Key Points
The CLL-IPI retains prognostic value for PFS, but its impact appears diminished in predicting survival with targeted drugs.
Improved survival with targeted therapies vs chemoimmunotherapy underscores the need to reevaluate prognostic tools amid treatment shifts.
Visual Abstract
We evaluated the chronic lymphocytic leukemia International Prognostic Index (CLL-IPI) in patients with CLL treated first line with targeted drugs (n = 991) or chemoimmunotherapy (n = 1256). With a median observation time of 40.5 months, the 3-year progression-free survival (PFS) rates for targeted drug–treated patients varied by CLL-IPI risk group: 96.5% (low), 87.6% (intermediate), 82.4% (high), and 78.7% (very high). Differences between consecutive CLL-IPI risk groups were observed for intermediate vs low and high vs intermediate, but not very high vs high. CLL-IPI factors β2-microglobulin, immunoglobulin heavy variable (IGHV) status, and TP53 status each retained prognostic value for PFS. The 3-year overall survival (OS) rates by CLL-IPI risk groups were 100%, 96%, 93.9%, and 89.4%, respectively, with no differences between consecutive risk groups. Age, Binet stage, β2-microglobulin, and TP53 status each retained prognostic value for OS. In chemoimmunotherapy patients (median observation time, 66.9 months), 3-year PFS rates for CLL-IPI risk groups were 78.1%, 51.4%, 40.1%, and 16.5%, respectively; corresponding 3-year OS rates were 97.4%, 93.1%, 81.8%, and 57.3%. In a matched-pair analysis, PFS differences in targeted therapies (n = 812) vs chemoimmunotherapy (n = 812) across all risk groups and OS differences in all but patients at low risk were demonstrated. The CLL-IPI maintains its prognostic value in predicting PFS outcomes with targeted drugs, but its impact in predicting survival appears diminished. Targeted therapies showed enhanced outcomes over chemoimmunotherapy, highlighting their effectiveness across various risk groups. Our findings support ongoing assessment of prognostic tools in CLL treatment evolution. These trials were registered at www.ClinicalTrials.gov as #NCT02345863, #NCT02401503, #NCT02689141, #NCT02445131, #NCT02758665, #NCT02950051, #NCT02242942, #NCT00262782, #NCT00281918, and #NCT01010061.
Introduction
The chronic lymphocytic leukemia International Prognostic Index (CLL-IPI) combines pretreatment genetic, biochemical, and clinical parameters into a prognostic model for overall survival (OS), discriminating 4 prognostic subgroups.1 However, the score was established in patients with CLL treated first line with chemoimmunotherapy, which are less efficacious in patients with CLL and high-risk features. The most adverse prognosticator is the loss of p53 function resulting from del(17p) and/or TP53 mutation that leads to refractoriness of chemotherapy. A growing understanding of B-cell receptor signaling as a central stimulator and survival pathway in CLL cells has led to the development of inhibitors that tackle enzymatic drivers of B-cell receptor effector signaling. Other new compounds are BH3 mimetics, small molecules that inhibit the antiapoptotic molecule B-cell lymphoma 2 (BCL2), and related family members. Targeted drugs significantly improve the outcome even in poor-risk CLL and might diminish the prognostic utility of CLL-IPI.2,3
The purpose of this analysis was to evaluate CLL-IPI in a pooled data set of clinical phase 2 and 3 trials in patients with CLL treated first line with targeted drugs or chemoimmunotherapy. For this purpose, the impact of the CLL-IPI on progression-free survival (PFS) and OS of patients with CLL was evaluated upon treatment with targeted drugs or chemoimmunotherapy.
Methods
Patients
All patients had a confirmed diagnosis of CLL, were previously untreated, and had an indication for treatment. The clinical trials were approved by the institutional review board and registered at www.ClinicalTrials.gov. All patients provided written informed consent in accordance with the Declaration of Helsinki. The CLL-IPI was calculated by using a weighted grading of the following 5 prognostic factors as previously published:1TP53 status (no abnormalities vs del(17p) or TP53 mutation or both), immunoglobulin heavy variable (IGHV) status (mutated vs unmutated), serum β2-microglobulin concentration (≤3.5 mg/L vs >3.5 mg/L), clinical stage (Binet A or Rai 0 vs Binet B-C or Rai I-IV), and age (≤65 years vs >65 years).
The primary data set included all patients treated first line with at least 1 dose of targeted drug and available information for the calculation of CLL-IPI treated within the CLL2-BIG (NCT02345863), CLL2-BAG (NCT02401503), CLL2-BIO (NCT02689141), CLL2-BCG (NCT02445131), CLL2-GIVe (NCT02758665), CLL13 (NCT02950051), and CLL14-trial (NCT02242942). An optional debulking with 2 cycles of bendamustine was recommended for patients with a high tumor burden defined by an absolute lymphocyte count ≥25 × 109/L and/or bulky disease before the start of treatment with a targeted agent in the CLL2-BIG, CLL2-BAG, CLL2-BIO, and CLL2-BCG trials.
The secondary data set included all patients treated first line with at least 1 dose of standard chemoimmunotherapy and available information for the calculation of CLL-IPI treated within the CLL1 (NCT00262782), CLL8 (NCT00281918), CLL11 (NCT01010061), CLL13 (NCT02950051), and CLL14 trials (NCT02242942).
Data analysis
Patient, CLL, and treatment characteristics were reported using counts and percentages for categorical variables and descriptive statistics for continuous variables. For demographics and CLL characteristics, values at the start of first line therapy were used.
Time-to-event analyses were performed applying Kaplan-Meier estimates. PFS was calculated from start of first line therapy to first disease progression or death. OS was defined as the time between start of first line therapy and death. Hazard ratios (HRs), 95% confidence intervals (CIs), and P values were determined using Cox proportional hazards regression modeling. C-statistics were calculated to assess the discriminatory value of the CLL-IPI.
To compare patients treated first line with at least 1 dose of targeted drug with those treated with chemoimmunotherapy, a matched-pair analysis was conducted. Patients from both data sets were matched on the CLL-IPI variables (CLL-IPI risk group, age group, Binet stage, serum β2-microglobulin, IGHV mutational status, and TP53 status), with no differences with regard to the selected variables allowed. Each patient was only matched once (matching without replacement). HRs, CIs, and P values were calculated to estimate the differences in PFS and OS regarding single CLL-IPI risk factors between both data sets.
All statistical tests were 2-sided with significance level being set at .05. All P values were considered descriptive without adjustments for multiple testing.
All analyses were performed using SPSS v27.
Results
Patients characteristics
Data from 2579 patients were exported from 10 trial data sets, in which 1053 patients were treated with targeted drugs (primary data set), and 1256 patients were treated with chemoimmunotherapy (secondary data set). Due to missing information for the calculation of CLL-IPI, 62 patients (5.9%) of the primary data set and 270 patients (17.7%) of the secondary data set were excluded from the analysis population.
Thus, the primary analysis population included 991 treatment-naïve patients who were treated with at least 1 dose of venetoclax (n = 653), ibrutinib (n = 68), ibrutinib plus venetoclax (n = 261) or idelalisib (n = 9). A total of 100 patients (10.1%) received at least 1 dose of bendamustine debulking.
The secondary analysis population included 1256 treatment-naïve patients who were treated with at least 1 dose of fludarabine and cyclophosphamide plus rituximab (n = 466), bendamustine plus rituximab (n = 77), chlorambucil plus rituximab (n = 263), or chlorambucil plus obinutuzumab (n = 450).
Due to the different inclusion criteria of the phase 2 and 3 trials, age and cumulative illness rating scale scores differed according to treatment exposure (Table 1). The median age was 64 (range, 27-89) and 68 years (range, 29-90), and the median cumulative illness rating scale score was 3 (range, 0-23) and 6 (range, 0-28) for patients treated with targeted drugs or chemoimmunotherapy, respectively.
Baseline demographic and disease characteristics of patients with CLL treated first line with targeted drugs or chemoimmunotherapy by the type of first line treatment
| Primary data set . | |||||
|---|---|---|---|---|---|
| Parameters . | Venetoclax (n = 653) . | Ibrutinib (n = 68) . | Venetoclax + ibrutinib (n = 261) . | Idelalisib (n = 9) . | Total (N = 991) . |
| Age, y | |||||
| Median (range) | 65 (27-89) | 63 (32-82) | 60 (30-85) | 70 (44-77) | 64 (27-89) |
| >65 y | 308 (47.2) | 26 (38.2) | 92 (35.2) | 6 (66.7) | 432 (43.6) |
| Male sex | 472 (72.3) | 42 (61.8) | 171 (65.5) | 8 (88.9) | 693 (69.9) |
| ECOG PS >0 | 234 (35.8) | 22 (32.4) | 80 (30.7) | 4 (44.4) | 340 (34.3) |
| Binet stage B-C | 498 (76.3) | 53 (77.9) | 191 (73.2) | 8 (88.9) | 750 (75.7) |
| CIRS score | |||||
| Median (range) | 3 (0-23) | 3 (0-16) | 2 (0-7) | 2 (0-9) | 3 (0-23) |
| >1 | 465 (71.2) | 53 (77.9) | 165 (63.2) | 6 (66.7) | 689 (69.5) |
| >6 | 156 (23.9) | 11 (16.2) | 3 (1.1) | 1 (11.1) | 171 (17.3) |
| Serum β2-microglobulin (mg/L) | |||||
| Median (range) | 4.0 (1-16.2) | 3.9 (1.8-11.8) | 4.2 (1.3-12.5) | 5.2 (3.4-10.3) | 4.0 (1-16.2) |
| >3.5 | 400 (61.3) | 43 (63.2) | 169 (64.8) | 8 (88.9) | 620 (62.6) |
| Type according to hierarchical model, N (%) | |||||
| del(17p) | 15 (2.3) | 9 (13.2) | 24 (9.2) | 0 (0.0) | 48 (4.8) |
| del(11q) | 117 (17.9) | 14 (20.6) | 35 (13.4) | 2 (22.2) | 168 (17.0) |
| Trisomy 12 | 111 (17.0) | 12 (17.6) | 35 (13.4) | 2 (22.2) | 160 (16.1) |
| No del(17p)/del(11q)/trisomy12/del(13q) | 134 (20.5) | 16 (23.5) | 59 (22.6) | 2 (22.2) | 211 (21.3) |
| del(13q) alone | 276 (42.3) | 17 (25.0) | 108 (41.4) | 3 (33.3) | 404 (40.8) |
| Unmutated IGHV status | 386 (59.1) | 47 (69.1) | 151 (57.9) | 4 (44.4) | 588 (59.3) |
| TP53 | |||||
| Mutated | 23 (3.5) | 11 (16.2) | 38 (14.6) | 0 (0.0) | 72 (7.3) |
| deleted and/or mutated | 24 (3.7) | 12 (17.6) | 39 (14.9) | 0 (0.0) | 75 (7.6) |
| Secondary data set | |||||
| Parameters | F/FCR (n = 466) | BR (n = 77) | CLB-rituximab (n = 263) | CLB-obinutuzumab (n = 450) | Total (n = 1256) |
| Age, y | |||||
| Median (range) | 59 (29-80) | 71 (66-84) | 73 (47-90) | 73 (39-89) | 68 (29-90) |
| >65 y | 79 (17.0) | 77 (100.0) | 208 (79.1) | 366 (81.3) | 730 (58.1) |
| Male sex | 333 (71.5) | 53 (68.8) | 161 (61.2) | 287 (63.8) | 834 (66.4) |
| ECOG PS >0 | 159 (34.1) | 28 (36.4) | 173 (65.8) | 269 (59.8) | 629 (50.1) |
| Binet stage B-C | 352 (75.5) | 58 (75.3) | 205 (77.9) | 355 (78.9) | 970 (77.2) |
| CIRS score | |||||
| Median (range) | 1 (0-7) | 3 (0-6) | 8 (0-18) | 8 (0-28) | 6 (0-28) |
| >1 | 188 (40.3) | 59 (76.6) | 255 (97.0) | 439 (97.6) | 941 (74.9) |
| >6 | 2 (0.4) | 0 (0.0) | 188 (71.5) | 352 (78.2) | 542 (43.2) |
| Serum β2-microglobulin, mg/L | |||||
| Median (range) | 3.1 (1.2-12.3) | 4.7 (1.9-15.5) | 2.9 (0.2-17.8) | 3.5 (0.2-16.7) | 3.3 (0.2-17.8) |
| >3.5 | 180 (38.6) | 61 (79.2) | 99 (37.6) | 222 (49.3) | 562 (44.7) |
| Type according to hierarchical model, n (%) | |||||
| del(17p) | 26 (5.6) | 0 (0.0) | 19 (7.2) | 31 (6.9) | 76 (6.1) |
| del(11q) | 107 (23.0) | 14 (18.2) | 48 (18.3) | 76 (16.9) | 245 (19.5) |
| Trisomy 12 | 53 (11.4) | 12 (15.6) | 42 (16.0) | 76 (16.9) | 183 (14.6) |
| No del(17p)/del(11q)/trisomy12/del(13q) | 114 (24.5) | 15 (19.5) | 68 (25.9) | 116 (25.8) | 313 (24.9) |
| del(13q) alone | 161 (34.5) | 36 (46.8) | 86 (32.7) | 151 (33.6) | 434 (34.6) |
| Unmutated IGHV status | 294 (63.1) | 47 (61.0) | 171 (65.0) | 285 (63.3) | 797 (63.5) |
| TP53 | |||||
| Mutated | 27 (5.8) | 0 (0.0) | 21 (8.0) | 45 (10.0) | 93 (7.4) |
| deleted and/or mutated | 30 (6.4) | 0 (0.0) | 29 (11.0) | 54 (12.0) | 113 (9.0) |
| Primary data set . | |||||
|---|---|---|---|---|---|
| Parameters . | Venetoclax (n = 653) . | Ibrutinib (n = 68) . | Venetoclax + ibrutinib (n = 261) . | Idelalisib (n = 9) . | Total (N = 991) . |
| Age, y | |||||
| Median (range) | 65 (27-89) | 63 (32-82) | 60 (30-85) | 70 (44-77) | 64 (27-89) |
| >65 y | 308 (47.2) | 26 (38.2) | 92 (35.2) | 6 (66.7) | 432 (43.6) |
| Male sex | 472 (72.3) | 42 (61.8) | 171 (65.5) | 8 (88.9) | 693 (69.9) |
| ECOG PS >0 | 234 (35.8) | 22 (32.4) | 80 (30.7) | 4 (44.4) | 340 (34.3) |
| Binet stage B-C | 498 (76.3) | 53 (77.9) | 191 (73.2) | 8 (88.9) | 750 (75.7) |
| CIRS score | |||||
| Median (range) | 3 (0-23) | 3 (0-16) | 2 (0-7) | 2 (0-9) | 3 (0-23) |
| >1 | 465 (71.2) | 53 (77.9) | 165 (63.2) | 6 (66.7) | 689 (69.5) |
| >6 | 156 (23.9) | 11 (16.2) | 3 (1.1) | 1 (11.1) | 171 (17.3) |
| Serum β2-microglobulin (mg/L) | |||||
| Median (range) | 4.0 (1-16.2) | 3.9 (1.8-11.8) | 4.2 (1.3-12.5) | 5.2 (3.4-10.3) | 4.0 (1-16.2) |
| >3.5 | 400 (61.3) | 43 (63.2) | 169 (64.8) | 8 (88.9) | 620 (62.6) |
| Type according to hierarchical model, N (%) | |||||
| del(17p) | 15 (2.3) | 9 (13.2) | 24 (9.2) | 0 (0.0) | 48 (4.8) |
| del(11q) | 117 (17.9) | 14 (20.6) | 35 (13.4) | 2 (22.2) | 168 (17.0) |
| Trisomy 12 | 111 (17.0) | 12 (17.6) | 35 (13.4) | 2 (22.2) | 160 (16.1) |
| No del(17p)/del(11q)/trisomy12/del(13q) | 134 (20.5) | 16 (23.5) | 59 (22.6) | 2 (22.2) | 211 (21.3) |
| del(13q) alone | 276 (42.3) | 17 (25.0) | 108 (41.4) | 3 (33.3) | 404 (40.8) |
| Unmutated IGHV status | 386 (59.1) | 47 (69.1) | 151 (57.9) | 4 (44.4) | 588 (59.3) |
| TP53 | |||||
| Mutated | 23 (3.5) | 11 (16.2) | 38 (14.6) | 0 (0.0) | 72 (7.3) |
| deleted and/or mutated | 24 (3.7) | 12 (17.6) | 39 (14.9) | 0 (0.0) | 75 (7.6) |
| Secondary data set | |||||
| Parameters | F/FCR (n = 466) | BR (n = 77) | CLB-rituximab (n = 263) | CLB-obinutuzumab (n = 450) | Total (n = 1256) |
| Age, y | |||||
| Median (range) | 59 (29-80) | 71 (66-84) | 73 (47-90) | 73 (39-89) | 68 (29-90) |
| >65 y | 79 (17.0) | 77 (100.0) | 208 (79.1) | 366 (81.3) | 730 (58.1) |
| Male sex | 333 (71.5) | 53 (68.8) | 161 (61.2) | 287 (63.8) | 834 (66.4) |
| ECOG PS >0 | 159 (34.1) | 28 (36.4) | 173 (65.8) | 269 (59.8) | 629 (50.1) |
| Binet stage B-C | 352 (75.5) | 58 (75.3) | 205 (77.9) | 355 (78.9) | 970 (77.2) |
| CIRS score | |||||
| Median (range) | 1 (0-7) | 3 (0-6) | 8 (0-18) | 8 (0-28) | 6 (0-28) |
| >1 | 188 (40.3) | 59 (76.6) | 255 (97.0) | 439 (97.6) | 941 (74.9) |
| >6 | 2 (0.4) | 0 (0.0) | 188 (71.5) | 352 (78.2) | 542 (43.2) |
| Serum β2-microglobulin, mg/L | |||||
| Median (range) | 3.1 (1.2-12.3) | 4.7 (1.9-15.5) | 2.9 (0.2-17.8) | 3.5 (0.2-16.7) | 3.3 (0.2-17.8) |
| >3.5 | 180 (38.6) | 61 (79.2) | 99 (37.6) | 222 (49.3) | 562 (44.7) |
| Type according to hierarchical model, n (%) | |||||
| del(17p) | 26 (5.6) | 0 (0.0) | 19 (7.2) | 31 (6.9) | 76 (6.1) |
| del(11q) | 107 (23.0) | 14 (18.2) | 48 (18.3) | 76 (16.9) | 245 (19.5) |
| Trisomy 12 | 53 (11.4) | 12 (15.6) | 42 (16.0) | 76 (16.9) | 183 (14.6) |
| No del(17p)/del(11q)/trisomy12/del(13q) | 114 (24.5) | 15 (19.5) | 68 (25.9) | 116 (25.8) | 313 (24.9) |
| del(13q) alone | 161 (34.5) | 36 (46.8) | 86 (32.7) | 151 (33.6) | 434 (34.6) |
| Unmutated IGHV status | 294 (63.1) | 47 (61.0) | 171 (65.0) | 285 (63.3) | 797 (63.5) |
| TP53 | |||||
| Mutated | 27 (5.8) | 0 (0.0) | 21 (8.0) | 45 (10.0) | 93 (7.4) |
| deleted and/or mutated | 30 (6.4) | 0 (0.0) | 29 (11.0) | 54 (12.0) | 113 (9.0) |
BR, bendamustine-rituximab; CLB, chlorambucil; F/FCR, fludarabine/fludarabine-cyclophosphamide-rituximab.
CLL-IPI risk factors in the primary and secondary data sets were distributed as follows: (1) age >65 years in 43.6% and 58.1%; (2) Binet stage B/C in 75.7% and 77.2%; (3) β2-microglobulin >3.5 mg/L in 62.6% and 44.7%; (4) unmutated IGHV status in 59.3% and 63.5%; and (5) del(17p) and/or TP53 mutations in 7.6% and 9.5%, respectively.
CLL-IPI risk groups in the primary and secondary data sets were low in 13.4% and 13%, intermediate in 31.1% and 32.4%, high in 48.7% and 46.5%, and very high in 6.8% and 8.1%, respectively.
PFS
With a median observation time of 40.5 months (range 1-75.9), the median PFS was not reached in the primary data set of patients treated with targeted drugs, with 215 documented PFS events (21.7%). In the secondary data set of patients treated with chemoimmunotherapy, the median PFS was 33.5 months after a median observation time of 66.9 months (range, 0-134.3), with 854 documented PFS events (68%).
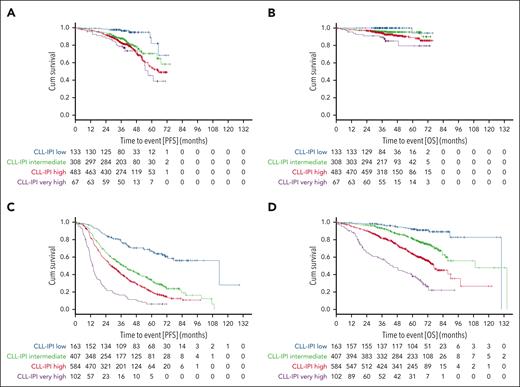
The estimated 36-month PFS rates of patients with CLL in the low, intermediate, high, and very high CLL-IPI risk groups were 96.5%, 87.6%, 82.4%, and 78.7% upon treatment with targeted drugs (Figure 1A) and 78.1%, 51.4%, 40.1%, and 16.5% upon treatment with chemoimmunotherapy, respectively (Figure 1C).
PFS and OS according to CLL-IPI risk groups. (A-B) PFS (A) and OS (B) in patients treated with targeted drugs. (C-D) PFS (C) and OS (D) in patients treated with chemoimmunotherapy.
PFS and OS according to CLL-IPI risk groups. (A-B) PFS (A) and OS (B) in patients treated with targeted drugs. (C-D) PFS (C) and OS (D) in patients treated with chemoimmunotherapy.
In patients treated with targeted drugs, 8 of 133 patients with low CLL-IPI (6%), 60 of 308 with intermediate CLL-IPI (19.5%), 125 of 483 with high CLL-IPI (25.9%) and 22 of 67 with very high CLL-IPI (32.8%) had a documented PFS event. In Cox proportional hazards regression analysis, PFS differences were demonstrated in patients treated with targeted drugs with regard to CLL-IPI risk groups intermediate vs low (HR, 3.296; 95% CI, 1.576-6.894; P = .002) and high vs intermediate (HR, 1.365; 95% CI, 1.003-1.858; P = .048), with no differences in very high vs high risk groups (HR, 1.314; 95% CI, 0.835-2.068; P = .238) (supplemental Table 1, available on the Blood website).
A sensitivity analysis excluding patients treated with bendamustine debulking (n = 891) similarly demonstrated PFS differences in CLL-IPI risk groups intermediate vs low (HR, 3.515; 95% CI, 1.596-7.734; P = .002) and high vs intermediate (HR, 1.406; 95% CI, 1.013-1.952; P = .042) and no differences in very high vs high risk groups (HR, 1.403; 95% CI, 0.862-2.282; P = .173; supplemental Figure 2).
In patients treated with chemoimmunotherapy, 56 of 163 patients with low CLL-IPI (34.4%), 267 of 407 patients with intermediate CLL-IPI (65.6%), 439 of 584 patients with high CLL-IPI (75.2%), and 92 of 102 patients with very high CLL-IPI (90.2%) had a documented PFS event. In Cox proportional hazards regression analyses, PFS differences were demonstrated with regard to CLL-IPI intermediate vs low risk groups (HR, 2.747; 95% CI, 2.053-3.676; P < .001), high vs intermediate (HR, 1.384; 95% CI, 1.188-1.612; P < .001), and very high vs high (HR, 2.172; 95% CI, 1.733-2.723; P < .001; supplemental Table 1).
C-statistics for PFS at 3 years was 0.6022 (95% CI, 0.5583-0.6429) for patients treated with targeted drugs and 0.6513 (95% CI, 0.6251-0.6794) for patients treated with chemoimmunotherapy.
PFS by CLL-IPI risk factors
When analyzing PFS by individual CLL-IPI risk factors, the estimated 36-month PFS rates were 85.6% and 85.7% for patients aged >65 vs ≤65 years treated with targeted drugs (HR, 0.791; 95% CI, 0.6-1.042; P = .095; supplemental Figure 1A; supplemental Table 3), and 39.4% and 57.3% for patients aged >65 vs ≤65 years treated with chemoimmunotherapy (HR, 1.579; 95% CI, 1.371-1.818; P < .001).
With regard to Binet stages A, B, and C, the PFS rates at 36 months in patients treated with targeted drugs were 88.6%, 84.2%, and 85.2%, respectively (HRBvsA, 1.369 [95% CIBvsA, 0.948-1.977; P = .094]; HRCvsB, 0.899 [95% CICvsB, 0.667-1.212; P = .484]; supplemental Figure 1B; supplemental Table 3), and 45.3%, 48.7%, and 45.4% for patients treated with chemoimmunotherapy (HRBvsA, 1.002 [95% CIBvsA, 0.839-1.197; P = .981]; HRCvsB, 1.055 [95% CICvsB, 0.906-1.229; P = .489]).
Regarding β2-microglobulin levels >3.5 vs ≤3.5 mg/dL, the PFS rates at 36 months were 83.1% and 89.8% for patients treated with targeted drugs (HR, 1.703; 95% CI, 1.266-2.292; P < .001; supplemental Figure 1C; supplemental Table 3) and 45.2% vs 48.1% for patients treated with chemoimmunotherapy, respectively (HR, 1.204; 95% CI, 1.052-1.379; P = .007).
For patients with CLL with unmutated vs mutated IGHV status, the PFS rates at 36 months were 81.4% and 92% in the group treated with targeted drugs (HR, 2.601; 95% CI, 1.886-3.587; P < .001; supplemental Figure 1D; supplemental Table 3) and 35.5% and 66.2% in the group treated with chemoimmunotherapy, respectively (HR, 2.563; 95% CI, 2.195-2.994; P < .001).
With regard to TP53 mutation and/or deletion vs no TP53 mutation/deletion, the PFS rates at 36 months were 80.9% and 86% for patients treated with targeted drugs (HR, 1.622; 95% CI, 1.06-2.482; P = .026; supplemental Figure 1E; supplemental Table 3) and 20.4% and 50.2% for patients treated with chemoimmunotherapy, respectively (HR, 2.433; 95% CI, 1.964-3.015, P < .001).
OS
The median OS was not reached in patients treated with targeted drugs with 64 documented events (16.5%; Figure 1B) and was 90.1 months with 395 documented events (31.4%) in patients treated with chemoimmunotherapy (Figure 1D).
With regard to CLL-IPI risk groups, the estimated survival rates at 36 months for low, intermediate, high, and very high CLL-IPI risk groups were 100%, 96%, 93.9%, and 89.4% for patients treated with targeted drugs (Table 2) and 97.4%, 93.1%, 81.8%, and 57.3% for patients treated with chemoimmunotherapy, respectively.
OS from start of targeted therapy by selected characteristics
| OS . | Pts, n . | Events, n (%) . | Median, mo . | 12-mo survival, % . | 24-mo survival, % . | 36-mo survival, % . | 48-mo survival, % . |
|---|---|---|---|---|---|---|---|
| All patients | 991 | ||||||
| CLL-IPI risk group | |||||||
| Low | 133 | 1 (0.8) | Not reached | 100.0 | 100.0 | 100.0 | 100.0 |
| Intermediate | 308 | 14 (4.5) | Not reached | 98.7 | 96.7 | 96.0 | 95.4 |
| High | 483 | 39 (8.1) | Not reached | 97.9 | 97.1 | 93.9 | 91.0 |
| Very high | 67 | 10 (14.9) | Not reached | 95.5 | 91.0 | 89.4 | 85.2 |
| Age, y | |||||||
| ≤65 | 559 | 22 (3.9) | Not reached | 98.7 | 98.0 | 96.9 | 96.0 |
| >65 | 432 | 42 (9.7) | Not reached | 97.7 | 95.6 | 92.6 | 89.7 |
| Binet stage | |||||||
| A | 241 | 10 (4.1) | Not reached | 98.3 | 97.5 | 97.0 | 95.1 |
| B | 374 | 21 (5.6) | Not reached | 98.4 | 96.8 | 95.5 | 93.8 |
| C | 376 | 33 (8.8) | Not reached | 98.1 | 96.8 | 93.5 | 91.1 |
| Serum β2-microglobulin, mg/L | |||||||
| ≤3.5 | 371 | 11 (3.0) | Not reached | 99.5 | 98.6 | 97.4 | 97.0 |
| >3.5 | 620 | 53 (8.5) | Not reached | 97.6 | 95.9 | 93.6 | 90.8 |
| IGHV status | |||||||
| Unmutated | 588 | 45 (7.7) | Not reached | 98.1 | 96.9 | 94.1 | 91.3 |
| Mutated | 400 | 19 (4.7) | Not reached | 98.5 | 97.0 | 96.4 | 95.8 |
| TP53 status | |||||||
| None | 916 | 54 (5.9) | Not reached | 98.5 | 97.4 | 95.4 | 93.7 |
| Deleted and/or mutated | 75 | 10 (13.3) | Not reached | 96.0 | 91.9 | 90.5 | 86.6 |
| OS . | Pts, n . | Events, n (%) . | Median, mo . | 12-mo survival, % . | 24-mo survival, % . | 36-mo survival, % . | 48-mo survival, % . |
|---|---|---|---|---|---|---|---|
| All patients | 991 | ||||||
| CLL-IPI risk group | |||||||
| Low | 133 | 1 (0.8) | Not reached | 100.0 | 100.0 | 100.0 | 100.0 |
| Intermediate | 308 | 14 (4.5) | Not reached | 98.7 | 96.7 | 96.0 | 95.4 |
| High | 483 | 39 (8.1) | Not reached | 97.9 | 97.1 | 93.9 | 91.0 |
| Very high | 67 | 10 (14.9) | Not reached | 95.5 | 91.0 | 89.4 | 85.2 |
| Age, y | |||||||
| ≤65 | 559 | 22 (3.9) | Not reached | 98.7 | 98.0 | 96.9 | 96.0 |
| >65 | 432 | 42 (9.7) | Not reached | 97.7 | 95.6 | 92.6 | 89.7 |
| Binet stage | |||||||
| A | 241 | 10 (4.1) | Not reached | 98.3 | 97.5 | 97.0 | 95.1 |
| B | 374 | 21 (5.6) | Not reached | 98.4 | 96.8 | 95.5 | 93.8 |
| C | 376 | 33 (8.8) | Not reached | 98.1 | 96.8 | 93.5 | 91.1 |
| Serum β2-microglobulin, mg/L | |||||||
| ≤3.5 | 371 | 11 (3.0) | Not reached | 99.5 | 98.6 | 97.4 | 97.0 |
| >3.5 | 620 | 53 (8.5) | Not reached | 97.6 | 95.9 | 93.6 | 90.8 |
| IGHV status | |||||||
| Unmutated | 588 | 45 (7.7) | Not reached | 98.1 | 96.9 | 94.1 | 91.3 |
| Mutated | 400 | 19 (4.7) | Not reached | 98.5 | 97.0 | 96.4 | 95.8 |
| TP53 status | |||||||
| None | 916 | 54 (5.9) | Not reached | 98.5 | 97.4 | 95.4 | 93.7 |
| Deleted and/or mutated | 75 | 10 (13.3) | Not reached | 96.0 | 91.9 | 90.5 | 86.6 |
No survival differences were demonstrated for patients with CLL-IPI intermediate vs low risk (HR, 6.013; 95% CI, 0.791-45.725; P = .083), high vs intermediate risk (HR, 1.743; 95% CI, 0.946-3.213, P = .075), and very high vs high risk (HR, 1.841; 95% CI, 0.918-3.69; P = .086) treated with targeted drugs by Cox regression analysis (supplemental Table 1).
A sensitivity analysis excluding patients treated with bendamustine debulking (n = 891) demonstrated only survival differences for high vs intermediate risk groups (HR, 1.954; 95% CI, 1.02-3.742; P = .043; supplemental Figure 3).
In patients treated with chemoimmunotherapy, 15 of 163 patients with low CLL-IPI (9.2%), 94 of 407 patients with intermediate CLL-IPI (23.1%), 220 of 584 with high CLL-IPI (37.7%), and 66 of 102 patients with very high CLL-IPI (64.7%) had a documented survival event.
In the Cox proportional hazards regression analysis, survival differences were demonstrated regarding intermediate vs low (HR, 2.756; 95% CI, 1.596-4.758; P < .001), high vs intermediate (HR, 2.063; 95% CI, 1.616-2.634; P < .001), and very high vs high IPI risk groups (HR, 2.366; 95% CI, 1.796-3.117; P < .001; supplemental Table 1).
C-statistics for OS at 3 years were 0.6334 (95% CI, 0.5709-0.6994) for patients treated with targeted drugs and 0.7058 (95% CI, 0.6702-0.7384) for patients treated with chemoimmunotherapy.
OS by CLL-IPI risk factors
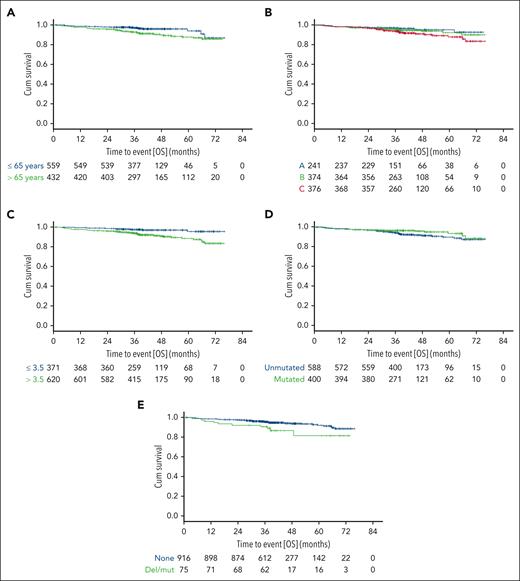
When analyzing OS by individual CLL-IPI risk factors (Table 2), the estimated survival rates at 36 months were 92.6% and 96.9% for patients aged >65 vs ≤65 years treated with targeted drugs (HR, 2.198; 95% CI, 1.305-3.703; P = .003; Figure 2A; supplemental Table 2) and 82.4% vs 90% for patients treated with chemoimmunotherapy (HR, 1.793; 95% CI, 1.444-2.228; P < .001).
OS in patients treated with targeted drugs by CLL-IPI risk factors. OS according to age (A), Binet stage (B), levels of serum β2-microglobulin (C), IGHV mutational status (D), and TP53 status (E).
OS in patients treated with targeted drugs by CLL-IPI risk factors. OS according to age (A), Binet stage (B), levels of serum β2-microglobulin (C), IGHV mutational status (D), and TP53 status (E).
With regard to Binet stages A, B, and C in patients treated with targeted drugs, the survival rates at 36 months were 97%, 95.5%, and 93.5%, respectively (HRBvsA, 1.329; 95% CIBvsA, 0.626-2.822; P = .459; HRCvsB, 1.543; 95% CICvsB, 0.893-2.667; P = .12; HRCvsA, 2.05; 95% CICvsA, 1.01-4.16; P = .047; Figure 2B; supplemental Table 2), and 90.9%, 88.2%, and 78.9% for patients treated with chemoimmunotherapy (HRBvsA, 1.216; 95% CIBvsA, 0.925-1.598; P = .162; HRCvsB, 1.245; 95% CICvsB, 0.998-1.553; P = .052; HRCvsA, 1.514; 95% CICvsA, 1.147-1.998; P = .003).
With regard to β2-microglobulin >3.5 vs ≤3.5 mg/dL, the survival rates at 36 months were 93.6% and 97.4% for patients treated with targeted drugs (HR, 3.053; 95% CI, 1.594-5.845; P < .001; Figure 2C; supplemental Table 2) and 81.7% and 88.7% for patients treated with chemoimmunotherapy, respectively (HR, 1.696; 95% CI, 1.389-2.07; P < .001).
For patients with CLL and with unmutated vs mutated IGHV status, the survival rates at 36 months were 94.1% and 96.4% for the group treated with targeted drugs (HR, 1.606; 95% CI, 0.939-2.745; P = .083; Figure 2D; supplemental Table 2) and 81.8% vs 92.1% for the group treated with chemoimmunotherapy (HR, 2.184; 95% CI, 1.726-2.764; P < .001).
With regard to TP53 mutation and/or deletion vs no mutation/deletion, the survival rates at 36 months were 90.5% and 95.4% for patients treated with targeted drugs (HR, 2.221; 95% CI, 1.13-4.366; P = .021; Figure 2E; supplemental Table 2), compared with 58.1% and 87.7% for patients treated with chemoimmunotherapy, respectively (HR, 3.1; 95% CI, 2.38-4.038; P < .001).
Matched-pair analyses
A total of 812 patients treated with targeted drugs were matched on selected variables (CLL-IPI risk groups, age groups, Binet stages, β2-microglobulin, IGHV mutational status, and TP53 status) to 812 patients treated with chemoimmunotherapy, with a median observation time of 41.4 months (range, 1-75.9) and 65.1 months (range, 0-95), respectively.
The PFS rates at 36 months for patients with low, intermediate, high, and very high risks treated with chemoimmunotherapy vs targeted drugs were 78.1% vs 97% (HR, 5.396; 95% CI, 2.285-12.742; P < .001), 58.8% vs 86.6% (HR, 2.952; 95% CI, 2.136-4.081; P < .001), 43.1% vs 80.4% (HR, 3.422; 95% CI, 2.744-4.267; P < .001), and 21.3% vs 71.5% (HR, 4.273; 95% CI, 2.442-7.474; P < .001), respectively.
At 36 months, respective OS rates for patients treated with chemoimmunotherapy vs targeted drugs were 97.5% vs 100% for low risk (HR, 5.2; 95% CI, 0.658-41.119; P = .118), 93.5% vs 95.8% for intermediate risk (HR, 2.932; 95% CI, 1.52-5.656; P = .001), 81.7% vs 93.2% for high risk (HR, 3.058; 95% CI, 2.126-4.398; P < .001), and 67.5% vs 84.7% for very high risk (HR, 2.357; 95% CI, 1.135-4.895; P = .021).
Assessing OS by individual CLL-IPI risk factors, the estimated survival rates at 36 months for patients aged >65 years were 83.1% and 92.4% (HR, 2.873; 95% CI, 2.037-4.05; P < .001), respectively, and 90.8% and 96.7% (HR, 2.949; 95% CI, 1.735-5.013; P < .001) for patients aged ≤65 years treated with chemoimmunotherapy compared with targeted drugs, respectively (Figure 3A; supplemental Table 4).
OS in patients treated with targeted drugs compared with chemoimmunotherapy matched on CLL-IPI risk factors. OS according to age (A), Binet stage (B), levels of serum β2-microglobulin (C), IGHV mutational status (D), and TP53 status (E).
OS in patients treated with targeted drugs compared with chemoimmunotherapy matched on CLL-IPI risk factors. OS according to age (A), Binet stage (B), levels of serum β2-microglobulin (C), IGHV mutational status (D), and TP53 status (E).
For patients categorized by Binet stages, the OS rates at 36 months were 91.3% and 96.6% (HR, 3.202; 95% CI, 1.546-6.63; P = .002) for stage A, 89.9% and 95.4% (HR, 3.21; 95% CI, 1.951-5.282; P < .001) for stage B, and 81% and 92.4% (HR, 2.478; 95% CI, 1.652-3.718; P < .001) for stage C, respectively (Figure 3B; supplemental Table 4).
Regarding β2-microglobulin levels, patients with levels >3.5 mg/L had 36-month OS rates of 81.5% and 92.6% (HR, 2.699; 95% CI, 1.952-3.732; P < .001), whereas those with levels ≤ 3.5 mg/L had rates of 94.3% and 97.2% (HR, 3.623; 95% CI, 1.903-6.9; P < .001) for chemoimmunotherapy vs targeted drugs, respectively (Figure 3C; supplemental Table 4).
For patients with unmutated IGHV status, OS rates at 36 months were 83% vs 93.2% (HR, 2.916; 95% CI, 2.078-4.092; P < .001), whereas for those with mutated IGHV status, the rates were 92.2% vs 96.3% (HR, 2.654; 95% CI, 1.529-4.606; P < .001) when comparing chemoimmunotherapy with targeted drugs (Figure 3D; supplemental Table 4).
The 36-month OS rates for patients with TP53 mutation and/or deletion were 68.9% and 86.2% (HR, 2.322; 95% CI, 1.126-4.792; P = .023), whereas for those without TP53 mutation and/or deletion, the rates were 88% and 95.1% (HR, 2.947; 95% CI, 2.147-4.044; P < .001) when treated with chemoimmunotherapy vs targeted drugs, respectively, as illustrated in Figure 3E; supplemental Table 4.
Discussion
The CLL-IPI combines genetic, biochemical, and clinical parameters into a prognostic model, describing 4 prognostic subgroups with significantly different 5-year survival rates ranging from 23.3% for patients at very high risk to 93.2% for patients at low risk.1 The score was calculated in patients with CLL treated first line with chemotherapy (83.5% of the analysis population) or chemoimmunotherapy (16.5% of the analysis population) recruited from 1997 to 2009.
However, with the approval of targeted drugs, the treatment and prognosis of patients with CLL has remarkably changed, particularly in those with high-risk features. Therefore, we wished to evaluate the prognostic value of CLL-IPI in 991 patients with CLL treated first line with targeted drugs and in 1256 patients treated with chemoimmunotherapy.
In the subset of patients treated with targeted drugs, the PFS rates varied by CLL-IPI risk groups, with differences in all but patients with very high vs high risks. The 3-year OS rates by CLL-IPI risk groups were 100%, 96%, 93.9%, and 89.4%, respectively, with no differences between consecutive risk groups. Specific CLL-IPI factors for PFS (β2-microglobulin, IGHV status, and TP53 status) and survival (β2-microglobulin, age, Binet stage, and TP53 status) did offer valuable risk information and retained prognostic value. Debulking with bendamustine before the start of targeted therapy was recommended in a subset of patients with a higher tumor burden. A sensitivity analysis that excluded patients who have been treated with bendamustine debulking reaffirmed our findings.
The applicability of the CLL-IPI was fully validated in the data set of patients treated with chemoimmunotherapy. When comparing both data sets by a matched-pair analysis, differences regarding PFS in targeted therapies vs chemoimmunotherapy across all risk groups and differences regarding OS in all but patients at low risk were demonstrated. This provides robust evidence of the substantial benefit of targeted drugs over chemoimmunotherapy. Notably, the low-risk group exhibited excellent outcomes with targeted drugs, with a 100% survival rate at 36 months.
Ibrutinib was approved in 2014 for the first line treatment of patients with CLL with TP53-aberration, and in 2016, the approval was extended irrespective of genetic features. Since then, many other targeted drugs have been approved. The CLL-IPI has been published in 2016, including patients treated first line until 2009. In the multivariable model that constituted the basis for the construction of the CLL-IPI, 5 individual weighed factors established the risk score groups.1 The factors of age and clinical stage received the lowest score (1 point), whereas the IGHV mutational status was double (2 points), and the weighted risk score of TP53 status was double of IGHV mutational status (4 points). Elevated β2-microglobulin levels were similarly graded as an unmutated IGHV status with 2 points.
In discussing our findings, it is essential to acknowledge that, despite the apparent weaknesses of CLL-IPI within a population of relatively short follow-up duration (∼40 months), specific factors for PFS (β2-microglobulin, IGHV status, and TP53 status) and survival (β2-microglobulin, age, Binet stage, and TP53 status) continued to offer valuable risk information and retained prognostic value. This prompts consideration of further investigation and reassessing the CLL-IPI score itself and the original weighting of individual factors.
Targeted drugs partially act in a TP53-independent manner that circumvent the refractoriness to chemotherapy conferred by TP53 disruption, which translates into excellent disease control and affects strongly on this poor prognostic subgroup of patients. Previously published long-term data of patients with CLL and TP53 aberration treated first line with ibrutinib reported, at 6 years, an estimated PFS and OS of 61% and 79%, respectively.2 The following reports confirmed excellent disease control in patients at high risk treated first line with targeted drugs.4-9 In venetoclax-based clinical trials, TP53 remained an adverse prognostic factor for PFS.10 In our analysis, the survival of patients with TP53 aberrant CLL had significantly improved with targeted therapies compared with chemoimmunotherapy. Despite the improved survival, TP53 retained prognostic impact in terms of PFS and OS. However, the observation time is relatively short and potentially not capturing the full impact of targeted therapies on survival in this high-risk population.
The IGHV mutational status as a reliable tool in predicting the time to first treatment in patients with treatment-naïve CLL has been implemented in various prognostic models.11,12 Many studies have confirmed the prognostic value of IGHV genes in patients treated with chemoimmunotherapy,13,14 with superior outcomes for patients with mutated IGHV.15,16 In patients treated with targeted drugs, PFS differences are mainly driven by differences among patients with IGHV unmutated status, which particularly benefits compared with that of patients treated with chemoimmunotherapy.3,17,18 In contrast, time-limited, venetoclax-based therapies have shown differences in the durability of response and disease control after the end of treatment3,19 and reported medians of PFS ranging between 57 months for patients with unmutated IGHV and not reached for patients with mutated IGHV.10 Our results confirm the ongoing prognostic value of IGHV for PFS in both subsets of patients treated with either chemoimmunotherapy or targeted drugs.
The serum parameter β2-microglobulin correlates with CLL disease burden and outcomes,20 maintaining its robust prognostic value for PFS and survival in our analysis. Similarly, age, as highlighted by the landmark publication by Rai et al in 1975,21 continues to be a prognostic indicator for survival in CLL, with younger patients showing superior outcomes compared with older age groups in our analysis.
The aim of our study was to provide a broad assessment of the CLL-IPI performance in the era of targeted therapies. However, we fully acknowledge the need for additional, future studies evaluating additional risk factors or focusing on individual agents, particularly Bruton tyrosine kinase inhibitor monotherapy because we did not include this subset of patients.
Our study has some limitations, including the short observation time with an insufficient maturity of OS data and a relatively small number of events in patients treated with targeted drugs. Therefore, our findings need a careful re-evaluation with longer follow-up.
In summary, our analysis of a pooled data set of clinical phase 2 and 3 trials suggests that CLL-IPI maintains its prognostic value in predicting outcomes for PFS in patients treated first line with targeted drugs. However, with a median observation time of 40 months, the impact of CLL-IPI was diminished in predicting survival, underscoring the potential of these targeted therapies to improve outcomes across different risk groups. Compared with chemoimmunotherapy, the survival after targeted therapies is improved. Our findings support the evolving landscape of CLL treatment and highlight the need for the ongoing evaluation of prognostic tools in the context of evolving treatment strategies.
Acknowledgment
This work was supported in part by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), SFB 1530 project C04 (K.F.).
Authorship
Contribution: P.L., K.F., and M. Hallek were responsible for the conception and design of the analysis; A.G., and S.R. performed the analysis; P.L., K.F., and M. Hallek wrote the first draft of the manuscript; and all authors revised the manuscript and approved the final version.
Conflict-of-interest disclosure: P.L. reports honoraria and personal fees from AbbVie, AstraZeneca, BeiGene, and Janssen; and research funding from Janssen. S.R. reports honoraria from AstraZeneca and Merck Sharp Dohme. P.C. reports research funding by Acerta, AstraZeneca, BeiGene, F. Hoffmann-La Roche, Gilead, Janssen-Cilag, and Novartis; honoraria by AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, F. Hoffmann-LaRoche, and Janssen-Cilag; advisory board fees from AbbVie, Acerta, AstraZeneca, BeiGene, Janssen-Cilag, and Novartis; travel support from AbbVie, AstraZeneca, BeiGene, CSL Behring, F. Hoffmann-La Roche, Gilead, Janssen-Cilag, and Novo Nordisk. J.v.T. reports research funding from F. Hoffmann-La Roche and Janssen-Cilag; honoraria for advisory boards from AbbVie, F. Hoffmann-LaRoche, and Janssen-Cilag; and travel grants by Celgene, F. Hoffmann-La Roche, and Janssen-Cilag. O.A.-S. reports honoraria and personal fees from AbbVie, Adaptive, Ascentage, AstraZeneca, BeiGene, Eli Lilly, Gilead, Janssen, and Roche; and research funding from AbbVie, BeiGene, Janssen, and Roche. A.M.F. reports research grants by Celgene; and travel grants by AbbVie, F. Hoffmann-La Roche, and Mundipharma. M.F. reports research grants from AbbVie, AstraZeneca, BeiGene, Janssen, and Roche; and honoraria from AbbVie. N.K. reports research grants from AstraZeneca and Gilead; and honoraria by AbbVie, AstraZeneca, Bristol Myers Squibb, and Kite/Gilead. F.S. reports honoraria from AstraZeneca; research funding from AstraZeneca; and travel support from Eli Lilly. V.G. has received honoraria (speaker and advisory board) from AstraZeneca, AbbVie, Janssen, Gilead, Roche, Bayer, Novartis, Heel, and Berlin Chemie. C.U.N. reports research grants by AstraZeneca and Gilead; and honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb, and Kite/Gilead. C.d.C.-B. reports consulting fee by Janssen; payment or honoraria for lectures, presentations from Octapharma; support for attending meetings and/or travel from AbbVie and Octapharma; and participation on a data safety monitoring board or advisory board for Janssen, AstraZeneca, and BeiGene. A.K. reports research grants from AbbVie, AstraZeneca, Bristol Myers Squibb, Janssen, and Roche; and advisory board for AbbVie, AstraZeneca, Bristol Myers Squibb, Janssen, LAVA, and Roche Genentech. J.D. reports research funding by Roche and Genentech. M.G. reports honoraria for advisory boards from AbbVie, Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen-Cilag, Roche, Sanofi, and Servier; and travel support by AbbVie and BeiGene. P.B.S. reports grants by Roche and Abbvie; honoraria from Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca, Takeda, BeiGene, Lilly, Incyte, and Merck Sharp Dohme. C.S. reports speakers bureau fees from AstraZeneca and AbbVie. S.S. reports advisory board honoraria, research support, travel support, and speaker fees from AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Hoffmann-La Roche, Janssen, Novartis, and Sunesis. B.E. reports grants and personal fees from Janssen-Cilag, Roche, AbbVie, and Gilead; personal fees from Novartis, Celgene, ArQule, AstraZeneca, and Oxford Biomedica (United Kingdom); and grants from BeiGene. K.F. reports honoraria from AbbVie and Roche; and advisory board fee from AstraZeneca. M. Hallek reports receiving personal fees and others from AbbVie, Celgene, Gilead Sciences, Janssen, Mundipharma, Pharmacyclics, and Roche; honoraria (speaker’s bureau and/or advisory board) from Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, and AstraZeneca; and research support from Roche, Gilead, Janssen, Bristol Myers Squibb, AbbVie, and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Petra Langerbeins, Department I of Internal Medicine and German CLL Study Group, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, University of Cologne, Faculty of Medicine and University Hospital Cologne, Kerpener Str 62, 50937 Cologne, Germany; email: petra.langerbeins@uk-koeln.de.
References
Author notes
Data are available upon reasonable request from the corresponding author, Petra Langerbeins (petra.langerbeins@uk-koeln.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal