25) | 2 (18) | 2 (25) | 28 (21) |
In ZUMA-7 (n = 159), CRS rates and neurologic events were also generally similar across groups (Table 3). CRS rates were 92% (grade ≥3, 5%) in non-Hispanic White, 88% (no grade ≥3) in non-Hispanic Black, and 100% in non-Hispanic Asian (grade ≥3, 9%) and Hispanic patients (grade ≥3, 13%). In total, 59% (grade ≥3, 21%) of non-Hispanic White, 50% (grade ≥3, 25%) of non-Hispanic Black, 45% (grade ≥3, 18%) of non-Hispanic Asian, and 100% (grade ≥3, 25%) of Hispanic patients developed neurologic events in the ZUMA-7 trial. In a sensitivity analysis with race and ethnicity separated as 2 variables, safety results were consistent with the main analysis (supplemental Table 6).
Discussion
In the real-world cohort and the ZUMA-1 and ZUMA-7 clinical trials, OS and most safety outcomes of axi-cel in patients with R/R LBCL were consistent across all racial and ethnic groups included in this analysis. In the real-world cohort, lower rates of response and PFS were observed among non-Hispanic Black patients than among non-Hispanic White patients, although these differences did not translate to a difference in OS. Additionally, non-Hispanic Asian patients had a significantly longer DOR than non-Hispanic White and Hispanic patients, with a trend toward longer DOR than non-Hispanic Black patients.
There were no significant differences in rates of CRS or prolonged neutropenia in patients treated with axi-cel by race or ethnicity in both the real-world and clinical trial analyses. However, in the real-world cohort, non-Hispanic Black and non-Hispanic Asian patients had a lower incidence of any-grade ICANS than non-Hispanic White patients. These results in non-Hispanic Asian patients were consistent with outcomes in a recent study of Chinese patients with R/R non-Hodgkin lymphoma treated with axi-cel, in which lower rates of any grade neurologic events (n = 17, 16.2%) were reported.20 Hispanic patients also had a lower incidence of any-grade and grade ≥3 ICANS. Furthermore, non-Hispanic Asian patients had a higher rate of prolonged thrombocytopenia than non-Hispanic White or non-Hispanic Black patients.
In this analysis, the proportions of non-Hispanic Black, non-Hispanic Asian, and Hispanic patients were comparable between the real-world setting (5%-12%) and clinical trials (5%-11%). The population expected to be Hispanic from the Surveillance, Epidemiology, and End Results (SEER) estimation (10.95% of 1500 patients) was similar to the proportion of Hispanic patients included in this analysis (11.78% in the real-world, and 10.55% in the clinical trial analyses). However, the proportion of non-Hispanic Black patients in the real-world analysis (5.27% of 1290) and clinical trial analysis (5.45% of 275 from ZUMA-1 and ZUMA-7 combined) was inconsistent with the proportion of the Black population in the United States diagnosed with LBCL, and numerically lower than a recent SEER estimation of disease prevalence in this population (7.24% of 1500 patients with LBCL were expected to be non-Hispanic Black).21 The sample size for other races (eg, Pacific Islander and Native Americans) was too small to be evaluated in this study. Differences in axi-cel treatment among racial and ethnic minorities may be further exacerbated by inaccuracies in the recording of patient race or ethnicity because of the lack of a standardized demographic data collection process.22
Age also affects cancer mortality, and the median age of non-Hispanic Black patients in the current analysis was numerically lower than that of non-Hispanic White patients.23 Generally, Black patients are more likely to be diagnosed with diffuse LBCL at a younger age, have stage III/IV disease, and have a worse 5-year survival rate relative to White patients.24 However, in the ZUMA-1 and ZUMA-7 clinical trials, older age did not affect efficacy with axi-cel and a manageable safety profile was maintained in all patients with R/R LBCL aged ≥65 years.25,26
Additionally, in the real-world cohort, a larger proportion of non-Hispanic Black patients had ≥12-month timeframes from diagnosis to infusion compared with all other patients, suggesting axi-cel may have been reserved as a treatment option in later lines of therapy. Barriers to treatment access among non-Hispanic Black patients may not be specific to CAR T-cell therapy alone but extend throughout the course of therapy.3,22 In light of this, and taking into consideration that non-Hispanic Black patients also experienced lower response rates, shorter PFS, and a greater proportion of patients waited ≥28 days from leukapheresis to lymphodepleting chemotherapy compared with other racial and ethnic groups, it is imperative to understand treatment access barriers that may differentially affect diverse patient populations.
Several factors disproportionately affect patients from racial and ethnic minority groups that may account for the underrepresentation of these patients in clinical trials.3,27-30 In the real-world cohort, numerically higher rates of non-Hispanic Black patients did not meet eligibility criteria for the ZUMA-1 trial compared with all other patients. Historically, factors such as higher poverty levels, poorer community health status, employment, education, and insufficient caregiver support27 were considered major barriers to clinical trial enrollment, particularly among non-Hispanic Black patients.3,27,28 In a SEER-based report, proximity to authorized treatment centers alone was insufficient to explain underrepresentation of minority patients in trial participation and optimal access to care.21 Furthermore, although race and ethnicity have been consistently associated with poorer outcomes and an absence of adequate treatment, a higher cancer burden and mortality rate may also be closely linked to negative social determinants of health. This includes low income (<$25 000 yearly), lack of health insurance, and living in states with poorer socioeconomic status and inadequate public health infrastructure.28
A study from the ASTCT-NMDP ACCESS Initiative assessing barriers to HCT and CAR T-cell therapy found that many states across the United States have potentially discriminatory policy restrictions and inadequate support for patients and their caregivers (eg, noncoverage if patient has a child with nonsuitable family support or history of mental illness) that result in financial burden when seeking these treatments.31 Timely allogeneic and autologous HCT is particularly low in Black patients compared with in White patients.32-34 This can affect rate of survival 1 year after transplant35,36 and access to proper posttransplant therapies.30,35,37 Access to timely HCT therapy is crucial for the post-HCT journey in patients with hematologic malignancies. With respect to CAR T-cell therapy, the ASTCT-NMDP ACCESS Initiative also found that Black patients were more likely to travel longer distances to receive this therapy and, in general, were less likely to actually be treated.38
CAR T-cell therapy is costly and, in the United States, medical insurance coverage varies by race and ethnicity.39 A recent report of United States patients showed that those receiving CAR T-cell therapy were more likely to have commercial insurance and less likely to be uninsured or covered by Medicare.40 Furthermore, given the high out-of-pocket cost for CAR T-cell therapy that includes expenses for travel or hospitalization, inadequate insurance coverage (which do not cover these expenses) further limit access to treatment for minority groups.40 Additional areas of inclusivity research related to CAR T-cell therapy are needed, with a focus on promoting timely referral of patients from racial/ethnic minority groups, reporting whether access for minority groups is improving over time, and describing any disparities related to delivery of the CAR T-cell product for infusion. Identification of other disparities related to race, ethnicity, and socioeconomic status in the setting of CAR T-cell therapy is also important to close treatment gaps and help promote equal, inclusive treatment strategies. Health care providers may consider developing active inclusion strategies to limit race- and ethnicity-based social deterrents to CAR T-cell therapy to benefit a broader range of patients.24,28,41 A successful treatment approach may also include patient-specific screening; for example, based on the safety findings with axi-cel in this study, risk factors for thrombocytopenia could be assessed in all non-Hispanic Asian patients.28
As with all observational studies, this analysis had some key limitations. In both the real-world and clinical trial assessments, race and ethnicity were self-reported, with no standardized definitions, potentially leading to biases and variability in reporting. CIBMTR reporting was not mandatory, and results may not be reflective of the entirety of axi-cel usage and outcomes in the United States. Because of the small sample size of racial/ethnic minority groups in the clinical trial analysis, CIs were wide and time-to-event analyses could not be conducted. The analysis only included patients treated in the United States and thus results may not be globally applicable but may provide context in different geographic locations.42 Finally, this analysis did not include patient-reported outcomes or quality-of-life end points.
Overall, outcomes with axi-cel CAR T-cell therapy in R/R LBCL were mostly consistent between races and ethnicities reported here, with some exceptions among non-Hispanic Black patients. These results provide important context regarding barriers of access to therapies for lymphoma to help develop interventions that improve health care access for all patients.
Acknowledgments
The authors thank the patients who participated in this study and their families, caregivers, and friends; and the study investigators, coordinators, and health care staff at each site. Medical writing support was provided by Hameda Khandaker, Nexus Global Group Science, funded by Kite, a Gilead company.
The CIBMTR is supported primarily by US Public Health Service U24CA076518 from the National Institutes of Health (NIH), National Cancer Institute (NCI), the NIH, National Heart, Lung, and Blood Institute (NHLBI), and the NIH, National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 and U24HL157560 from NIH, NHLBI and NCI; U24CA233032 from the NIH, NCI; OT3HL147741 and U01HL128568 from the NIH, NHLBI; HHSH250201700005C, HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration; and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research. Additional federal support is provided by grants from the NIH, NCI, NIAID, and NHLBI: P01CA111412, R01CA100019, R01CA152108, R01CA218285, R01CA231141, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01HL155741, R01HL131731, SC1MC31881, UM1CA121947, U01AI069197, U01AI126612, and UG1HL06924. Support is also provided by the Be the Match Foundation, Boston Children's Hospital, Dana-Farber Cancer Institute, St. Baldrick's Foundation, Pediatric Blood and Marrow Transplant Consortium; Stanford University, Medical College of Wisconsin, and the National Marrow Donor Program; and from the following commercial entities: AbbVie; Actinium Pharmaceuticals; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc; Amgen; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb; CareDx; CRISPR; CSL Behring; CytoSen Therapeutics; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida Cell, Ltd; Gilead Sciences; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals; Kadmon, a Sanofi company; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; medac GmbH; Medexus Pharma; Merck & Co; Millennium, the Takeda Oncology Co; Miltenyi Biotec; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer; Pharmacyclics, LLC, an AbbVie company; Priothera; Sanofi; Sanofi Aventis US Inc; Sobi; StemCyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; and Xenikos BV. F.L.L. is supported by the Leukemia and Lymphoma Society as a Scholar in Clinical Research, and NIH, NCI grant R01CA244328.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: F.L.L., T.S., Z.-H.H., H.-L.W., E.B., H.M., C.S., H.X., and M.C.P. designed the study; M.C.P., H.M., H.-L.W., Z.-H.H., and H.X. acquired and assembled data; Z.-H.H., H.-L.W., and E.B. analyzed data and prepared the analysis reports; and all authors were involved in the interpretation of the data and writing of the article, and provided final approval to submit for publication.
Conflict-of-interest disclosure: F.L.L. reports a scientific advisory role/consulting role with A2, Allogene, Amgen, bluebird bio, Bristol Myers Squibb/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; reports patents, royalties, and other intellectual property in several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; reports travel support from A2 Bio; and reports other relationships with Allogene (institutional), Aptitude Health, American Society of Hematology, bluebird bio (institutional), BioPharma Communications CARE Education, Bristol Myers Squibb (institutional), CERo Therapeutics (institutional), Clinical Care Options Oncology, Imedex, Kite (a Gilead company; institutional), Novartis (institutional), National Cancer Institute, Leukemia and Lymphoma Society, and Society for Immunotherapy of Cancer. T.S. held a consultancy or advisory role with AbbVie, AstraZeneca, BeiGene, Celgene, Juno, and Kite (a Gilead company), and Pharmacyclics; reports speakers' bureau participation for AstraZeneca, BeiGene, and Bristol Myers Squibb; and reports institutional research funding from Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb, Celgene, Juno, Kite (a Gilead company), Oncternal, Pharmacyclics, and TG Therapeutics. C.A.J. held a consulting/advisory role with AbbVie, Abintus Bio, ADC Therapeutics, Bristol Myers Squibb/Celgene, Caribou Bio, Daiichi Sankyo, ImmPACT Bio, Instil Bio, Ipsen, Kite (a Gilead company), Miltenyi Biotec, MorphoSys, Novartis, and Synthekine; and reports research funding from Kite (a Gilead company) and Pfizer. A.G. received honoraria from Kite (a Gilead company); served in a consulting/advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc; and reports research funding from Amgen, Genentech, and Kite. S.A. received research funding from Bristol Myers Squibb, Merck, Nektar, Tessa Therapeutics, and Xencor. D.B.M. received honoraria and travel support from Janssen; reports a consulting/advisory role for Adaptive Biotechnologies, Bristol Myers Squibb, Janssen, Kite (a Gilead company), and Miltenyi Biotec; reports research funding from 2Seventy Bio, Adicet, Allogene, Fate Therapeutics, Kite, and Miltenyi Biotec; and reports patents, royalties, or other intellectual property from chronic graft-versus-host disease (cGVHD) patent holder for ibrutinib as cGVHD therapy but no compensation. M.-A.P. received honoraria from AbbVie, Astellas, Celgene, Bristol Myers Squibb, Incyte, Karyopharm, Kite (a Gilead company), Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, and Takeda; served in a consulting/advisory role for Merck and Omeros; reports institutional research funding for clinical trials from Incyte, Kite, Miltenyi Biotec, and Novartis; and reports other relationship with a Data Safety Monitoring Board: Cidara Therapeutics, Medigene, and Servier. J.M. received honoraria from Curio, Kyowa Kirin, OncView, Physicians' Education Resource, Targeted Oncology, and Seagen; reports consulting/advisory role for ADC Therapeutics, Alexion, Bayer, BeiGene, Bristol Myers Squibb, Debiopharm, Epizyme, Fosunkite, Genmab, Innovent, Janssen, Juno/Celgene, Karyopharm, Kite (a Gilead company), Kyowa Kirin, Lilly/Loxo, MEI Pharma, MorphoSys/Incyte, Novartis, Pfizer, Pharmacyclics/AbbVie, Seagen, Servier, TG Therapeutics, and Zodiac; reports speakers’ bureau participation for Acrotech/Aurobindo, AstraZeneca, Bayer, BeiGene, Celgene/Bristol Myers Squibb, Genentech/Roche, Kite (a Gilead company), Kyowa Kirin, Pharmacyclics/Janssen, Seagen, and Verastem; and reports research funding from Bayer, Celgene, Genentech, Incyte, Janssen, Kite, Merck, Millennium, Pharmacyclics, Portola, and Seagen. M.P. reports honoraria from Bristol Myers Squibb; served in a consulting/advisory role for Nektar Therapeutics; and declares travel support from Novartis. J.G. had a consulting/advisory role with Janssen, Kite (a Gilead company), Legend Biotech, MorphoSys, and Sobi; and received research funding from Angiocrine Bioscience, Celgene, Juno Therapeutics (a Bristol Myers Squibb company), and Sobi. M.S. had employment with Bristol Myers Squibb (spouse); served in a consulting/advisory role for AbbVie, Adaptimmune, Adaptive Biotechnologies, AstraZeneca, Atara Biotherapeutic, BeiGene, Bristol Myers Squibb, Eli Lilly, Epizyme, Fate Therapeutics, Genentech, Innate Pharma, Kite (a Gilead company), MEI Pharma, Merck, MorphoSys/Incyte, Mustang Bio, Pharmacyclics, Regeneron, Sound Biologics, and TG Therapeutics; and reports research funding from AbbVie, AstraZeneca, Atara Biotherapeutics, BeiGene, Bristol Myers Squibb, Celgene, Genentech, Genmab, Gilead, MorphoSys/Incyte, Mustang Bio, Pharmacyclics, Sunesis, and TG Therapeutics. L.G. received honorarium from Bristol Myers Squibb. M.B.A. received research funding from Ansun, BioPharma Inc, and Janssen. S.H. had employment with Adaptive Biotechnologies. N.S.M. had employment with, and stock or other ownership in, HCA Healthcare; and reports a consulting/advisory role for Anthem, Inc. M.A.K.-D. received research funding from Bristol Myers Squibb, Pharmacyclics, and Novartis. T.B. had stock or other ownership in Aprea Therapeutics; received honoraria from Pfizer Hematology-Oncology; and reports research funding from Mayo Clinic, Cancer Center Support Grant. Y.L. reports a consulting/advisory role for Kite/Gilead, Celgene/Bristol Myers Squibb, Juno/Bristol Myers Squibb, bluebird bio, Janssen, Legend Biotech, Gamida Cell, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; and received research funding from Kite/Gilead, Celgene/Bristol Myers Squibb, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. N.N.B. had a consulting/advisory role for Acrotech, Affimed, Astellas, Kymera, and Secura Bio; and received research funding from Affimed, Daiichi Sankyo, and Kite (a Gilead company). Z.-H.H. had employment with Kite (a Gilead company); and stock or other ownership in Gilead Sciences. H.-L.W. had employment with Kite (a Gilead company). A.B. had employment with, and stock or other ownership in, Gilead Sciences. E.B. had employment with, stock or other ownership in, and consulting/advisory role for, Kite (a Gilead company). H.M. had employment with Kite (a Gilead company); and stock or ownership in Gilead Sciences. C.S. had employee with Kite (a Gilead company); and stock or other ownership in Gilead Sciences. H.X. had employment with Kite (a Gilead company). M.C.P. received honoraria from Celgene; reports consulting/advisory role for Amgen, Medigene, and Pfizer; and reports research funding from Bristol Myers Squibb, Kite (a Gilead company), and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Marcelo C. Pasquini, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; email: mpasquini@mcw.edu.
References
Author notes
F.L.L. and T.S. are joint first authors.
H.X. and M.C.P. are joint senior authors.
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal