In this issue of Blood, Usart et al1 provide strong experimental evidence that heterozygous and homozygous deletion of DNA methyltransferase 3 alpha (Dnmt3a) prevents JAK2V617F hematopoietic stem cell (HSC) exhaustion due to interferon alfa (IFN-α) in mouse models and human cells (see figure). This result explains the resistance to IFN-α therapy in JAK2V617F patients with DNMT3A-mutated hematopoietic cells.
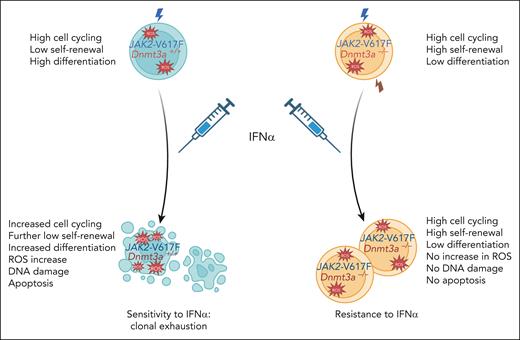
Effects of Dnmt3a deletion on the proliferation, self-renewal, differentiation, and fitness of long-term HSCs carrying JAK2V617F in the absence and presence of treatment with IFN-α. Compared with wild-type JAK2 HSCs, JAK2V617F HSCs exhibit higher cycling, higher proliferation, lower self-renewal, and increased differentiation. After IFN-α treatment, JAK2V617F HSCs increase their cell cycling, with a higher proliferation leading to both a further decrease in self-renewal capacity and an increase in differentiation and probably of apoptosis. This increased proliferation is associated with a replicative stress, including a higher level of reactive oxygen species (ROS) and DNA damage. In contrast, the Dnmt3a–/–JAK2V617F HSCs, although exhibiting a similar cell cycling, exhibit proliferation oriented toward self-renewal at the expense of differentiation, which is not modified by IFN-α.
Effects of Dnmt3a deletion on the proliferation, self-renewal, differentiation, and fitness of long-term HSCs carrying JAK2V617F in the absence and presence of treatment with IFN-α. Compared with wild-type JAK2 HSCs, JAK2V617F HSCs exhibit higher cycling, higher proliferation, lower self-renewal, and increased differentiation. After IFN-α treatment, JAK2V617F HSCs increase their cell cycling, with a higher proliferation leading to both a further decrease in self-renewal capacity and an increase in differentiation and probably of apoptosis. This increased proliferation is associated with a replicative stress, including a higher level of reactive oxygen species (ROS) and DNA damage. In contrast, the Dnmt3a–/–JAK2V617F HSCs, although exhibiting a similar cell cycling, exhibit proliferation oriented toward self-renewal at the expense of differentiation, which is not modified by IFN-α.
At the moment, the only treatment that can induce deep molecular remissions (MRs) in some patients with polycythemia vera and essential thrombocythemia harboring JAK2V617F is type 1 IFN, such as IFN-α. IFN-α induces cycling of normal HSCs,2 but physiologically nonmutated HSCs reenter quiescence despite IFN-α treatment pressure,3 whereas JAK2V617F mutated HSCs are already in cycle and are further stimulated by IFN-α, leading to a replication stress with the acquisition of DNA breaks, exhaustion, and apoptosis.4 This multistep mechanism leads to a slow depletion of mutated HSCs, explaining why MRs are detected after 7 to 12 months of treatment in patients and 14 to 18 weeks of treatment in mice.5 The mechanisms of resistance to IFN-α therapy in DNMT3A-mutated patients remained elusive until this article.
Here, the authors have used elegant mouse models to demonstrate the effects of Dnmt3a loss on JAK2V617F disease development and the response to IFN-α therapy. The development of a mouse model of a Dnmt3a knockout on a JAK2V617F transgenic background is applicable to heterozygous DNMT3A mutations in myeloproliferative neoplasms (MPNs), as the most frequent DNMT3A mutations (around residue R882) behave as a dominant negative. Surprisingly, they found that Dnmt3a loss mitigated the JAK2V617F disease in the absence of any treatment but did increase the hematopoietic stem progenitor cell (HSCP) compartment in the spleen. This result differs from an earlier report by Jacquelin et al6 where Dnmt3a loss in the setting of Jak2V617F induces myelofibrosis. The reasons explaining these differences are not known, but the mouse models are different (human JAK2V617F transgenic vs murine Jak2V617F knock in, Dnmt3a–/– vs CRISPR Cas9 genome editing, and essentially native mice vs transplantation). In the present work, the Dnmt3a loss attenuates the MPN phenotype, confers resistance to IFN-α treatment, but with an increase in splenomegaly, and has no effect on JAK2V617F HSCP in chimeric mice. The authors could show using secondary transplants that Dnmt3a loss induced a 10-fold expansion of JAK2V617F long term (LT)-HSC. This result is reminiscent of the effects of Dnmt3a loss in normal HSCs, which results in a dramatic increase in their self-renewal capacity in serial bone marrow transplantation experiments.7 In addition, they could show that IFN-α treatment did not alter this enhanced self-renewal capacity in JAK2V617F HSC induced by Dnmt3a loss. Furthermore, in the absence of IFN-α, the percentage of LT-HSC in cell cycle was similar in JAK2V617F and Dnmt3a–/–JAK2V617F mice. IFN-α induced cell cycle entry of a large fraction of JAK2V617F LT-HSC, but not of their Dnmt3a–/–JAK2V617F counterparts. Moreover, this increase in cell cycle entry of JAK2V617F LT-HSC was associated with replicative stress, as attested to by an increase in double-stranded DNA breaks and a marked increase in reactive oxygen species, demonstrating the cellular basis of this resistance to IFN-α therapy (see figure). Of interest, replicative stress was not observed after Dnmt3a loss in the wild-type JAK2 setting.
The authors then investigated the molecular mechanisms of IFN-α resistance by using single-cell RNA sequencing (scRNA-seq) at short and long intervals of IFN-α treatment. Surprisingly, resistance did not involve a block in the ability of IFN-α to induce the expression of interferon-stimulated genes (ISGs). To the contrary, signaling and induction of ISGs and genes involved in inflammation, such as those induced by tumor necrosis factor, were higher in Dnmt3a–/–JAK2V617F than in JAK2V617F quiescent LT-HSCs, showing than the resistance to IFN-α therapy is not related to an impaired IFN response. Similarly, it has been shown that Dnmt3a–/– young HSCs are resistant to the proinflammatory action of oncostatin M, due to an increased expression of anti-inflammatory genes, and not due to a defect in the induction of oncostatin M target genes.8 In the present study, the pathway by which Dnmt3a loss induces resistance to IFN-α action could not be completely elucidated by the scRNA-seq approach because of either lack of sensitivity of the method or potential RNA modifications, including splicing and/or deregulation at the protein level. These results indicate the need for single-cell proteomics approaches able to distinguish the protein effectors involved in cell cycle entry and DNA repair.
Finally, the authors investigated whether it was possible to undo the resistance to IFN-α treatment induced by Dnmt3a loss. They could show that a combination of IFN-α with 5-azacytidine nearly completely exhausted JAK2V617F LT-HSCs. However, this combination of IFN-α and 5-azacytidine only partially rescued the molecular resistance to IFN-α induced by Dnmt3a loss by stimulating cell cycle entry and DNA damage of Dnmt3a–/–JAK2V617F HSCs, which may be through the induction of high levels of the Myc/E2F pathways. Such a combination, if not too toxic, might be of interest in the treatment of IFN-α–resistant patients with MPN. Another molecule that could be tested in the future is metformin, which is capable of decreasing the fitness of murine Dnmt3a-mutated HSCs.9
DNMT3A is a key gene mutated in clonal hematopoiesis of undetermined potential. However, with age, the proliferative advantage of DNMT3A-mutated HSCs decreases, whereas TET2 mutations are able to maintain the advantage in such conditions.10 Therefore, new effector mechanisms will need to be identified that explain how DNMT3A gates response to IFN-α and how age regulates and impacts this process.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal