Visual Abstract
Over the last decades, significant improvements in reducing the toxicities of allogeneic hematopoietic cell transplantation (allo-HCT) have widened its use as consolidation or salvage therapy for high-risk hematological malignancies. Nevertheless, relapse of the original malignant disease remains an open issue with unsatisfactory salvage options and limited rationales to select among them. In the last years, several studies have highlighted that relapse is often associated with specific genomic and nongenomic mechanisms of immune escape. In this review we summarize the current knowledge about these modalities of immune evasion, focusing on the mechanisms that leverage antigen presentation and pathologic rewiring of the bone marrow microenvironment. We present examples of how this biologic information can be translated into specific approaches to treat relapse, discuss the status of the clinical trials for patients who relapsed after a transplant, and show how dissecting the complex immunobiology of allo-HCT represents a crucial step toward developing new personalized approaches to improve clinical outcomes.
Introduction
Over the last decades, the treatment-related mortality of allogeneic hematopoietic cell transplantation (allo-HCT) has decreased significantly.1 Nevertheless, these improvements have not been paralleled by similar achievements in preventing relapse of the underlying disease. Moreover, although patients who relapse are now more fit to receive salvage treatments and the therapeutic portfolio has significantly broadened, none of these therapies has significantly modified the long-term outcomes, and rationales to choose the best therapy for a given patient are still lacking.2
Starting from this unmet critical issue, relevant recent studies, mostly conducted in the setting of allo-HCT for acute myeloid leukemia (AML), have transformed our view on the biology that underpins relapse, shifting the paradigm from treatment failure to the appreciation of relapse as an active and dynamic evolutionary process that leads to the selection of tumor variants resistant to allo-HCT therapeutic activity. The seminal observations that emerged from these studies indicate that most posttransplant relapses represent the end-result of tumor immune escape and that evasion from the graft-versus-leukemia (GVL) effect can occur by different modalities, often nonoverlapping, including alterations in the antigen presentation machinery and in the leukemia microenvironment.3-6 Notably, these mechanisms have no clear association with the mutational background of the original disease, supporting that posttransplantation relapse leverages biologic drivers that are unique and different from those involved in leukemia pathogenesis.
This review summarizes the current knowledge about these different patterns of immune escape, focusing on how the biologic characterization of relapse could inform the design of tailored therapies.
Alterations in antigen presentation machinery: leukemia becomes invisible to T-cell alloreactivity
Genomic loss of one HLA haplotype (HLA loss)
The therapeutic efficacy of allo-HCT relies on recognition by the donor-derived T cells of incompatible, patient-specific antigens presented on the surface of leukemic cells, which are recognized as foreign by the transplanted immune system (alloreactivity). These antigens, recognized by donor T cells through their T-cell receptors (TCRs), encompass polymorphic proteins that are processed and presented by HLA molecules matched between the donor and the recipient (minor histocompatibility antigens) and by HLA molecules mismatched between the donor and the recipient. Incompatible HLAs are the most potent targets of alloreactivity, being naturally recognized by a high number of individual T cells without need of previous priming.
The genomic loss of one HLA haplotype through copy neutral loss of heterozygosity (CN-LOH) represents the first evidence of a recurrent modality through which leukemia evades immune recognition after allo-HCT.7 CN-LOH, also known as uniparental disomy, is a genetic alteration in which deletion of a usually large genomic region is rapidly counterbalanced by duplication of its homologous region on the other chromosome, leading to a homozygous genotype for all genes encompassing the alteration without changes in the gene content or expression levels.8
Relapses after allo-HCT from partially HLA-incompatible donors often display CN-LOH events that encompass the entire HLA region, leading to irreversible loss of the haplotype that encodes for mismatched HLA (commonly referred to as HLA loss). As mentioned above, HLA incompatibilities are immunodominant targets of T-cell alloreactivity, thus, losing mismatched HLAs by duplicating a matched haplotype reduces the immunogenicity of leukemic cells, rendering them invisible to the preexisting immunity. Retrospective single-center studies have explored the incidence and features of HLA loss in different settings and reported that this modality accounts for no less than 30% of relapses after haploidentical HCT, apparently regardless of the underlying disease and strategy to prevent graft-versus-host disease (GVHD).9-13 Although the studies that evaluated HLA loss frequency after unrelated donor allo-HCT are fewer and less solid, they converge toward a frequency ranging between 10% and 15%.14-16 Unrelated donor allo-HCT is often performed from a donor with a few HLA mismatches, and a lower incidence of HLA loss in this setting suggests that a lower extent of HLA incompatibility might reduce the overall strength of anti-HLA T-cell alloreactivity and the selection pressure toward immune escape.
Several methods have been developed to detect genomic loss of HLA haplotypes, originally performed through HLA typing of purified leukemia blasts17 and single nucleotide polymorphism arrays7,10-12 and now possible through quantitative polymerase chain reaction (HLA-KMR18) and next-generation sequencing.13,19
Since their initial description, HLA loss relapses have prompted considerations about salvage therapeutic approaches. The first clinically relevant observation is that, especially in the haploidentical setting, the use of donor lymphocyte infusions (DLIs) has to be avoided because leukemia (but not other organs) has lost the main targets of alloreactivity, thereby decreasing their therapeutic index. For this reason, the European Society for Blood and Bone Marrow Transplantation recommends HLA loss testing for relapse management before proceeding with DLIs.20
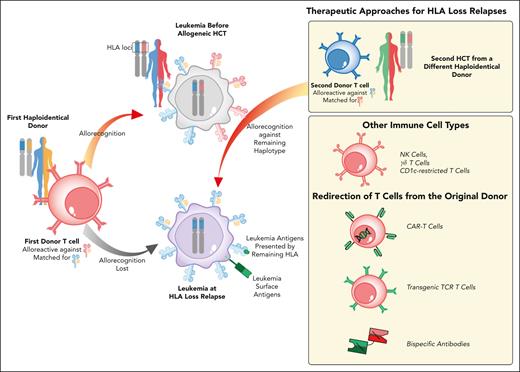
Besides avoiding ineffective and potentially harmful DLIs, it is also relevant to identify alternatives that are effective against HLA loss variants. One of the first options proposed is a second allo-HCT from a different, partially HLA-incompatible donor, selected for being HLA mismatched against the leukemia. Although this could be achieved with umbilical cord blood units and unrelated adult donors, the best example comes from choosing a second haploidentical donor, matched with the patient for the other haplotype (Figure 1).21 Although previous studies have shown limited benefit when changing the donor for the second transplant,22,23 a small, yet more recent study from Imus and collaborators has shown that in patients who relapsed after haploidentical HCT, re-transplanting from a donor who share a different haplotype with the patient can improve the outcome.24
Therapeutic interventions to counteract genomic loss of the mismatched HLA haplotype after allo-HCT. Schematic representation of the genomic HLA loss relapse modality and of available therapeutic strategies to restore donor T-cell recognition of leukemia. Genomic loss of one HLA haplotype by CN-LOH under allo-immune pressure leads to leukemia immune evasion from donor T cells (in red) and subsequently to relapse (leukemia at diagnosis in gray, relapse in violet). To counteract HLA loss relapse, one of the therapies available is a second allo-HCT using an alternative, partially HLA-incompatible donor. A different haploidentical donor is, in fact, expected to have T cells (in blue) alloreactive against the haplotype conserved and duplicated by the relapsed leukemic blasts. Other potential approaches that are gaining increased attention include non-HLA restricted immunotherapies, including NK cells or CD1c-restricted lymphocytes, and redirection of T cells from the stem cell donor through bispecific antibodies or transgenic receptors.
Therapeutic interventions to counteract genomic loss of the mismatched HLA haplotype after allo-HCT. Schematic representation of the genomic HLA loss relapse modality and of available therapeutic strategies to restore donor T-cell recognition of leukemia. Genomic loss of one HLA haplotype by CN-LOH under allo-immune pressure leads to leukemia immune evasion from donor T cells (in red) and subsequently to relapse (leukemia at diagnosis in gray, relapse in violet). To counteract HLA loss relapse, one of the therapies available is a second allo-HCT using an alternative, partially HLA-incompatible donor. A different haploidentical donor is, in fact, expected to have T cells (in blue) alloreactive against the haplotype conserved and duplicated by the relapsed leukemic blasts. Other potential approaches that are gaining increased attention include non-HLA restricted immunotherapies, including NK cells or CD1c-restricted lymphocytes, and redirection of T cells from the stem cell donor through bispecific antibodies or transgenic receptors.
Still, despite the improvements in reducing the toxicity of allo-HCT, a sizable proportion of patients at the time of HLA loss relapse are too old or frail to undergo a second transplant. Thus, the growing armamentarium of immunotherapeutic approaches that do not rely on conventional TCR-HLA interactions may come to aid.
For instance, although the genetic event at the basis of HLA loss does not decrease the overall cell surface expression levels of HLA class I molecules of leukemic blasts, it eliminates specific alleles. This frequently modifies the asset of killer cell immunoglobulin-like receptor ligands, potentially exposing leukemia to natural killer (NK) cell alloreactivity.25,26 Beyond NK cells, a number of other adaptive or innate-like cell types that do not rely on conventional TCR-HLA interactions are gaining attention for leukemia immunotherapy, including invariant NKT cells, CD1c-restricted T cells, and γδ T cells, which represents an additional option for this relapse type.27-29 Although no trials are currently ongoing for HLA loss relapses, several of these cell subsets have been employed already as adoptive cell therapies after allo-HCT with promising evidence of safety and efficacy.30-32
Another potential option is the redirection of conventional donor-derived T cells, including those from the original stem cell donor that drove the selection of the resistant variants. T cells can be redirected either through a chimeric antigen receptor, currently available only for selected diseases, or with a TCR restricted for HLAs conserved on leukemic cells, an approach that has shown signs of efficacy in the posttransplantation context.33 Finally, bispecific antibodies deserve a specific mention. In fact, we showed that, at the time of HLA loss (usually many months after allo-HCT), most patients have recovered T-cell counts and phenotype, which enable effective bridging of these lymphocytes to leukemia via an anti-CD3/CD33 bispecific antibody. This induces potent T-cell activation in vitro and in animal models, leading to the elimination of HLA loss blasts (Figure 1).34 In the setting of B-cell acute lymphoblastic leukemia, Wu and coworkers administrated the anti-CD19/CD3 bispecific T-cell engager blinatumomab to 4 patients with HLA loss relapses. All of them achieved complete remission with minimal residual disease negativity reached in 3.35 Blinatumomab demonstrated promising efficacy and safety as both a preemptive and maintenance therapy after allo-HCT for B-cell acute lymphoblastic leukemia,36 further supporting the idea that bispecifics may represent an attractive option, even for patients with minimal residual disease reappearance or those at very high risk for HLA loss relapse (Figure 1). Clinical trials that explored the use of bispecifics and cell therapies for posttransplantation relapses are summarized in Table 1.5,37-47
Selected clinical trials that are testing new approaches to overcome immune escape in posttransplantation relapses
| Relapse modality . | Therapy . | Therapeutic intervention . | Trial number . | Phase . | Disease . | N . | Clinical outcomes . | Reference . |
|---|---|---|---|---|---|---|---|---|
| HLA loss | Cellular immunotherapies | CIML NK cell infusion | NCT03068819 | 1/2 | AML | 10 | CR: 25%; PR: 12.5%; PD: 50%; OS at 2 y follow-up: 42% | 37 |
| CART-38 | NCT04351022 | 1/2 | AML | 6 | CR: 66.7% (CR = 1; CRi = 3); OS and LFS at 6 mo follow-up: 50% | 38 | ||
| HLA loss and HLA class II downregulation | Bispecific antibodies | Flotetuzumab | NCT05506956 | 1b | AML | N/A | N/A | N/A |
| Flotetuzumab + DLI | NCT04582864 | 2 | AML, MDS | N/A | N/A | N/A | ||
| HLA class II downregulation | IFN-γ delivery | IFN-γ | NCT04628338 | Early phase 1 | AML, MDS | 4 | CR: 75% | 39 |
| p53-MDM2 inhibitors | Siremadlin (HDM201) ± DLI | NCT05447663 | 1/2 | AML | 38 | N/A | 40 | |
| Upregulation of T-cell-inhibitory ligands | ICI | Sabatolimab (MBG453/anti-TIM3) | NCT04623216 | 1/2 | AML | 21 | CR: 30%; no GVHD | 41 |
| Pembrolizumab | NCT02981914 | Early phase 1 | AML, MDS, HL, B-NHL | 12 | ORR: 22% (2/9), CR = 2, SD = 2, PD = 5; median follow-up: 4.4 y; PFS: 2.9 mo, OS: 23.3 mo | 42 | ||
| IPI or nivolumab | NCT01822509 | 1 | CLL, NHL, HL, MM, AML, ALL, MDS | 28 | ORR: 29%; OS and PFS at 1 y follow-up: 56% and 23%; GVHD: 39% | 43 | ||
| ICI + HMAs | Nivolumab + HMA | EudraCT 2017-002194-18 | 1/2 | AML | 16 | ORR: 25%; SD: 25%; PD: 12.5%; median PFS: 1.8 mo; median OS: 15.6 mo; aGVHD: 31.25%, cGVHD: 25% | 44 | |
| Nivolumab + Aza | NCT03825367 | 1/2 | AML | N/A | N/A | N/A | ||
| IPI + decitabine | NCT02890329 | 1 | AML, MDS | 25/48 | ORR = 20% | 45 | ||
| HMAs | Guadecitabine + DLI | NCT02684162 | 2 | AML, MDS | N/A | N/A | N/A | |
| Aza, lenalidomide + DLIs | NCT02472691 | 2 | MDS, sAML, CMML | 50 | ORR: 56%; 1-y OS: 65%; aGVHD: 46%, cGVHD: 52% | 46 | ||
| Azacytidine | NCT00887068 | 3 | AML, MDS | 187 | No benefit in treatment arm (RFS, OS rate); no difference in aGVHD-cGVHD | 47 | ||
| Metabolic rewiring of TME | Metabolic modulators | Bicanorm (NaBi) + DLIs | NCT04321161 | Early phase 1 | AML | 10 | Recovery of T-cell fitness, rewiring of acidic pH | 5 |
| Relapse modality . | Therapy . | Therapeutic intervention . | Trial number . | Phase . | Disease . | N . | Clinical outcomes . | Reference . |
|---|---|---|---|---|---|---|---|---|
| HLA loss | Cellular immunotherapies | CIML NK cell infusion | NCT03068819 | 1/2 | AML | 10 | CR: 25%; PR: 12.5%; PD: 50%; OS at 2 y follow-up: 42% | 37 |
| CART-38 | NCT04351022 | 1/2 | AML | 6 | CR: 66.7% (CR = 1; CRi = 3); OS and LFS at 6 mo follow-up: 50% | 38 | ||
| HLA loss and HLA class II downregulation | Bispecific antibodies | Flotetuzumab | NCT05506956 | 1b | AML | N/A | N/A | N/A |
| Flotetuzumab + DLI | NCT04582864 | 2 | AML, MDS | N/A | N/A | N/A | ||
| HLA class II downregulation | IFN-γ delivery | IFN-γ | NCT04628338 | Early phase 1 | AML, MDS | 4 | CR: 75% | 39 |
| p53-MDM2 inhibitors | Siremadlin (HDM201) ± DLI | NCT05447663 | 1/2 | AML | 38 | N/A | 40 | |
| Upregulation of T-cell-inhibitory ligands | ICI | Sabatolimab (MBG453/anti-TIM3) | NCT04623216 | 1/2 | AML | 21 | CR: 30%; no GVHD | 41 |
| Pembrolizumab | NCT02981914 | Early phase 1 | AML, MDS, HL, B-NHL | 12 | ORR: 22% (2/9), CR = 2, SD = 2, PD = 5; median follow-up: 4.4 y; PFS: 2.9 mo, OS: 23.3 mo | 42 | ||
| IPI or nivolumab | NCT01822509 | 1 | CLL, NHL, HL, MM, AML, ALL, MDS | 28 | ORR: 29%; OS and PFS at 1 y follow-up: 56% and 23%; GVHD: 39% | 43 | ||
| ICI + HMAs | Nivolumab + HMA | EudraCT 2017-002194-18 | 1/2 | AML | 16 | ORR: 25%; SD: 25%; PD: 12.5%; median PFS: 1.8 mo; median OS: 15.6 mo; aGVHD: 31.25%, cGVHD: 25% | 44 | |
| Nivolumab + Aza | NCT03825367 | 1/2 | AML | N/A | N/A | N/A | ||
| IPI + decitabine | NCT02890329 | 1 | AML, MDS | 25/48 | ORR = 20% | 45 | ||
| HMAs | Guadecitabine + DLI | NCT02684162 | 2 | AML, MDS | N/A | N/A | N/A | |
| Aza, lenalidomide + DLIs | NCT02472691 | 2 | MDS, sAML, CMML | 50 | ORR: 56%; 1-y OS: 65%; aGVHD: 46%, cGVHD: 52% | 46 | ||
| Azacytidine | NCT00887068 | 3 | AML, MDS | 187 | No benefit in treatment arm (RFS, OS rate); no difference in aGVHD-cGVHD | 47 | ||
| Metabolic rewiring of TME | Metabolic modulators | Bicanorm (NaBi) + DLIs | NCT04321161 | Early phase 1 | AML | 10 | Recovery of T-cell fitness, rewiring of acidic pH | 5 |
aGVHD, acute graft-versus-host disease; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CART, chimeric antigen receptor T cell; cGVHD, chronic GVHD; CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; CRi, complete remission with incomplete recovery; DLI, donor lymphocyte infusion; HL, Hodgkin lymphoma; LFS, leukemia-free survival; MDM2, mouse-double-minute-2; MM, multiple myeloma; N/A, not available; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RFS, relapse-free survival; SD, stable disease.
In addition, in a recent report, Pagliuca and coworkers reported that relapses may also carry clonal point mutations and indels in individual HLAs, which might lead to the loss of specific alleles and, consequently, of immunodominant responses across those HLA restrictions.48 Moreover, because it is becoming clear that changes in the HLA immunopeptidome can heavily impact the outcome of allo-HCT,49,50 it will be relevant to address whether and how somatic mutations in the binding groove alter the peptide repertoire presented. Although the prevalence and significance of HLA point mutations need to be addressed further and may be challenging to detect in routine clinical practice, we can speculate that the therapeutic approaches mentioned for HLA loss relapses should also represent viable options for these relapse variants.
Transcriptional downregulation of HLA class II
After the discovery of HLA loss, 2 independent studies identified a different modality through which leukemia can alter its HLA asset, thereby hiding from T-cell recognition. By comparing paired patient leukemia samples collected before and after transplantation, both studies provided evidence of abolished expression of HLA class II genes (HLA-DR, -DQ, -DP) and of their master regulator (the class II major histocompatibility transactivator [CIITA]) in up to 40% of AML relapses after transplant.4,6 Of note, different from its genetic counterpart, this mechanism was apparently not correlated with the number of donor-recipient incompatibilities, occurring with similar frequencies after HLA-compatible and -incompatible transplants. Sequencing of HLA loci and their regulatory network revealed no mutations that could explain the abrogated expression of class II, hinting that this phenotype has a primarily epigenetic origin. A recent study analyzed the HLA class II transcriptional network of these relapses in detail and identified a tetrad of transcription factors (IRF8, MYB, MEF2C, and MEIS1) that control HLA expression levels, partly independent of the interferon gamma (IFN-γ)/CIITA pathway.51
The nongenetic and dynamic nature of this immune escape modality has the direct and natural consequence that it could be reversible by appropriate interventions. Different approaches have been successfully tested, either based on the rationale that IFN-γ can act on alternative regulatory elements of CIITA to recover the phenotype or with the more ambitious goal to rewire the altered epigenetic network.
Regarding the former approach, an ongoing phase 1 trial is testing, with promising results, the administration of recombinant IFN-γ in patients with AML/MDS who relapsed after allo-HCT (NCT04628338) (Table 1). Preliminary results of the first 4 patients who were treated showed surprising IFN-γ tolerability, even when combined with DLIs, and complete molecular remission in 3 of 4 treated patients.39 Still, one of the main concerns regarding direct IFN-γ administration relates to its systemic effects, which might include clinical manifestations of variable severity including hemophagocytic lymphohistiocytosis. To increase the safety of IFN-γ administration and the concentration of the cytokine in the target site, gene therapy- and nanoparticle–based delivery strategies are being actively investigated.52-54
Importantly, the recognition by T cells of leukemia cells and other targets in their microenvironment physiologically leads to IFN-γ release, which, in turn, can recover HLA class II expression on leukemic cells. Consequently, we could speculate that, in this relapse setting, a moderate degree of inflammation, as observed in nonsevere GVHD, could be associated with the reinstatement of a proficient antileukemia response. Rimando and collaborators have recently developed an approach to control the IFN-γ release only upon engagement with leukemic cells. They demonstrated that flotetuzumab, an anti-CD123XCD3 bispecific dual-affinity retargeting molecule, and chimeric antigen receptor T cells directed against distinct AML antigens (CD123, CD33, or CD371) could upregulate HLA class II expression on relapsed leukemic blasts through local IFN-γ release, in addition to their direct antitumor effect.55 These observations provide an additional framework for the implementation of AML-directed T-cell immunotherapies in the posttransplant relapse setting (Table 1).
With respect to strategies against the epigenetic alterations that underpin HLA class II downregulation, our group has identified polycomb repressive complex 2 (PRC2) as a key epigenetic driver of this immune escape modality by showing PRC2-mediated chromatin compaction at HLA class II and CIITA loci in leukemic blasts at relapse. PRC2 is a multisubunit complex that regulates chromatin accessibility through the methylation of H3K27. Studies on leukemia models showed that the inhibition of EZH2, the catalytic subunit of PRC2, impairs leukemia cell growth and reduces the number of leukemic stem cells, supporting oncogenic functions in AML.56,57 Notably, in our setting, blockade of PRC2 with inhibitors of the PRC2 complex (including tazemetostat, already clinically approved for hematological and solid tumors) was able to rescue HLA class II expression both in vitro and in vivo, reinstating leukemic recognition by T cells.58 The use of PRC2 inhibitors or other epigenetic therapies to revert immune escape holds many potential advantages when compared with other, more direct approaches, because it acts on hub regulators rather than downstream effectors, and it is theoretically capable of mediating more durable effects. However, it should be noted that PRC2, like many other epigenetic complexes, has such widespread relevance in the biology of living organism and thus its durable high-affinity inhibition might have unexpected and potentially severe long-term effects.
Furthermore, Chan and coworkers, by using an unbiased in vitro CRISPR-Cas9 screen, identified additional PRC2-independent transcriptional and posttranslational mechanisms of HLA class II/CIITA regulation based on the C-terminal-binding protein (CtBP) complex and FBXO11, a component of the E3 ubiquitin ligase complex. Although CtBP transcriptionally repressed HLA class II genes, FBXO11 facilitated CIITA proteasomal degradation. Their genetic knockout and inhibition of the euchromatic histone-lysine N-methyltransferase 1 and 2 (EHMT1/2) complex by A-366 and UNC0638 led to HLA class II upregulation on leukemic blasts, thereby promoting CD4+ T-cell activation and antileukemic responses59 (Figure 2). Finally, in a recent study that used both human samples and mouse models, Ho and collaborators demonstrated that inhibition of MDM2 ubiquitin ligase, a negative regulator of p53, favored the upregulation of HLA class II and TNF-related apoptosis-inducing ligand (TRAIL) receptor 1 and 2 (TRAIL-R1/2) on leukemia cells, thereby boosting the GVL effect.60 Accordingly, Langenbach and colleagues showed that MDM2 inhibition in murine melanoma cells stimulated p53-dependent increase of IL-15 and HLA class II, thereby enhancing tumor immunogenicity. They also showed that MDM2 inhibition synergized with immune checkpoint blockade, providing the rationale for combinatorial approaches.61 MDM2 inhibitors, such as HDM201, are being tested in clinical trials for AML and solid tumors, and a study is currently assessing the efficacy and safety of HDM201 in the prevention of AML relapse after allo-HCT (NCT05447663) (Table 1).
Immunotherapeutic and pharmacologic approaches to restore HLA class II expression and leukemia recognition after allo-HCT. The upper panel shows immunotherapeutic interventions based on IFN-γ, either directly administered or released by immune cells, to recover HLA class II expression via activation of IFN-responsive elements in alternative regulatory regions of CIITA. The recognition by donor T cells (in red) of minor histocompatibility antigens (MiHAs) and tumor antigens on leukemia cells (in violet) upon HLA class I presentation and of HLA class I/II restricted MiHAs on other patient tissues in the setting of GVHD can lead to IFN-γ release. Bispecifics and CAR T cells can have both direct antileukemic effects (redirecting T-cell responses) and indirect effects, which leads to release of IFN-γ and restoration of anti-HLA alloreactivity. The lower panel shows interventions aimed at restoring HLA expression by acting on epigenetic mechanisms that lead to HLA class II downregulation. Reduced accessibility of HLA class II and CIITA genes at the promoter level are mediated by different chromatin regulators, such as PRC2 and CtBP complex, which rely respectively on the methytransferases EZH2 and EHMT1/2 to catalyze the trimethylation Lys-27 (H3K27me3) of histone H3 and the monomethylation and dimethylation of Lys-9 (H3K9me1-2). Targeting the main components of the PRC2 complex, namely EZH2, EED, and JARID2 with the respective inhibitors tazemetostat, EED226, and JIB-04 and EHMT1/2 with UNC0638/A-366, can reinstate HLA class II and CIITA expression. In addition, although RREB1 knockout leads to the activation of both CIITA and HLA class II expression, FBXO11 loss can activate HLA class II genes with no changes in CIITA mRNA levels, thereby blocking CIITA polyubiquitination and proteasomal degradation. Finally, the inhibition of MDM2 ubiquitin ligase by HDM201 also has been shown to induce the upregulation of TRAIL1/2 and HLA class II genes on leukemia cells.
Immunotherapeutic and pharmacologic approaches to restore HLA class II expression and leukemia recognition after allo-HCT. The upper panel shows immunotherapeutic interventions based on IFN-γ, either directly administered or released by immune cells, to recover HLA class II expression via activation of IFN-responsive elements in alternative regulatory regions of CIITA. The recognition by donor T cells (in red) of minor histocompatibility antigens (MiHAs) and tumor antigens on leukemia cells (in violet) upon HLA class I presentation and of HLA class I/II restricted MiHAs on other patient tissues in the setting of GVHD can lead to IFN-γ release. Bispecifics and CAR T cells can have both direct antileukemic effects (redirecting T-cell responses) and indirect effects, which leads to release of IFN-γ and restoration of anti-HLA alloreactivity. The lower panel shows interventions aimed at restoring HLA expression by acting on epigenetic mechanisms that lead to HLA class II downregulation. Reduced accessibility of HLA class II and CIITA genes at the promoter level are mediated by different chromatin regulators, such as PRC2 and CtBP complex, which rely respectively on the methytransferases EZH2 and EHMT1/2 to catalyze the trimethylation Lys-27 (H3K27me3) of histone H3 and the monomethylation and dimethylation of Lys-9 (H3K9me1-2). Targeting the main components of the PRC2 complex, namely EZH2, EED, and JARID2 with the respective inhibitors tazemetostat, EED226, and JIB-04 and EHMT1/2 with UNC0638/A-366, can reinstate HLA class II and CIITA expression. In addition, although RREB1 knockout leads to the activation of both CIITA and HLA class II expression, FBXO11 loss can activate HLA class II genes with no changes in CIITA mRNA levels, thereby blocking CIITA polyubiquitination and proteasomal degradation. Finally, the inhibition of MDM2 ubiquitin ligase by HDM201 also has been shown to induce the upregulation of TRAIL1/2 and HLA class II genes on leukemia cells.
Thwarting immune recognition through T-cell impairment and rewiring of the microenvironment
Enforcement of inhibitory checkpoints
Upregulation of molecules that inhibit T-cell responses on leukemic cells represents another mechanism of posttransplantation relapse.6,62-65 Recent studies that compared patient AML blasts that were collected at relapse with the initial diagnosis showed that, after allo-HCT, leukemic cells displayed significantly increased expression of ligands for inhibitory TCRs, such as programmed death ligand 1 (PD-L1), B7-H3, and PVRL2, in composite and variable patterns.6
Notably, immunophenotypic changes in the profile of ligands expressed by leukemia cells were mirrored by corresponding alterations in the cognate receptors expressed by T cells as showed by our group for PD-L1 on leukemia and programmed death 1 (PD-1) on T cells.6,63 PD-L1–positive leukemic blasts elicit poor responses by donor-derived PD-1-positive T cells and recognition can be restored, at least partially, by anti–PD-L1 antibodies.
Concordant with these findings, several studies highlighted the coexpression of multiple inhibitory checkpoint receptors on donor-derived T cells after allo-HCT and their association with posttransplantation relapse. In the context of HLA-matched transplants, Jain and colleagues reported that PD-1 was similarly expressed on peripheral blood (PB) T cells of patients who relapsed and those who did not and found T-cell immunoglobulin and mucin domain 3 (TIM-3) and lymphocyte activation gene 3 (LAG-3) overexpression on leukemia antigen-specific T cells, suggesting that PD-1 is not the sole marker of T-cell exhaustion that is able to predict leukemia relapse.66 Coherently, Kong et al found that PD-1high/TIM-3+ PB T cells of patients who underwent transplantation, showed features of functional exhaustion and accumulated before clinically evident relapse, suggesting that they may serve as biomarkers to predict recurrence.67 Additional studies reported that in patients who relapsed, PD-1, T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT), and killer cell lectin-like receptor G1 (KLRG1) were highly coexpressed on T cells that were reactive against patient-specific minor histocompatibility antigens.65,68
These changes in the costimulatory interactions between leukemia and donor T cells seem even more prominent in the bone marrow (BM) of patients who relapsed. In fact, Williams et al reported a higher frequency of CD8+PD-1+/TIM-3+ and PD-1+/LAG-3+ in the BM of patients with AML who relapsed.69 Accordingly, Noviello et al identified specific features of exhaustion in central memory and memory stem T cells that infiltrated the BM of patients who relapsed with coexpression of multiple inhibitory receptors such as CTLA-4, PD-1, and TIM-3.63
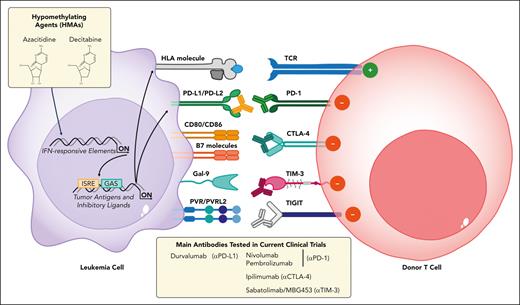
Therefore, targeting inhibitory checkpoints with monoclonal antibodies represents an attractive strategy to rewire the immunosuppressive environment and to re-establish antitumor immunity in selected patients. Initial reports showed interesting results when using the anti-CTLA-4 antibody ipilimumab (IPI) as monotherapy for treating posttransplantation relapses (Figure 3), demonstrating its safety and efficacy in stimulating antitumor responses especially in lymphoid malignancies and extramedullary AML despite some immune-related adverse events and GVHD.70-72 Penter et al investigated the biology that underlies these clinical observations and demonstrated that the response is accompanied by a specific transcriptomic signature of CD8+ T-cell activation. In particular, IPI treatment systemically alters the composition of peripheral T-cell populations with a concomitant increase in the expression of PD-1, HLA-DR, and ICOS on CD8+ cells.73
Immune checkpoint blockade to reinstate the GVL effect. Schematic representation of inhibitory-costimulatory immune receptors, their ligands, and the available monoclonal antibodies (MoAbs) to counteract T-cell inhibition. Upregulation of PD-1, CTLA-4, TIM-3, and TIGIT receptors on T cells (in red) mirrors the immunophenotypic changes observed in leukemic blasts (in violet) ligands because PD-L1/PD-L2, CD80/CD86, B7 molecules, Gal-9, and PVR/PVRL-2 and their interactions are responsible for T-cell impairment. Monoclonal antibodies can be used to block these interactions, either in monotherapy or in combination with HMAs (azacitidine, decitabine), which can potentiate these effects through upregulation of the relevant ligands, HLAs, and of tumor antigens in leukemic cells.
Immune checkpoint blockade to reinstate the GVL effect. Schematic representation of inhibitory-costimulatory immune receptors, their ligands, and the available monoclonal antibodies (MoAbs) to counteract T-cell inhibition. Upregulation of PD-1, CTLA-4, TIM-3, and TIGIT receptors on T cells (in red) mirrors the immunophenotypic changes observed in leukemic blasts (in violet) ligands because PD-L1/PD-L2, CD80/CD86, B7 molecules, Gal-9, and PVR/PVRL-2 and their interactions are responsible for T-cell impairment. Monoclonal antibodies can be used to block these interactions, either in monotherapy or in combination with HMAs (azacitidine, decitabine), which can potentiate these effects through upregulation of the relevant ligands, HLAs, and of tumor antigens in leukemic cells.
Given the promise shown by IPI monotherapy and that hypomethylating agents (HMAs) have been shown to promote the upregulation of both tumor antigens and inhibitory ligands on leukemic blasts,74,75 Garcia et al tested the combined administration of IPI with decitabine in transplant-naïve and posttransplant patients in a recent multicenter phase 1 trial (ETCTN/CTEP 10026 study, NCT02890329) (Figure 3; Table 1). In patients who underwent transplantation, the overall response rate was 20% with rather short-lived remissions. Immune-related adverse events occurred in 44% of patients and did not seem to be associated with differential response. Although multiplexed immunofluorescence staining on serial BM biopsies provided striking examples of individual responders with increased cytotoxic T-cell infiltrate and globally increased CD3+ density after IPI plus decitabine administration, no distinct pattern of local T-cell infiltration was associated with response, thereby highlighting the underlying tumor and immune heterogeneity.45 In parallel, Penter et al employed single-cell transcriptomics to analyze BM samples of patients treated in this trial and showed a strong association with response when the baseline ratio of T cells to AML cells was high. Immune activation was only evident after IPI exposure, which drove CD4+ T-cell differentiation and increased the frequency of marrow-infiltrating regulatory T cells. Of note, immune changes were more evident in extramedullary leukemia sites when compared with the BM, suggesting a relevant role of microenvironmental niches in shaping the GVL effect.76
In addition, PD-1 inhibitors, such as nivolumab or pembrolizumab, have been tested in the posttransplant relapse setting with interesting response rates in Hodgkin Lymphoma and Anaplastic Large Cell Lymphoma but with much less convincing results in AML.43,77-79 Moreover, PD-1 inhibitors seem to have an increased ability to cause or reactivate severe GVHD when compared with CTLA-4 inhibitors.80 Given the unsatisfactory results of monotherapy with PD-1 inhibitors in treating patients with AML after transplantation, recent studies have tested these checkpoint blockers in combination with HMAs,44,81 which were also followed with low-dose DLI82 (Figure 3). In particular, Apostolova and colleagues tested the combination of nivolumab with HMA in patients with relapsed AML in the phase 2 NIFAR study (Eudra-CT 2017-002194-18). The overall response rate was 25%, and another 25% of the patients achieved stable disease. Immune profiling of patients who enrolled in the trial documented a higher frequency of activated nonsenescent CD8+ effector T cells in responders. Single-cell transcriptomics revealed a proinflammatory rewiring of the expression profile of T and myeloid cells in responders.44
Additional clinical trials are currently ongoing to assess the tolerability and efficacy of single or combined immune checkpoint inhibitors alone or in association with epigenetic therapies or chemotherapy (Table 1). Although the expression of inhibitory receptors on T cells or their ligands on tumor cells did not seem to be reliable predictors of response in the small studies conducted to date, new studies may clarify whether the selection of patients with significant enforcement of inhibitory T-cell/leukemia interactions will improve the benefit-to-risks ratio of these approaches. Moreover, the studies presented previously have variably analyzed the expression of immune checkpoints and response to their blockade in PB and in BM. More work should be conducted to clarify which compartment provides the best information regarding the potential efficacy of treatments and should be assessed for emergence of resistance mechanisms.
Metabolic rewiring of the TME
It is increasingly recognized that altered metabolism represents a hallmark of cancer and that tumor cells can rewire the metabolic state of surrounding cells to facilitate their growth. Although a wealth of evidence shows the role of metabolic changes in promoting AML onset and progression,83 studies only recently started to link these processes to immune evasion and posttransplantation relapse.
Hypoxia, altered levels of metabolically active molecules, and competition for nutrient availability are distinctive features of the tumor microenvironment (TME). These alterations dramatically impact antitumor immune responses, in particular T-cell effector functions.84 In addition, because both tumor cells and T lymphocytes rely on a glycolytic metabolism with the consequent production of lactic acid (LA), alterations in this energetic interplay inevitably determine TME acidosis, thereby hampering the effector functions of different immune subsets and thus GVL responses.85
Fischer et al reported that LA production by tumor cells can suppress cytotoxic T-lymphocyte activity by obstructing LA efflux, thereby altering T-cell metabolism.86 Uhl et al recently showed that at relapse after allo-HCT, AML blasts can impair T-cell proliferation and antitumor activity by producing LA. Interestingly, the authors showed that this condition can be reversed by metabolic reprogramming with sodium bicarbonate (NaBi), which can restore T-cell functional fitness and an effective GVL (Figure 4). This was rapidly translated into an early phase 1 clinical trial that tested the administration of Bicanorm (NaBi) following DLIs (NCT04321161, Table 1).5
Strategies to rewire the metabolic asset of the TME. Schematic illustration of the different metabolic alteration in the TME and the available options to rewire its physiological status. Upon relapse after allo-HCT, AML cells (in violet) can impair donor T-cell (in red) proliferative potential and cytotoxic functions through LA release. Metabolic reprogramming through the administration of Bicanorm (NaBi), which antagonizes LA-induced effects, represent an optimal strategy to restore T-cell fitness and functions. In addition, azithromycin administration is directly involved in T-cell global impairment because it specifically inhibits T-cell effector functions and alters the gut microbiome with defects being associated with specific plasma metabolite signatures and with the accumulation of exhausted T cells. To counteract these effects, administration of probiotics and fecal microbiota transplantation can be useful approaches to restore the composition of the microbiome. The conditioning regimen and GVHD can alter the plasmatic oxidative balance, which can lead to an accumulation of ROS that, in turn, hampers T-cell functions. Administration of antioxidants can be a rational therapeutic approach to decrease ROS levels and reinstate redox balance.
Strategies to rewire the metabolic asset of the TME. Schematic illustration of the different metabolic alteration in the TME and the available options to rewire its physiological status. Upon relapse after allo-HCT, AML cells (in violet) can impair donor T-cell (in red) proliferative potential and cytotoxic functions through LA release. Metabolic reprogramming through the administration of Bicanorm (NaBi), which antagonizes LA-induced effects, represent an optimal strategy to restore T-cell fitness and functions. In addition, azithromycin administration is directly involved in T-cell global impairment because it specifically inhibits T-cell effector functions and alters the gut microbiome with defects being associated with specific plasma metabolite signatures and with the accumulation of exhausted T cells. To counteract these effects, administration of probiotics and fecal microbiota transplantation can be useful approaches to restore the composition of the microbiome. The conditioning regimen and GVHD can alter the plasmatic oxidative balance, which can lead to an accumulation of ROS that, in turn, hampers T-cell functions. Administration of antioxidants can be a rational therapeutic approach to decrease ROS levels and reinstate redox balance.
In addition, Vallet and coworkers documented an unexpected role of azithromycin in promoting relapse after allo-HCT. Their investigation started from a phase 3 trial that tested the effectiveness of posttransplantation azithromycin in preventing bronchiolitis obliterans syndrome (ALLOZITHRO trial) and that was stopped before completion because of an excess of relapse mortality in the azithromycin arm.87 By combining multiomic profiling and functional experiments, the authors showed that azithromycin alters the proportion of immune subsets that circulate in patients and inhibits T-cell cytotoxicity against tumor cells, mainly by altering their metabolism. Main alterations included glycolysis inhibition, downregulation of mitochondrial genes, and upregulation of immunomodulatory genes, specifically including SOCS1.88 This is in line with a previous study by Ansari et al who showed how azithromycin can alter the T helper cell subset in terms of phenotype, proliferation, and effector functions, supporting a role of this drug in inducing T-cell impairment.89 Besides its direct effect on immune cells, azithromycin also dramatically alters the gut microbiome. Also starting from the ALLOZITHRO trial samples, Vallet and coworkers identified a Bacteroides taxon that was significantly enriched in the enterobiome of patients who relapsed and that had an association with a specific plasma metabolite signature that ultimately favored the accumulation of exhausted T cells.90 These results corroborate and complement existing evidence on the role of the enterobiome in modulating the GVL effect. Peled et al showed in a large cohort of patients who underwent transplantation that a higher abundance of a bacterial group mostly composed of Eubacterium limosum associates with relapse protection,91 suggesting that therapeutic strategies aimed at rewiring the microbiome (eg, probiotics or fecal microbiota transplantation) might also play a role in preventing or treating relapse.
In addition, in the complex setting of allo-HCT, the tissue damage associated with the conditioning regimen and immune complications (eg, GVHD) leads to oxidative stress as shown by the increased level of oxidative markers and altered antioxidant balance in the serum of patients who underwent transplantation.92,93 The main cause of oxidative stress is the altered regulation of reactive oxygen species levels in the cells, which culminate in a high degree of oxidative DNA damage and dramatically hamper proper T-cell activation.94 Karl et al reported that allo-HCT recipients display a high level of the oxidative DNA damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG), both in serum and immune cells (T and NK cells). During posttransplant immune reconstitution, T cells with high levels of 8-OHdG (8-OHdGhi) not only displayed enhanced proliferation/activation rates but also premature exhaustion and reduced GvT effect.95 Therefore, patients with 8-OHdGhi T cells had a higher relapse incidence and inferior overall survival after allo-HCT, supporting the rationale for implementing antioxidant therapies.
Finally, although mainly in the nontransplant setting, some studies highlighted that leukemic cells reprogram the TME by producing different immunosuppressive enzymes, such as IDO-1,96 arginase,97 the ectonucleotidase CD73,98 and CD39.99 Indeed, studies performed mostly in solid tumors showed the effective rewiring of T-cell metabolism toward an antitumoral function, achieved either as indirect effect of other targeted approaches100-102 or directly by specific inhibitors.103,104
Conclusions
In a relatively short time, our understanding of the biologic mechanisms at the basis of posttransplantation relapse has significantly increased. Still, to translate this progress into benefit for patients, there are critical issues yet to be faced.
First, diagnostic assays that are easy to perform, affordable, and reliable should be developed to provide a rapid idea of the mechanism that is driving the relapse to help untangle the extensive interpatient heterogeneity. Specific assays are becoming available to detect genomic HLA loss18 but assay for the other relapse modalities are yet to be developed.
Crucially, therapeutic trials should be designed based on the biologic knowledge and should enroll patients based on relapse immune features. We can envisage that the availability of rapid and accessible assays to identify the relapse modality might facilitate the design of trials specifically directed to patient with a specific type of relapse. This would expectedly improve the efficacy and prevent unnecessary toxicities, such as GVHD among those patients whose relapse is driven by permanent genetic loss of HLA.7 This change of approach would shift results of posttransplantation salvage treatments from small benefit in a large cohort to a more sizable effect in a limited and carefully selected population. However, to make this possible and attractive to the relevant stakeholders, it is imperative to devise multicenter trials and to accrue cases in a reasonable time. This would also allow us to better appreciate the toxicities of the approaches presented in this review, which is difficult to ascertain in small, single-center series.
Finally, the most important objective is to prevent relapse rather than to treat it. Technologic improvements offer increasing opportunities to detect the reappearance of disease-specific mutations, immunophenotypic marker combinations, or host chimerism before clinical relapse. Still, at these early stages, it is not possible to retrieve any information on the biology that underlies impeding relapse and thus to act according to the principles outlined in this review. Large, multicentric studies on the topic of relapse might also help to move toward this direction. By collecting clinical and biologic data, these studies might help to identify risk factors for each modality and, on this basis, to generate artificial intelligence–powered predictors that are able to call the most likely relapse pattern for a specific patient and, based on this information, to suggest the most appropriate way to counteract it.
Acknowledgments
The authors thank Katharina Fleischhauer (University of Essen, Essen, Germany) and Nicoletta Cieri (Dana-Farber Cancer Institute, Boston, MA) for critical reading the manuscript and for their advice. The authors apologize to all authors who were not quoted in this review because of space constraints.
Work in the Vago laboratory was supported by the Associazione Italiana per la Ricerca sul Cancro (investigator grants #22197 and #28953 to L.V.), the European Commission and the Fondazione Regionale per la Ricerca Biomedica (ERA-NET-JTC2021 TRANSCAN-3 “PIXEL” to L.V.), the Italian Ministry of Health (GR-2016-02364847 to C.T. and GR-2018-12367860 to L.V.), the Cariplo Foundation (CARIPLO Giovani Ricercatori 2019-1708 to C.T.), the DKMS Stiftung Leben Spenden (John Hansen Grant 2022 to C.T.), and the Leukemia & Lymphoma Society (LLS SCOR to Robert Soiffer).
Authorship
Contribution: A.T., C.T., and L.V. jointly reviewed the literature and wrote the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: L.V. receives royalties from GenDx, B.V. (Utrecht, The Netherlands). The remaining authors declare no competing financial interests.
Correspondence: Luca Vago, Unit of Immunogenetics, Leukemia Genomics and Immunobiology, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; email: vago.luca@hsr.it.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal